The Effects of the Marination Process with Different Vinegar Varieties on Various Quality Criteria and Heterocyclic Aromatic Amine Formation in Beef Steak
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Chemicals
2.3. Methods
2.3.1. Marinating Process
2.3.2. Cooking Process
2.3.3. Water Content
2.3.4. Cooking Loss
2.3.5. pH Analysis
2.3.6. Lipid Oxidation
2.3.7. Color Analysis
2.3.8. Texture Profile Analysis
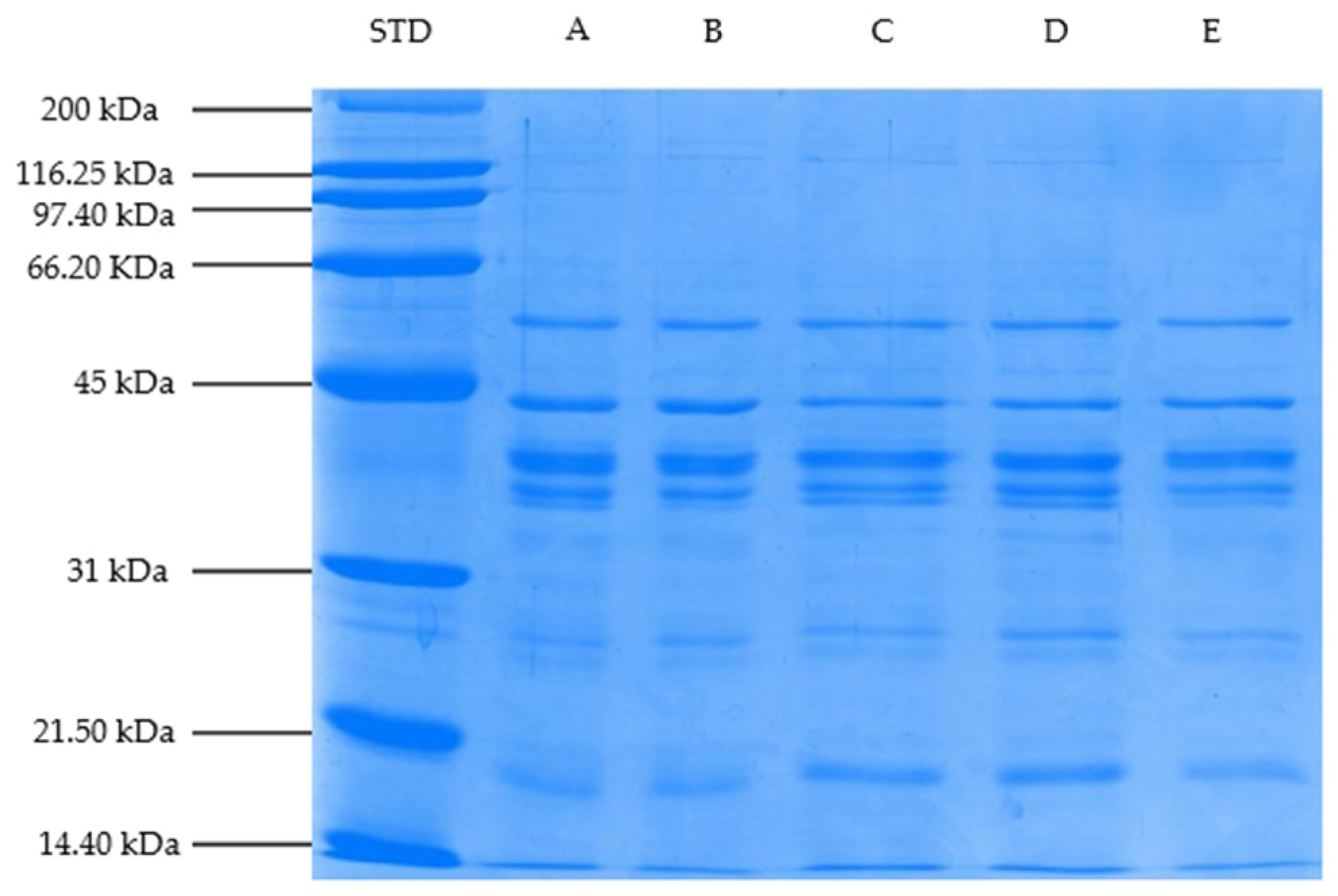
2.3.9. SDS-PAGE Profile of Myofibrillar Proteins
2.3.10. Heterocyclic Aromatic Amine Analysis
2.3.11. Statistical Analysis
3. Results and Discussion
3.1. Some Chemical and Physicochemical Properties of the Raw Material
3.2. Water Contents of the Cooked Steaks
3.3. Cooking Loss Values of Steaks
3.4. pH Values of Steaks
3.5. TBARS Values of Steaks
3.6. Color Values of Steak
3.7. Texture Profile Analysis Results of Steaks
3.8. Changes in Myofibrillar Proteins (SDS-PAGE Profile)
3.9. Heterocyclic Aromatic Amine (HAA) Contents of Steaks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, Y.C.; Choi, Y.M.; Kim, B.C. Variations in metabolite contents and protein denaturation of the longissimus dorsi muscle in various porcine quality classifications and metabolic rates. Meat Sci. 2005, 71, 522–529. [Google Scholar] [CrossRef]
- Lefaucheur, L. A second look into fibre typing–Relation to meat quality. Meat Sci. 2010, 84, 257–270. [Google Scholar] [CrossRef]
- Goli, T.; Ricci, J.; Bohuon, P.; Marchesseau, S.; Collignan, A. Influence of sodium chloride and pH during acidic marination on water retention and mechanical properties of turkey breast meat. Meat Sci. 2014, 96, 1133–1140. [Google Scholar] [CrossRef]
- Bor, Y. Application of Some Natural Antioxidant Sources on Marination of Turkey Meat. Master’s Thesis, Afyon Kocatepe University, Afyonkarahisar, Turkey, 2011. [Google Scholar]
- Cesur, E. Effects of Marinating with Sourcherry, Pomegranate, Orange, Grape or Apple Juice on the Chemical, Sensorial and Textural Properties of Chicken Breast Meat. Master’s Thesis, Celal Bayar University, Manisa, Turkey, 2009. [Google Scholar]
- Onenç, A.; Serdaroğlu, M.; Abdraimov, K. Effect of various additives to marinating baths on some properties of cattle meat. Eur. Food Res. Technol. 2004, 218, 114–117. [Google Scholar] [CrossRef]
- Serdaroglu, M.; Abdraimov, K.; Oenenc, A. The effects of marinating with citric acid solutions and grapefruit juice on cooking and eating quality of turkey breast. J. Muscle Foods 2007, 18, 162–172. [Google Scholar] [CrossRef]
- Sharedeh, D.; Gatellier, P.; Astruc, T.; Daudin, J.-D. Effects of pH and NaCl levels in a beef marinade on physicochemical states of lipids and proteins and on tissue microstructure. Meat Sci. 2015, 110, 24–31. [Google Scholar] [CrossRef]
- Wongmaneepratip, W.; Vangnai, K. Effects of oil types and pH on carcinogenic polycyclic aromatic hydrocarbons (PAHs) in grilled chicken. Food Control. 2017, 79, 119–125. [Google Scholar] [CrossRef]
- Akbaş, M. A Research on the Determination of Composition of Grape Vinegars Produced in Turkey and Their Conformity to Food Legislation. Master’s Thesis, Çukurova University, Adana, Turkey, 2008. [Google Scholar]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar functions on health: Constituents, sources, and formation mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Bozdemir, M.; Kamer, D.D.A.; Akgül, G.; Gümüş, T. Farklı hammaddelerden üretilen sirkelerin bazı fizikokimyasal ve fonksiyonel özellikleri. Tekirdağ Ziraat Fakültesi Derg. 2021, 18, 32–44. [Google Scholar]
