Novel Insight into the Formation of Odour—Active Compounds in Sea Buckthorn Wine and Distilled Liquor Based on GC–MS and E–Nose Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of SW and DL
2.2. Determination of Antioxidant Activity
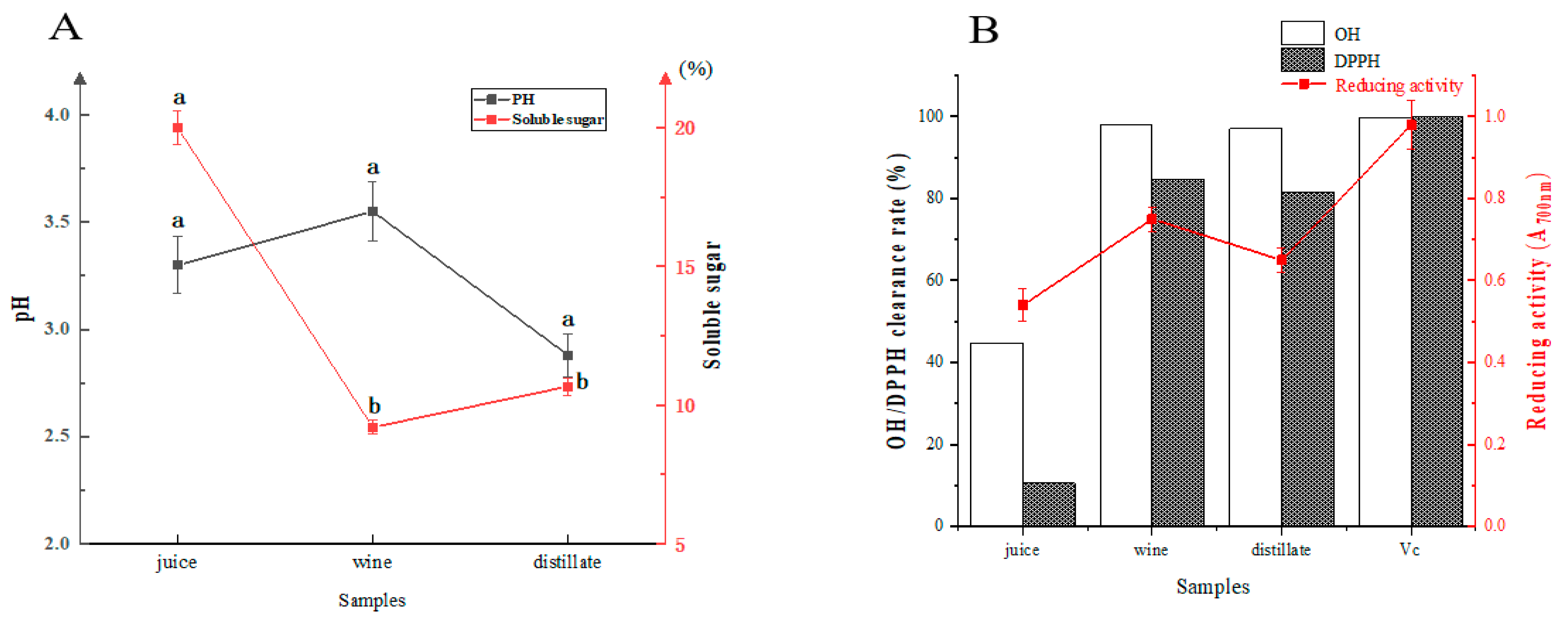
2.2.1. OH· Radical Scavenging Rate
2.2.2. DPPH Clearance Rate
2.2.3. Reducing Activity
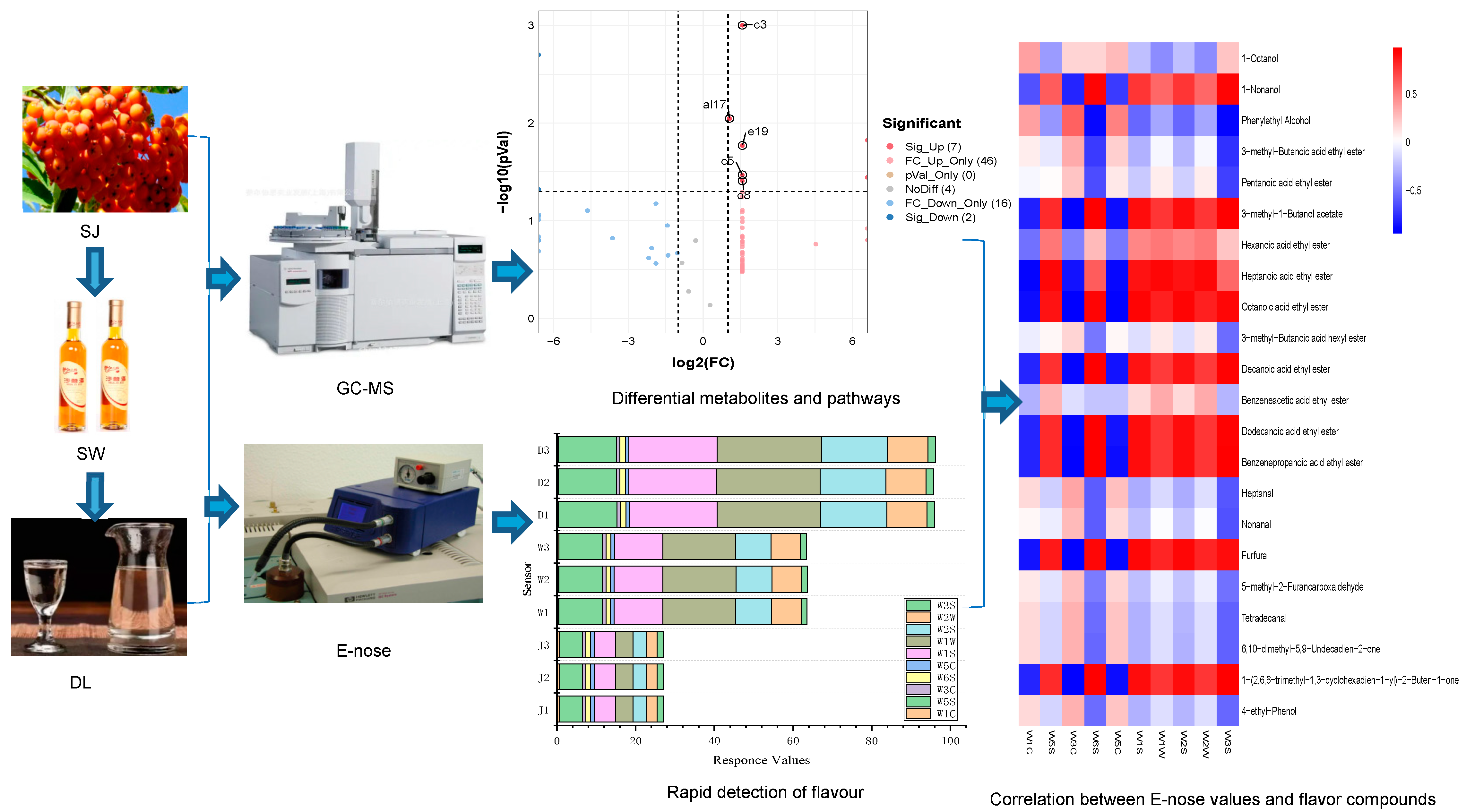
2.3. GC–MS Detection
2.4. E–Nose Measurement
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Sea Buckthorn Wine
3.2. Comparison of VOCs
3.3. Odour–Active Compounds
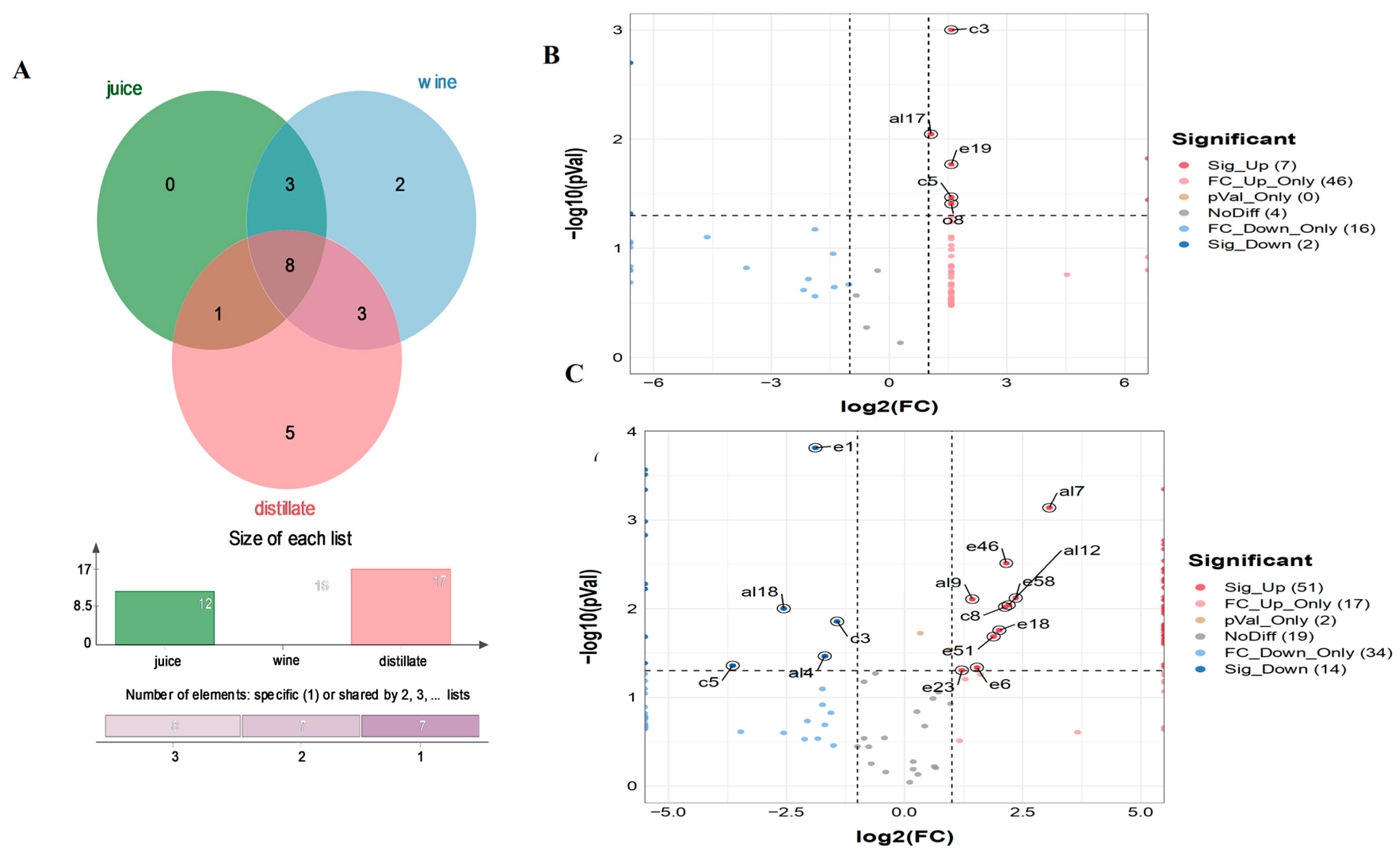
3.4. Differential Metabolites
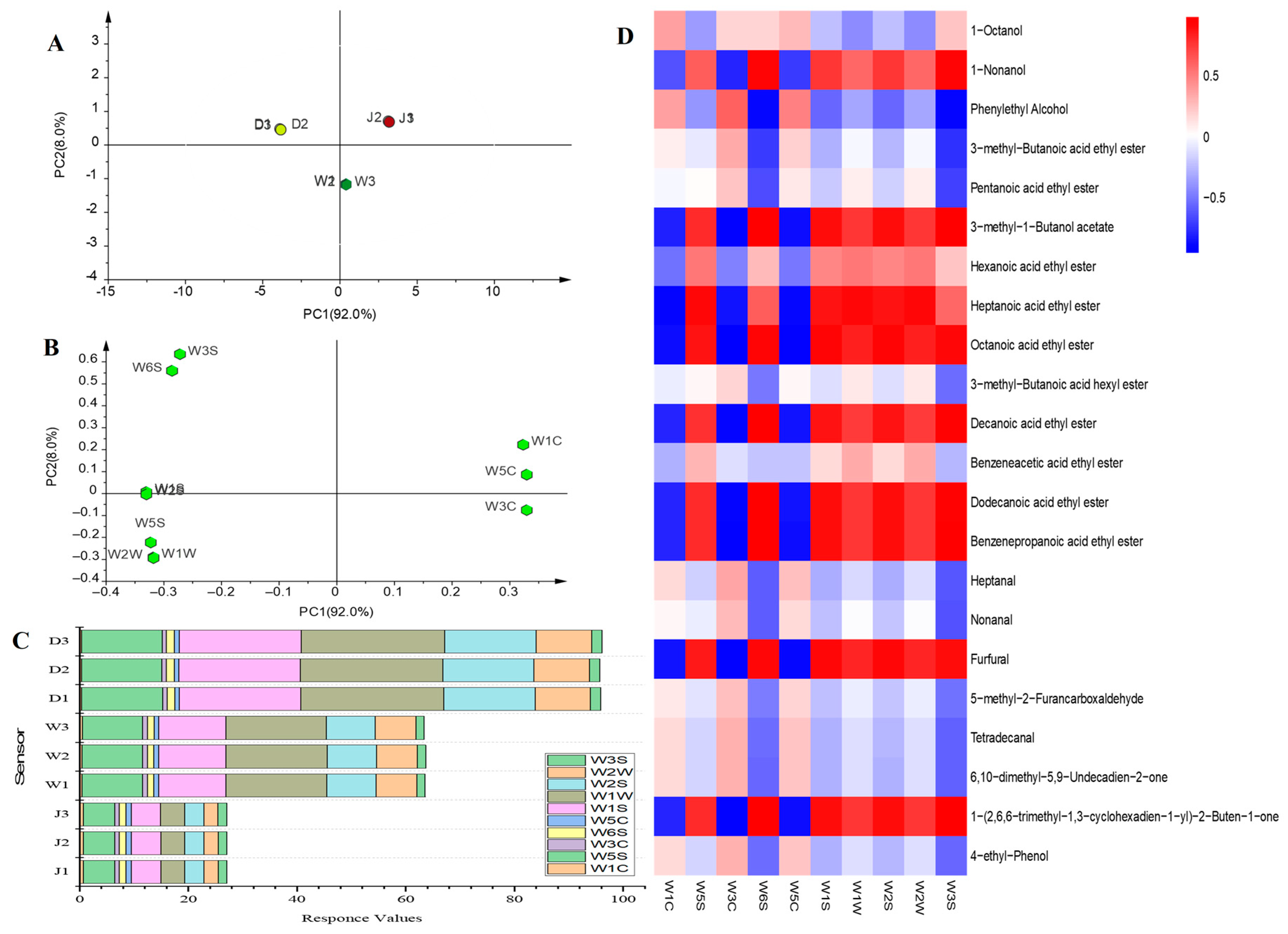
3.5. Rapid Detection of Flavour
3.6. Correlation between E–Nose Values and Key Flavour Compounds
4. Discussion
4.1. Fewer Differential Metabolites before and after Fermentation
4.2. Metabolic Pathways before and after Fermentation
4.3. Abundant Differential Metabolites before and after Distillation
4.4. Combined Use and Analysis of GC–MS and E–Nose
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ollinger, N.; Neuhauser, C.; Schwarzinger, B.; Wallner, M.; Schwarzinger, C.; Blank–Landeshammer, B.; Hager, R.; Sadova, N.; Drotarova, I.; Mathmann, K.; et al. Anti–hyperglycemic effects of oils and extracts derived from Sea Buckthorn—A comprehensive analysis utilizing in vitro and in vivo models. Mol. Nutr. Food Res. 2022, 66, 2101133. [Google Scholar] [CrossRef] [PubMed]
- Ran, B.-B.; Li, W.-D. Research progress on chemical constituents and their differences between sea buckthorn berries and leaves. China J. Chin. Mater. Med. 2019, 44, 1767–1773. [Google Scholar] [CrossRef]
- Christaki, E. Hippophae Rhamnoides L. (sea buckthorn): A potential source of nutraceuticals. Food Public Health 2012, 2, 69–72. [Google Scholar] [CrossRef]
- Krejcarová, J.; Straková, E.; Suchý, P.; Herzig, I.; Karásková, K. Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities—A review. Acta Vet. Brno 2015, 84, 257–268. [Google Scholar] [CrossRef]
- Negi, B. Protective effects of a novel seabuckthorn wine on oxidative stress and hypercholesterolemia. Food Funct. 2013, 4, 240–248. [Google Scholar] [CrossRef]