- Sengün, İ.Y.; Kılıc, G. Microflora, Bioactive Components and Health Effects of Different Vinegar Varieties. Akad. Food J. 2019, 17, 89–101. [Google Scholar]
- Budak, H.N. A Research on Compositional and Functional Properties of Vinegars Produced from Apple and Grape. Ph.D. Thesis, Suleyman Demirel University, Kaskelen, Kazakhstan, 2010. [Google Scholar]
- Oz, F. Mutagenic and Carcinogenic Compounds in Foods; Ataturk University: Erzurum, Turkey, 2020. [Google Scholar]
- Tornberg, E. Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kızıl, M.; Çelık, T. Effects of different cooking methods on the formation of heterocyclic aromatic amines in goose meat. J. Food Process. Preserv. 2016, 40, 1047–1053. [Google Scholar] [CrossRef]
- Oz, F.; Kızıl, M.; Zaman, A.; Turhan, S. The effects of direct addition of low and medium molecular weight chitosan on the formation of heterocyclic aromatic amines in beef chop. LWT-Food Sci. Technol. 2016, 65, 861–867. [Google Scholar] [CrossRef]
- Skog, K.; Johansson, M.; Jägerstad, M. Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef]
- Sugimura, T. History, present and future, of heterocyclic amines, cooked food mutagens. In Heterocyclic Amines in Cooked Foods: Possible Human Carcinogens; Princeton Scientific Pub: Princeton, NJ, USA, 1995. [Google Scholar]
- Savaş, A.; Oz, E.; Oz, F. Is oven bag really advantageous in terms of heterocyclic aromatic amines and bisphenol-A? Chicken meat perspective. Food Chem. 2021, 355, 129646. [Google Scholar] [CrossRef]
- Skog, K.; Solyakov, A. Heterocyclic amines in poultry products: A literature review. Food Chem. Toxicol. 2002, 40, 1213–1221. [Google Scholar] [CrossRef]
- Sugimura, T. Overview of carcinogenic heterocyclic amines. Mutat. Res. 1997, 376, 211–219. [Google Scholar] [CrossRef]
- Oz, F.; Kaya, M. The inhibitory effect of black pepper on formation of heterocyclic aromatic amines in high-fat meatball. Food Control. 2011, 22, 596–600. [Google Scholar] [CrossRef]
- Klassen, R.; Lewis, D.; Lau, B.-Y.; Sen, N. Heterocyclic aromatic amines in cooked hamburgers and chicken obtained from local fast food outlets in the Ottawa region. Food Res. Int. 2002, 35, 837–847. [Google Scholar] [CrossRef]
- Knize, M.G.; Kulp, K.S.; Salmon, C.P.; Keating, G.A.; Felton, J.S. Factors affecting human heterocyclic amine intake and the metabolism of PhIP. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2002, 506, 153–162. [Google Scholar] [CrossRef]
- Kujawa, M. IARC. Monographs on the evaluation of carcinogenic risks to humans. In Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Shin, H.-S.; Rodgers, W.J.; Gomaa, E.A.; Strasburg, G.M.; Gray, J.I. Inhibition of heterocyclic aromatic amine formation in fried ground beef patties by garlic and selected garlic-related sulfur compounds. J. Food Prot. 2002, 65, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.; Viegas, O.; Petisca, C.; Pinho, O.; Ferreira, I.M. Effect of beer/red wine marinades on the formation of heterocyclic aromatic amines in pan-fried beef. J. Agric. Food Chem. 2008, 56, 10625–10632. [Google Scholar] [CrossRef]
- Quelhas, I.; Petisca, C.; Viegas, O.; Melo, A.; Pinho, O.; Ferreira, I. Effect of green tea marinades on the formation of heterocyclic aromatic amines and sensory quality of pan-fried beef. Food Chem. 2010, 122, 98–104. [Google Scholar] [CrossRef]