- Chung, N.; Jo, Y.; Joe, M.H.; Jeong, M.H.; Jeong, Y.J.; Kwon, J.H. Rice vinegars of different origins: Discriminative characteristics based on solid–phase microextraction and gas chromatography with mass spectrometry, an electronic nose, electronic tongue and sensory evaluation. J. Inst. Brew. 2017, 123, 159–166. [Google Scholar] [CrossRef]
- Shi, J.; Nian, Y.; Da, D.; Xu, X.; Zhou, G.; Zhao, D.; Li, C.B. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography–mass spectrometry and electronic nose. LWT 2020, 124, 109182. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Y.R.; Luo, R.; Ding, D.; Bai, H.; Shen, F. Characterization of flavor volatile compounds in industrial stir–frying mutton sao zi by GC–MS, E–nose, and physicochemical analysis. Food Sci. Nutr. 2021, 9, 499–513. [Google Scholar] [CrossRef]
- Kimura, K.; Kubo, Y. Flavor development during natto fermentation. J. Jpn. Soc. Food Sci–Nippon. Shokuhin Kagaku Kogaku Kaishi 2017, 64, 379–384. [Google Scholar] [CrossRef]
- Socaci, S.A.; Socaciu, C.; Tofană, M.; Raţi, I.V.; Pintea, A. In–tube extraction and GC–MS analysis of volatile components from wild and cultivated sea buckthorn (Hippophae rhamnoides L. ssp. Carpatica) berry varieties and juice. Phytochem. Anal. 2013, 24, 319–328. [Google Scholar] [CrossRef]
- Tian, C.; Nan, P.; Chen, J.; Zhong, Y. Volatile composition of Chinese Hippophae rhamnoides and its chemotaxonomic implications. Biochem. Syst. Ecol. 2004, 32, 431–441. [Google Scholar] [CrossRef]
- Lopez, J.; Kerley, T.; Jenkinson, L.; Luckett, C.R.; Munafo, J.P. Odorants from the thermal treatment of hydrolyzed mushroom protein and cysteine enhance saltiness perception. J. Agric. Food Chem. 2019, 67, 11444–11453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Bratcher, C.L. Differentiation of commercial ground beef products and correlation between metabolites and sensory attributes: A metabolomic approach. Food Res. Int. 2016, 90, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Windes, S.; Bettenhausen, H.M.; Van Simaeys, K.R.; Clawson, J.; Fisk, S.; Heuberger, A.L.; Lim, J.; Queisser, S.H.; Shellhammer, T.H.; Hayes, P.M. Comprehensive analysis of different contemporary barley genotypes enhances and expands the scope of barley contributions to beer flavor. J. Am. Soc. Brew. Chem. 2021, 79, 281–305. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xing, L. Effects of ultrasound on the taste components from aqueous extract of unsmoked bacon. Food Chem. 2021, 365, 130411. [Google Scholar] [CrossRef]