- Oz, F.; Kaya, M. The inhibitory effect of red pepper on heterocyclic aromatic amines in fried beef Longissimus dorsi muscle. J. Food Process. Preserv. 2011, 35, 806–812. [Google Scholar] [CrossRef]
- Jamali, M.A.; Zhang, Y.; Teng, H.; Li, S.; Wang, F.; Peng, Z. Inhibitory effect of rosa rugosa tea extract on the formation of heterocyclic amines in meat patties at different temperatures. Molecules 2016, 21, 173. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.M.; Hamzalioglu, A.; Gokmen, V.; Kizil, M. Inhibitory effect of hawthorn extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Food Res. Int. 2017, 99, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Weiss, J. Inhibitory effect of cellulose fibers on the formation of heterocyclic aromatic amines in grilled beef patties. Food Chem. 2017, 229, 828–836. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.M.; Kizil, M. Reducing effect of artichoke extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Meat Sci. 2017, 134, 68–75. [Google Scholar] [CrossRef]
- Uzun, I.; Oz, F. Effect of basil use in meatball production on heterocyclic aromatic amine formation. J. Food Sci. Technol. 2021, 58, 3001–3009. [Google Scholar] [CrossRef]
- Gokalp, H.Y.; Kaya, M.; Tülek, Y.; Zorba, O. Quality Control and Laboratory Application Guide in Meat and Products; Ataturk University: Erzurum, Turkey, 2010. [Google Scholar]
- Oz, F.; Kızıl, M. Determination of heterocyclic aromatic amines in cooked commercial frozen meat products by ultrafast liquid chromatography. Food Anal. Methods 2013, 6, 1370–1378. [Google Scholar] [CrossRef]
- Kilic, B.; Richards, M. Lipid oxidation in poultry döner kebab: Pro-oxidative and anti-oxidative factors. J. Food Sci. 2003, 68, 686–689. [Google Scholar] [CrossRef]
- Oztürk, T.; Turhan, S. Physicochemical properties of pumpkin (Cucurbita pepo L.) seed kernel flour and its utilization in beef meatballs as a fat replacer and functional ingredient. J. Food Process. Preserv. 2020, 44, e14695. [Google Scholar] [CrossRef]
- Molina, I.; Toldra, F. Detection of proteolytic activity in microorganisms isolated from dry-cured ham. J. Food Sci. 1992, 57, 1308–1310. [Google Scholar] [CrossRef]
- Oz, E. The Effect of Muscle Type on Proteolytic Changes and Some Qualitative Properties of Pastirma. Doctorate Thesis, Ataturk University, Erzurum, Turkey, 2018. [Google Scholar]
- Messner, C.; Murkovic, M. Evaluation of a new model system for studying the formation of heterocyclic amines. J. Chromatogr. B 2004, 802, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Elbir, Z.; Oz, F. The assessment of commercial beef and chicken bouillons in terms of heterocyclic aromatic amines and some of their precursors. Int. J. Food Sci. Technol. 2021, 56, 504–513. [Google Scholar] [CrossRef]
- Oz, F. The Effects of Different Spices on Formation of Heterocyclic Aromatic Amines in Fresh Meat Products Which Cooked at Different Temperatures. Master’s Thesis, Ataturk University, Erzurum, Turkey, 2006. [Google Scholar]
- Oz, F.; Zikirov, E. The effects of sous-vide cooking method on the formation of heterocyclic aromatic amines in beef chops. LWT-Food Sci. Technol. 2015, 64, 120–125. [Google Scholar] [CrossRef]
- Zıkırov, E. The Effects of Sous-Vide Cooking Method on the Formation of Heterocyclic Aromatic Amines and Some Quaiative Criteria in Beef. Master’s Thesis, Ataturk University, Erzurum, Turkey, 2014. [Google Scholar]