- Zawirska–Wojtasiak, R.; Mildner–Szkudlarz, S. From the human nose to artificial olfaction. Agro Food Ind. Hi–Tech 2010, 21, 38–43. [Google Scholar]
- Liu, Y.; Sheng, J.; Li, J.; Zhang, P.; Tang, F.; Shan, C. Influence of lactic acid bacteria on physicochemical indexes, sensory and flavor characteristics of fermented sea buckthorn juice. Food Biosci. 2022, 46, 101519. [Google Scholar] [CrossRef]
- Da, D.; Nian, Y.; Shi, J.; Li, Y.; Zhao, D.; Zhang, G.; Li, C. Characterization of specific volatile components in braised pork with different tastes by SPME–GC/MS and electronic nose. J. Food Process. Preserv. 2021, 45, e15492. [Google Scholar] [CrossRef]
- He, J. Research of Carotenoids, Polyphenols and Antioxidant Activity of Sea Buckthorn Wines During Fermentation. Master’s thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2015. [Google Scholar]
- Xu, H.; Yan, N.; Chen, W.; Liu, L. Ultrasonic assisted extraction technology and its antioxidative activity of blackberry anthocyanin. Trans. CSAE 2008, 24, 264–269. [Google Scholar]
- Liu, F.; Ooi, V.; Chang, S. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Choonpicharn, S.; Tanreuan, K.; Lumyong, S. Characterization of polysaccharides from wild ddible mushrooms from Thailand and their antioxidant, antidiabetic, and antihypertensive activities. Int. J. Med. Mushrooms 2020, 22, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, Y.; Wang, J.; Shuang, Q. Assessment of key aroma compounds in fresh jujube brandy by GC–O–MS and odor activity value. J. Food Process. Preserv. 2020, 44, e14494. [Google Scholar] [CrossRef]
- Cui, D.D.; Liu, Y.; Chen, Y.P.; Feng, X.; Lu, Y.; Yu, B. Application of SPME–GC–TOFMS, E–nose, and sensory evaluation to investigate the flavor characteristics of Chinese Yunnan coffee at three different conditions (beans, ground powder, and brewed coffee). Flavour Fragr. J. 2020, 35, 541–560. [Google Scholar] [CrossRef]