- Sun, S.; Rasmussen, F.D.; Cavender, G.A.; Sullivan, G.A. Texture, color and sensory evaluation of sous-vide cooked beef steaks processed using high pressure processing as method of microbial control. LWT 2019, 103, 169–177. [Google Scholar] [CrossRef]
- Del Pulgar, J.S.; Gázquez, A.; Ruiz-Carrascal, J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef]
- Kotula, K.L.; Thelappurate, R. Microbiological and sensory attributes of retail cuts of beef treated with acetic and lactic acid solutions. J. Food Prot. 1994, 57, 665–670. [Google Scholar] [CrossRef]
- Mikel, W.; Goddard, B.; Bradford, D. Muscle microstructure and sensory attributes of organic acid-treated beef strip loins. J. Food Sci. 1996, 61, 1058–1062. [Google Scholar] [CrossRef]
- Seong, P.-N.; Kim, J.-H.; Cho, S.-H.; Kang, G.-H.; Park, B.-Y.; Park, K.-M.; Kim, D.-H.; Kim, D. The effects of marinating with commercial vinegars on the quality characteristics of biceps femoris muscle on Hanwoo. Ann. Anim. Resour. Sci. 2012, 23, 26–32. [Google Scholar]
- Young, L.; Lyon, C. Effect of calcium marination on biochemical and textural properties of peri-rigor chicken breast meat. Poult. Sci. 1997, 76, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, D.; Pasquini, M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT-Food Sci. Technol. 2005, 38, 895–901. [Google Scholar] [CrossRef]
- Koeipudsa, C.; Malila, Y.; Limpisophon, K. Improving tenderness of breast meat of spent-laying hens using marination in alkaline or acidic solutions. Asia-Pac. J. Sci. Technol. 2019, 24, 1–8. [Google Scholar]
- Aktaş, N.; Kaya, M. The influence of marinating with weak organic acids and salts on the intramuscular connective tissue and sensory properties of beef. Eur. Food Res. Technol. 2001, 213, 88–94. [Google Scholar] [CrossRef]
- Burke, R.; Monahan, F. The tenderisation of shin beef using a citrus juice marinade. Meat Sci. 2003, 63, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Estrada, M.; Penazzi, G.; Caboni, M.; Bertacco, G.; Lercker, G. Effect of different cooking methods on some lipid and protein components of hamburgers. Meat Sci. 1997, 45, 365–375. [Google Scholar] [CrossRef]
- LaRoche, A.P.; Boyer, R.F.; Clark, H.M. Reduction and release of ferritin iron by plant phenolics. J. Inorg. Biochem. 1988, 32, 171–181. [Google Scholar]
- Girard, P.J. Cooking. In Technology of Meat and Meat Products; Ellis Horwood: Chichester, UK, 1992; pp. 32–83. [Google Scholar]
- Jones, M.; Arnaud, E.; Gouws, P.; Hoffman, L.C. Effects of the addition of vinegar, weight loss and packaging method on the physicochemical properties and microbiological profile of biltong. Meat Sci. 2019, 156, 214–221. [Google Scholar] [CrossRef]
- Tänavots, A.; Põldvere, A.; Kerner, K.; Veri, K.; Kaart, T.; Torp, J. Effects of mustard-honey, apple vinegar, white wine vinegar and kefir acidic marinades on the properties of pork. Vet. Ir Zootech. 2018, 76, 98. [Google Scholar]
- Aktaş, N.; Aksu, M.; Kaya, M. The effect of organic acid marination on tenderness, cooking loss and bound water content of beef. J. Muscle Foods 2003, 14, 181–194. [Google Scholar] [CrossRef]
- Ertbjerg, P.; Mielche, M.M.; Larsen, L.M.; Møller, A.J. Relationship between proteolytic changes and tenderness in prerigor lactic acid marinated beef. J. Sci. Food Agric. 1999, 79, 970–978. [Google Scholar] [CrossRef]