- Wang, N.; Wen, X.; Gao, Y.; Lu, S.; Li, Y.; Shi, Y.; Yang, Z. Identification and Characterization of the Bioactive Polyphenols and Volatile Compounds in Sea Buckthorn Leaves Tea Together With Antioxidant and α–Glucosidase Inhibitory Activities. Front. Nutr. 2022, 29, 890486. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z. Functional ingredients and in vitro antioxidative activity of aloe liquor. China Brew. 2014, 33, 57–61. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhou, L.; Zhang, R. Study on the influences of ultrasound on the flavor profile of unsmoked bacon and its underlying metabolic mechanism by using HS–GC–IMS. Ultrason Sonochem. 2021, 80, 105807. [Google Scholar] [CrossRef]
- Xing, W. Research on Sea Buckthorn Wine Malolactic Fermentation Conditions and Characteristics. Master’s thesis, Northeast Agricultural University, Harbin, China, 2011. [Google Scholar]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography–mass spectrometry–olfactometry (GC–MS–O) and comprehensive two–dimensional gas chromatography mass spectrometry (GC×GC–qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Rekha, M.N.; Ramteke, R.S.P.; Eipeson, W.E. Seabuckthorn (Hippophae rhamnoides Lin.) berries: Harnessing its potential for processing. J. Food Sci. Technol. 2003, 40, 349–356. [Google Scholar]
- Niu, Y.; Liu, Y.; Xiao, Z. Evaluation of perceptual interactions between ester aroma components in Langjiu by GC–MS, GC–O, sensory analysis, and vector model. Foods 2020, 9, 183. [Google Scholar] [CrossRef]
- Jha, S.K.; Zhang, J.; Hayashi, K.; Liu, C. Identification of discriminating chemical compounds in banana species and their odor characterization using GC–MS, statistical, and clustering analysis. J. Food Sci. Technol. Mys. 2022, 59, 402–408. [Google Scholar] [CrossRef]
- Ma, N. Comparative analysis of nutritional components and flavoring components of seabuckthorn wine & grape wine. Liquor. Sci. Technol. 2014, 02, 92–97. [Google Scholar] [CrossRef]
- Lukša, J.; Vepštaitė-Monstavičė, I.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Stanevičienė, R.; Strazdaitė-Žielienė, Z.; Ravoitytė, B.; Aleknavičius, D.; Būda, V.; Mozūraitis, R.; et al. Fungal microbiota of sea buckthorn berries at two ripening stages and volatile profiling of potential biocontrol yeasts. Microorganisms 2020, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.; Bauer, F.E.; Panzeri, V.; Buica, A. Testing the sensitivity of potential panelists for wine taint compounds using a simplified sensory strategy. Foods 2018, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Buica, A. Investigating the effects of two volatile phenols on aroma perception of four red wine cultivars using projective mapping. J. Sens. Stud. 2021, 36, e12616. [Google Scholar] [CrossRef]
- Maturano, Y.; Nally, M.; Assof, M.; Toro, M.; de Figueroa, L.C.; Jofré, V.; Vazquez, F. Free volatile compounds of cv. Pedro Gimenez (Vitis vinifera L.) white grape must grown in San Juan, Argentina. South Afr. J. Enol. Vitic. 2018, 39, 27–34. [Google Scholar] [CrossRef]
- Martín, D.; Osorio, C. Identification of aroma–active volatile compounds in Pouteria sapota fruit by aroma extraction dilution analysis (AEDA). Quim. Nova 2019, 42, 607–610. [Google Scholar] [CrossRef]
- Meng, X.; Wu, Q.; Wang, L.; Wang, D.; Chen, L.; Xu, Y. Improving flavor metabolism of Saccharomyces cerevisiae by mixed culture with bacillus licheniformis for Chinese Maotai–flavor liquor making. J. Ind. Microbiol. Biotechnol. 2015, 42, 1601–1608. [Google Scholar] [CrossRef]
- Hua, J.; Li, J.; Ouyang, W.; Wang, J.; Yuan, H.; Jiang, Y. Effect of Strobilanthes tonkinensis Lindau addition on black tea flavor quality and volatile metabolite content. Foods 2022, 11, 1678. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, W.; Yu, Y.; Wang, J.; Yuan, H.; Hua, J.; Jiang, Y. Analysis of non–volatile and volatile metabolites reveals the influence of second–drying heat transfer methods on green tea quality. Food Chem. X 2022, 14, 100354. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, E.J.; Park, S.E.; Park, D.H.; Park, K.M.; Na, C.S.; Son, H.S. GC/MS–based metabolomics study to investigate differential metabolites between ale and lager beers. Food Biosci. 2020, 36, 100671. [Google Scholar] [CrossRef]
- Nawade, B.; Yahyaa, M.; Reuveny, H.; Shaltiel-Harpaz, L.; Eisenbach, O.; Faigenboim, A.; Bar-Yaakov, I.; Holland, D.; Ibdah, M. Profiling of volatile terpenes from almond (Prunus dulcis) young fruits and characterization of seven terpene synthase genes. Plant Sci. 2019, 287, 110187. [Google Scholar] [CrossRef] [PubMed]