- Morris, C.; Theis, R.; Miller, R.; Acuff, G.; Savell, J. Improving the flavor of calcium chloride and lactic acid injected mature beef top round steaks. Meat Sci. 1997, 45, 531–537. [Google Scholar] [CrossRef]
- Oreskovich, D.; Bechtel, P.; McKeith, F.; Novakofski, J.; Basgall, E. Marinade pH affects textural properties of beef. J. Food Sci. 1992, 57, 305–311. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Esfahani Mehr, A. The effect of meat marinating with lactic and citric acid on some physicochemical and electrophoretic pattern of beef burger. Iran. J. Vet. Med. 2015, 9, 103–108. [Google Scholar]
- Seuss, I.; Martin, M. The Influence of Marinating with Food Acids on the Composition and Sensory Properties of Beef. Fleischwirtschaft 1993, 73, 295. [Google Scholar]
- Yusop, S.M.; O’Sullivan, M.G.; Kerry, J.F.; Kerry, J.P. Effect of marinating time and low pH on marinade performance and sensory acceptability of poultry meat. Meat Sci. 2010, 85, 657–663. [Google Scholar] [CrossRef]
- Ramírez, M.; Morcuende, D.; Estévez, M.; López, R.C. Fatty acid profiles of intramuscular fat from pork loin chops fried in different culinary fats following refrigerated storage. Food Chem. 2005, 92, 159–167. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D. Mechanism of lipid peroxidation in meat and meat products-A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Hayes, J.; Stepanyan, V.; O’Grady, M.; Allen, P.; Kerry, J. Evaluation of the effects of selected phytochemicals on quality indices and sensorial properties of raw and cooked pork stored in different packaging systems. Meat Sci. 2010, 85, 289–296. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Alves, S.P.; Lopes, A.F.; Fernandes, M.J.; Costa, A.S.; Fontes, C.M.; Castro, M.L.; Bessa, R.J.; Prates, J.A. Effect of cooking methods on fatty acids, conjugated isomers of linoleic acid and nutritional quality of beef intramuscular fat. Meat Sci. 2010, 84, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Dal Bello, F.; Meineri, G. Effect of cooking method on carnosine and its homologues, pentosidine and thiobarbituric acid-reactive substance contents in beef and turkey meat. Food Chem. 2012, 132, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Librelotto, J.; Cofrades, S.; Sánchez-Muniz, F.; Jiménez-Colmenero, F. Composition and physicochemical characteristics of restructured beef steaks containing walnuts as affected by cooking method. Meat Sci. 2007, 77, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victório, A.d.M.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Meinert, L.; Andersen, L.T.; Bredie, W.L.; Bjergegaard, C.; Aaslyng, M.D. Chemical and sensory characterisation of pan-fried pork flavour: Interactions between raw meat quality, ageing and frying temperature. Meat Sci. 2007, 75, 229–242. [Google Scholar] [CrossRef]
- Campo, M.; Nute, G.; Hughes, S.; Enser, M.; Wood, J.; Richardson, R. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- Bilek, A.E.; Turhan, S. Enhancement of the nutritional status of beef patties by adding flaxseed flour. Meat Sci. 2009, 82, 472–477. [Google Scholar] [CrossRef]
- Oz, F. Effects of Water Extract of U rtica dioica L. on the Quality of Meatballs. J. Food Process. Preserv. 2014, 38, 1356–1363. [Google Scholar] [CrossRef]
- Yıldız-Turp, G.; Serdaroglu, M. Effects of using plum puree on some properties of low fat beef patties. Meat Sci. 2010, 86, 896–900. [Google Scholar] [CrossRef]