- López–Gresa, M.P.; Lisón, P.; Campos, L.; Rodrigo, I.; Rambla, J.L.; Granell, A.; Conejero, V.; Bellés, J.M. A non–targeted metabolomics approach unravels the VOCs associated with the tomato immune response against Pseudomonas syringae. Front. Plant Sci. 2017, 8, 1188. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zeng, L.; Yu, Z.; Li, J.; Tang, J.; Su, X.; Yang, Z. Differential accumulation of aroma compounds in normal green and Albino–induced yellow tea (Camellia sinensis) leaves. Molecules 2018, 23, 2677. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, F.; Zhong, K.; Huang, C.; Yu, Z.; Peng, Z.; Wu, Y.; Bu, Q.; Gao, H. Effect of Bacillus subtilis Y61 inoculation on bacterial community and metabolic profile of sichuan paocai fermentation. LWT 2021, 137, 110393. [Google Scholar] [CrossRef]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial activity and mechanism of linalool against shewanella putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.; Zhao, Y.; Wang, J.; Shuang, Q. Nitrogen metabolism of branched–chain alcohols acetates in jujube wine assessed by 13C–labeling. J. Food Process. Preserv. 2020, 44, e14741. [Google Scholar] [CrossRef]
- Celińska, E.; Borkowska, M.; Białas, W.; Kubiak, M.; Korpys, P.; Archacka, M.; Ledesma-Amaro, R.; Nicaud, J.M. Genetic engineering of Ehrlich pathway modulates production of higher alcohols in engineered Yarrowia lipolytica. FEMS Yeast Res. 2019, 19, foy122. [Google Scholar] [CrossRef]
- Yu, J.; Xu, X.; Guo, Z.; Teng, F.; Shuang, Q.; Xia, Y. Effect of ultra high pressure on seabuckthorn wine aging. China Brew. 2020, 39, 131–135. [Google Scholar] [CrossRef]
- Long, Q.; Li, Z.; Han, B.; Hosseini, H.G.; Zhou, H.; Wang, S.; Luo, D. Discrimination of two cultivars of Alpinia Officinarum Hance using an electronic nose and gas chromatography–mass spectrometry coupled with chemometrics. Sensors 2019, 19, 572. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.T.; Lu, Z.M.; Zhang, X.J.; Chai, L.J.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Metagenomics unveils microbial roles involved in metabolic network of flavor development in medium–temperature daqu starter. Food Res. Int. 2021, 140, 110037. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Key aroma compounds in fermented Forastero cocoa beans and changes induced by roasting. Eur. Food Res. Technol. 2019, 245, 1907–1915. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Zeng, L.; Wang, S.; Tang, Q.; Wu, Z.; Zhang, W. Discrimination of Luzhou–flavoured fresh raw liquor distilled from Zaopei fermented in new, trend to–be aged and aged pit mud based on their aroma and flavour compounds. J. Inst. Brew. 2017, 123, 242–251. [Google Scholar] [CrossRef]
- Zheng, J.; Liang, R.; Wu, C.; Zhou, R.; Liao, X. Discrimination of different kinds of Luzhou–flavor raw liquors based on their volatile features. Food Res. Int. 2014, 56, 77–84. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, D.; Niu, Y.; Chen, F.; Song, S.; Zhu, J.; Zhu, G. Characterization of aroma compounds of Chinese famous liquors by gas chromatography–mass spectrometry and flash GC electronic–nose. J. Chromatogr. B 2014, 945, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.L.; An, Y.; Chen, S.; Qian, M.C. Characterization of Qingke Liquor Aroma from Tibet. J. Agric. Food Chem. 2019, 67, 13870–13881. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, D.; Liu, F.; Yang, K.; Qiao, Z.; Zheng, J. Variation of grain aroma compounds in five grains during distillation process. Food Ferment. Sci. Technol. 2022, 58, 42–47. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. Change rules of flavoring compositions and sensory differences in different distillate during the distillation of Fen–flavor liquor. Liquor Mak. 2022, 49, 82–86. [Google Scholar] [CrossRef]
- Huang, H.; Deng, W.; Chen, J.; Zhang, L.; Li, M.; Wang, L. Analysis of the effect of cold plasma sterilization on the volatile compounds of sugarcane juice based on HS–SPME–GC–MS and electronic nose. Food Ferment. Ind. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Yu, H.; Yu, X.; Yu, Z. Analysis of volatile components of different dried Chrysanthemum Nankingense based on headspace solid phase microextraction–gas chromatography–mass spectrometry and OAV. Food Sci. 2022, 6, 1–17. Available online: https://kns.cnki.net/kcms/detail/11.2206.TS.20220621.1606.029.html (accessed on 20 September 2022).

| No. | Compounds | Threshold (μg/kg) | Content (mg/L) | OAV | ||||
|---|---|---|---|---|---|---|---|---|
| SJ | SW | DL | SJ | SW | DL | |||
| al9 | 1–Octanol | 54.000 | 0.098 ± 0.050 | 0.026 ± 0.002 | 0.070 ± 0.008 | 1.8 | 0.5 | 1.3 |
| al12 | 1–Nonanol | 2.000 | 0.120 ± 0.072 | 0.067 ± 0.005 | 0.306 ± 0.042 | 60.0 | 33.3 | 152.8 |
| al17 | Phenylethyl alcohol | 45.000 | 0.448 ± 0.132 | 0.936 ± 0.118 | –– | 10.0 | 20.8 | –– |
| e1 | 3–Methyl–butanoic acid ethyl ester | 6.890 | 0.129 ± 0.006 | 0.387 ± 0.018 | 0.067 ± 0.007 | 18.7 | 56.2 | 9.7 |
| e2 | Pentanoic acid ethyl ester | 94.000 | 0.153 ± 0.010 | 0.458 ± 0.029 | 0.123 ± 0.030 | 1.6 | 4.9 | 1.3 |
| e3 | 3–Methyl–1–butanol acetate | 3.000 | –– | –– | 0.118 ± 0.022 | –– | –– | 39.3 |
| e11 | Hexanoic acid ethyl ester | 0.500 | 0.103 ± 0.001 | 0.309 ± 0.003 | 0.335 ± 0.005 | 206.0 | 618.0 | 670.0 |
| e14 | Heptanoic acid ethyl ester | 170.000 | 0.067 ± 0.020 | 0.201 ± 0.060 | 0.270 ± 0.053 | 0.4 | 1.2 | 1.6 |
| e19 | Octanoic acid ethyl ester | 0.100 | 0.137 ± 0.038 | 0.411 ± 0.114 | 1.647 ± 0.348 | 1370.0 | 4110.0 | 16,466.7 |
| e21 | 3–Methyl–butanoic acid hexyl ester | 0.150 | 0.040 ± 0.019 | 0.120 ± 0.057 | 0.036 ± 0.010 | 266.7 | 800.0 | 240.0 |
| e27 | Decanoic acid ethyl ester | 20.000 | –– | –– | 1.233 ± 0.336 | –– | –– | 61.7 |
| e37 | Benzeneacetic acid ethyl ester | 100.000 | 0.087 ± 0.048 | 0.261 ± 0.044 | 0.143 ± 0.019 | 0.9 | 2.6 | 1.4 |
| e40 | Dodecanoic acid ethyl ester | 330.000 | –– | –– | 1.110 ± 0.276 | –– | –– | 3.4 |
| e44 | Benzenepropanoic acid ethyl ester | 0.060 | –– | –– | 0.029 ± 0.004 | –– | –– | 488.9 |
| c4 | Heptanal | 10.000 | 0.005 ± 0.001 | 0.015 ± 0.002 | –– | 0.5 | 1.5 | –– |
| c6 | Nonanal | 15.000 | 0.088 ± 0.035 | 0.264 ± 0.105 | 0.052 ± 0.012 | 5.9 | 17.6 | 3.5 |
| c8 | Furfural | 100.000 | 0.034 ± 0.003 | 0.102 ± 0.009 | 0.444 ± 0.090 | 0.3 | 1.0 | 4.4 |
| c10 | 5–Methyl–2–furancarboxaldehyde | 50.000 | 0.053 ± 0.011 | 0.159 ± 0.053 | 0.015 ± 0.001 | 1.1 | 3.2 | 0.3 |
| c13 | Tetradecanal | 60.000 | 0.097 ± 0.009 | 0.284 ± 0.069 | –– | 1.6 | 4.7 | –– |
| c14 | 6,10–dimethyl–5,9–Undecadien–2–one | 100.000 | 0.056 ± 0.013 | 0.169 ± 0.058 | –– | 0.5 | 1.7 | –– |
| c15 | 1–(2,6,6–Trimethyl–1,3–cyclohexadien–1–yl)–2–buten–1–one | 0.001 | –– | –– | 0.133 ± 0.029 | –– | –– | 132,666.7 |
| o3 | 4–Ethylphenol | 10.000 | 0.085 ± 0.012 | 0.254 ± 0.045 | –– | 8.4 | 25.4 | –– |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Zha, M.; Liu, H.; Shuang, Q.; Chen, Y.; Yang, X. Novel Insight into the Formation of Odour—Active Compounds in Sea Buckthorn Wine and Distilled Liquor Based on GC–MS and E–Nose Analysis. Foods 2022, 11, 3273. https://doi.org/10.3390/foods11203273
Xia Y, Zha M, Liu H, Shuang Q, Chen Y, Yang X. Novel Insight into the Formation of Odour—Active Compounds in Sea Buckthorn Wine and Distilled Liquor Based on GC–MS and E–Nose Analysis. Foods. 2022; 11(20):3273. https://doi.org/10.3390/foods11203273
Chicago/Turabian StyleXia, Yanan, Musu Zha, Hao Liu, Quan Shuang, Yongfu Chen, and Xujin Yang. 2022. "Novel Insight into the Formation of Odour—Active Compounds in Sea Buckthorn Wine and Distilled Liquor Based on GC–MS and E–Nose Analysis" Foods 11, no. 20: 3273. https://doi.org/10.3390/foods11203273
APA StyleXia, Y., Zha, M., Liu, H., Shuang, Q., Chen, Y., & Yang, X. (2022). Novel Insight into the Formation of Odour—Active Compounds in Sea Buckthorn Wine and Distilled Liquor Based on GC–MS and E–Nose Analysis. Foods, 11(20), 3273. https://doi.org/10.3390/foods11203273