- Aberle, E.D.; Forrest, J.C.; Gerrard, D.E.; Mills, E.W.; Hedrick, H.B.; Judge, M.D. Structure and composition of animal tissues. In Principles of Meat Science; Kendall and Hunt: Dubuque, IA, USA, 2001; Volume 116. [Google Scholar]
- Roldán, M.; Antequera, T.; Martín, A.; Mayoral, A.I.; Ruiz, J. Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci. 2013, 93, 572–578. [Google Scholar] [CrossRef]
- Arganosa, G.C.; Marriott, N.G. Organic acids as tenderizers of collagen in restructured beef. J. Food Sci. 1989, 54, 1173–1176. [Google Scholar] [CrossRef]
- Bell, M.; Marshall, R.; Anderson, M. Microbiological and sensory tests of beef treated with acetic and formic acids. J. Food Prot. 1986, 49, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jin, S.; Park, W.; Kim, B.; Joo, S.; Yang, H. The effect of garlic or onion marinade on the lipid oxidation and meat quality of pork during cold storage. J. Food Qual. 2010, 33, 171–185. [Google Scholar] [CrossRef]
- Oztürk, T. Potential Use of Pumpkin (Cucurbita pepo L.) Seed Flour as Fat Replacer and Functional Ingredient in the Meatball Production. Master’s Thesis, Ondokuz Mayıs University, Atakum, Samsun, Turkey, 2019. [Google Scholar]
- Gault, N. The relationship between water-holding capacity and cooked meat tenderness in some beef muscles as influenced by acidic conditions below the ultimate pH. Meat Sci. 1985, 15, 15–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Huang, Y.; Chen, L.; Bao, P.; Fang, H.; Xu, B.; Zhou, C. Effects of basic amino acid on the tenderness, water binding capacity and texture of cooked marinated chicken breast. LWT 2020, 129, 109524. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Stanley, D.W. Microstructural Principles of Food Processing and Engineering; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Anderson, M.J. Identification of Proteins and Biological Processes Associated with Tenderness in Beef Muscles; Iowa State University: Ames, IA, USA, 2011. [Google Scholar]
- Kajak-Siemaszko, K.; Aubry, L.; Peyrin, F.; Bax, M.-L.; Gatellier, P.; Astruc, T.; Przybylski, W.; Jaworska, D.; Gaillard-Martinie, B.; Santé-Lhoutellier, V. Characterization of protein aggregates following a heating and freezing process. Food Res. Int. 2011, 44, 3160–3166. [Google Scholar] [CrossRef]
- Dai, Y.; Miao, J.; Yuan, S.-Z.; Liu, Y.; Li, X.-M.; Dai, R.-T. Colour and sarcoplasmic protein evaluation of pork following water bath and ohmic cooking. Meat Sci. 2013, 93, 898–905. [Google Scholar] [CrossRef]
- Murkovic, M.; Steinberger, D.; Pfannhauser, W. Antioxidant spices reduce the formation of heterocyclic amines in fried meat. Z. Für Lebensm. Und-Forsch. A 1998, 207, 477–480. [Google Scholar] [CrossRef]
- Lan, C.; Chen, B. Effects of soy sauce and sugar on the formation of heterocyclic amines in marinated foods. Food Chem. Toxicol. 2002, 40, 989–1000. [Google Scholar] [CrossRef]
- Knize, M.G.; Salmon, C.P.; Mehta, S.S.; Felton, J.S. Analysis of cooked muscle meats for heterocyclic aromatic amine carcinogens. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1997, 376, 129–134. [Google Scholar] [CrossRef]
- Toribio, F.; Busquets, R.; Puignou, L.; Galceran, M.T. Heterocyclic amines in griddled beef steak analysed using a single extract clean-up procedure. Food Chem. Toxicol. 2007, 45, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Jinap, S.; Mohd-Mokhtar, M.; Farhadian, A.; Hasnol, N.; Jaafar, S.; Hajeb, P. Effects of varying degrees of doneness on the formation of heterocyclic aromatic amines in chicken and beef satay. Meat Sci. 2013, 94, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Szterk, A. Heterocyclic aromatic amines in grilled beef: The influence of free amino acids, nitrogenous bases, nucleosides, protein and glucose on HAAs content. J. Food Compos. Anal. 2015, 40, 39–46. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Inhibitory effect of marinades with hibiscus extract on formation of heterocyclic aromatic amines and sensory quality of fried beef patties. Meat Sci. 2010, 85, 735–742. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kataoka, H.; Ishihara, J.; Takachi, R.; Hamada, G.S.; Sharma, S.; Le Marchand, L.; Tsugane, S. Heterocyclic amines content of meat and fish cooked by Brazilian methods. J. Food Compos. Anal. 2010, 23, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.S.; Ameri, F.; Gadgil, P. Effect of marinades on the formation of heterocyclic amines in grilled beef steaks. J. Food Sci. 2008, 73, T100–T105. [Google Scholar] [CrossRef]
- Bakir, S.; Devecioglu, D.; Kayacan, S.; Toydemir, G.; Karbancioglu-Guler, F.; Capanoglu, E. Investigating the antioxidant and antimicrobial activities of different vinegars. Eur. Food Res. Technol. 2017, 243, 2083–2094. [Google Scholar] [CrossRef]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Chen, B.; Lee, K.; Tai, C.-Y. Formation of heterocyclic amines in fried fish fiber during processing and storage. J. Food Prot. 2000, 63, 1415–1420. [Google Scholar] [CrossRef]
- Kilic, S.; Oz, E.; Oz, F. Effect of turmeric on the reduction of heterocyclic aromatic amines and quality of chicken meatballs. Food Control. 2021, 128, 108189. [Google Scholar] [CrossRef]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Jägerstad, M. Influence of pro-and antioxidants on the formation of mutagenic-carcinogenic heterocyclic amines in a model system. Food Chem. 1996, 56, 69–75. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Impact of precursors creatine, creatinine, and glucose on the formation of heterocyclic aromatic amines in grilled patties of various animal species. J. Food Sci. 2015, 80, C2430–C2439. [Google Scholar] [CrossRef] [PubMed]
- Emamgholizadeh, F. Effects of Marinades on the Formation of Heterocyclic Amines in Grilled Beef Steaks. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2008. [Google Scholar]
- Lee, J.; Dong, A.; Jung, K.; Shin, H.-S. Influence of extra virgin olive oil on the formation of heterocyclic amines in roasted beef steak. Food Sci. Biotechnol. 2011, 20, 159–165. [Google Scholar] [CrossRef]
- Oz, F.; Kizil, M.; Cakmak, I.; Aksu, M. The effect of direct addition of conjugated linoleic acid on the formation of heterocyclic aromatic amines in beef chops. J. Food Process. Preserv. 2015, 39, 2820–2833. [Google Scholar] [CrossRef]
- Viegas, O.; Novo, P.; Pinto, E.; Pinho, O.; Ferreira, I.M. Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem. Toxicol. 2012, 50, 2128–2134. [Google Scholar] [CrossRef]
- Oz, F.; Kaban, G.; Kaya, M. Effects of cooking methods and levels on formation of heterocyclic aromatic amines in chicken and fish with Oasis extraction method. LWT-Food Sci. Technol. 2010, 43, 1345–1350. [Google Scholar] [CrossRef]
- Oz, E. Comparison of Sous-Vide Cooking Method with Some Traditional Cooking Methods in Terms of Meat Texture and SDS-PAGE profile; Ataturk University: Erzurum, Turkey, 2021. [Google Scholar]
- Robbana-Barnat, S.; Rabache, M.; Rialland, E.; Fradin, J. Heterocyclic amines: Occurrence and prevention in cooked food. Environ. Health Perspect. 1996, 104, 280–288. [Google Scholar] [CrossRef]
- Skog, K. Problems associated with the determination of heterocyclic amines in cooked foods and human exposure. Food Chem. Toxicol. 2002, 40, 1197–1203. [Google Scholar] [CrossRef]


| Analysis | Results |
|---|---|
| Water (%) | 78.09 ± 0.33 |
| pH | 5.76 ± 0.09 |
| TBARS (mg MDA/kg) | 0.181 ± 0.05 |
| L* | 31.67 ± 1.52 |
| a* | 16.42 ± 0.96 |
| b* | 6.17 ± 0.54 |
| Treatment | Water (%) | Cooking Loss (%) | pH | TBARS | L* | a* | b* |
|---|---|---|---|---|---|---|---|
| Control | 62.41 ± 2.02 a | 46.83 ± 13.48 a | 5.90 ± 0.06 a | 0.271 ± 0.06 a | 26.07 ± 2.33 b | 7.23 ± 0.68 ab | 8.04 ± 0.82 b |
| Balsamic | 64.02 ± 1.93 a | 46.52 ± 11.81 a | 5.60 ± 0.11 b | 0.353 ± 0.05 a | 24.41 ± 1.67 b | 6.54 ± 0.60 b | 7.46 ± 0.76 b |
| Pomegranate | 61.68 ± 3.34 a | 49.18 ± 11.29 a | 5.67 ± 0.07 b | 0.326 ± 0.10 a | 22.23 ± 1.64 c | 5.39 ± 0.46 c | 6.13 ± 0.80 c |
| Apple | 63.26 ± 3.83 a | 46.90 ± 12.12 a | 5.67 ± 0.04 b | 0.294 ± 0.08 a | 30.37 ± 3.38 a | 7.47 ± 0.41 a | 9.66 ± 0.78 a |
| Grape | 62.29 ± 3.84 a | 47.60 ± 13.66 a | 5.68 ± 0.08 b | 0.275 ± 0.19 a | 25.16 ± 1.80 b | 7.26 ± 0.67 ab | 8.04 ± 0.59 b |
| Sign | ns | ns | ** | ns | ** | ** | ** |
| Treatment | Hardness (N) | Springiness (mm) | Cohesiveness | Chewiness (N.mm) |
|---|---|---|---|---|
| Control | 215.37 ± 140.74 a | 0.89 ± 0.08 a | 0.79 ± 0.10 a | 157.77 ± 120.28 a |
| Balsamic | 216.35 ± 55.61 a | 0.80 ± 0.03 b | 0.74 ± 0.03 a | 128.43 ± 36.62 a |
| Pomegranate | 211.28 ± 109.02 a | 0.89 ± 0.08 a | 0.80 ± 0.11 a | 157.61 ± 107.95 a |
| Apple | 237.90 ± 65.65 a | 0.87 ± 0.07 ab | 0.78 ± 0.04 a | 161.06 ± 45.17 a |
| Grape | 137.26 ± 84.37 a | 0.80 ± 0.06 b | 0.73 ± 0.09 a | 84.75 ± 65.64 a |
| Sign | ns | * | ns | ns |
| Treatment | MeIQx (ng/g) | Total HAAs (ng/g) |
|---|---|---|
| Control | 0.95 ± 0.62 b | 1.04 ± 0.71 b |
| Balsamic | 0.67 ± 0.50 b | 0.67 ± 0.50 b |
| Pomegranate | 2.22 ± 1.14 a | 2.22 ± 1.11 a |
| Apple | 0.59 ± 0.84 b | 0.59 ± 0.85 b |
| Grape | 1.34 ± 1.00 ab | 1.34 ± 1.00 ab |
| Sign | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fencioglu, H.; Oz, E.; Turhan, S.; Proestos, C.; Oz, F. The Effects of the Marination Process with Different Vinegar Varieties on Various Quality Criteria and Heterocyclic Aromatic Amine Formation in Beef Steak. Foods 2022, 11, 3251. https://doi.org/10.3390/foods11203251
Fencioglu H, Oz E, Turhan S, Proestos C, Oz F. The Effects of the Marination Process with Different Vinegar Varieties on Various Quality Criteria and Heterocyclic Aromatic Amine Formation in Beef Steak. Foods. 2022; 11(20):3251. https://doi.org/10.3390/foods11203251
Chicago/Turabian StyleFencioglu, Halenur, Emel Oz, Sadettin Turhan, Charalampos Proestos, and Fatih Oz. 2022. "The Effects of the Marination Process with Different Vinegar Varieties on Various Quality Criteria and Heterocyclic Aromatic Amine Formation in Beef Steak" Foods 11, no. 20: 3251. https://doi.org/10.3390/foods11203251
APA StyleFencioglu, H., Oz, E., Turhan, S., Proestos, C., & Oz, F. (2022). The Effects of the Marination Process with Different Vinegar Varieties on Various Quality Criteria and Heterocyclic Aromatic Amine Formation in Beef Steak. Foods, 11(20), 3251. https://doi.org/10.3390/foods11203251







