Use of Near-Infrared Spectroscopy to Discriminate DFD Beef and Predict Meat Quality Traits in Autochthonous Breeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Meat Sampling
2.2. Meat Quality Measurements
2.3. NIR Spectra Collection and Spectral Pretreatment
2.4. Chemometric Analysis
2.4.1. NIR Classification (Normal vs. DFD)
Partial Least Squares-Discriminant Analysis (PLS-DA)
Soft Independent Modelling of Class Analogies (SIMCA)
2.4.2. Quantitative NIR Analysis of Quality Traits: Partial Least Squares (PLS)
3. Results and Discussion
3.1. Meat Quality Traits
3.2. NIRS Classification (Normal and DFD)
3.2.1. Partial Least Squares-Discriminant Analysis (PLS-DA)
3.2.2. SIMCA Analyses
3.3. NIR Predictive Models for Quality Traits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, S.; Roy, B.C.; Larsen, I.L.; Aalhus, J.L.; Dixon, W.T.; Bruce, H.L. Understanding the quality of typical and atypical dark cutting beef from heifers and steers. Meat Sci. 2017, 133, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Loudon, K.; Lean, I.; Pethick, D.; Gardner, G.; Grubb, L.J.; Evans, A.C.; Mcgilchrist, P. On farm factors increasing dark cutting in pasture finished beef cattle. Meat Sci. 2018, 144, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Bach, A.; Velarde, A.; Devant, M. Association between animal, transportation, abattoir practices, and meat pH in beef. Meat Sci. 2008, 78, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, P.V.; Mothersill, C. Glycolysis and associated changes in beef carcasses. J. Sci. Food Agric. 1977, 28, 739–749. [Google Scholar] [CrossRef]
- Silva, J.A.; Patarata, L.; Martins, C. Influence of ultimate pH on bovine meat tenderness during ageing. Meat Sci. 1999, 53, 453–459. [Google Scholar] [CrossRef]
- Adzitey, F.; Nurul, H. Pale soft exudative (PSE) and dark firm dry (DFD) meats: Causes and measures to reduce these incidences-a mini review. Int. Food Res. J. 2011, 18, 11–20. [Google Scholar]
- García-Torres, S.; de Vaca, M.C.; Tejerina, D.; Romero-Fernández, M.P.; Ortiz, A.; Díaz, F.; Sierra, V.; González, P.; Franco, D.; Zapata, C.; et al. ¿Es el pH un Criterio Suficiente para la Clasificación de Carne DFD en Vacuno? In Proceedings of the I Congreso Iberoamericano de Marcas de Calidad de Carne y de Productos Cárnicos, Bragança, Portugal, 24–25 October 2019; pp. 73–77. [Google Scholar]
- Jiang, H.; Yoon, S.C.; Zhuang, H.; Wang, W.; Yang, Y. Evaluation of factors in development of Vis/NIR spectroscopy models for discriminating PSE, DFD, and normal broiler breast meat. Br. Poult. Sci. 2017, 58, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Downey, G.; Hildrum, K.I. Analysis of Meats. In Near-Infrared Spectroscopy in Agriculture, 3rd ed.; Al-Amoodi, L., Craig, R., Workman, J., Reeves, J., Eds.; ASA; CSSA; SSSA: Madison, WI, USA, 2004; pp. 599–632. [Google Scholar]
- Prieto, N.; Roehe, R.; Lavín, P.; Batten, G.; Andrés, S. Application of near infrared reflectance spectroscopy to predict meat and meat products quality: A review. Meat Sci. 2009, 83, 175–186. [Google Scholar] [CrossRef]
- Prieto, N.; Pawluczyk, O.; Dugan, M.; Aalhus, J. A review of the principles and applications of near-infrared spectroscopy to characterize meat, fat, and meat products. Appl. Spectrosc. 2017, 71, 1403–1426. [Google Scholar] [CrossRef]
- Reis, M.; Rosenvold, K. Early on-line classification of beef carcasses based on ultimate pH by near infrared spectroscopy. Meat Sci. 2014, 96, 862–869. [Google Scholar] [CrossRef]
- Prieto, N.; López-Campos, Ó.; Zijlstra, R.T.; Aalhus, J.L. Beef dark cutter segregation by visible and near infrared spectroscopy. Meat Sci. 2015, 99, 147. [Google Scholar] [CrossRef]
- Nubiato, K.E.; Mazon, M.; Antonelo, D.; Silva, S. Classifying of Nellore cattle beef on Normal and DFD applying a non-conventional technique. Infrared Phys. Technol. 2016, 78, 195–199. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2017. [Google Scholar]
- Honikel, K. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Faber, N.K.M. A closer look at the bias-variance trade-off in multivariate calibration. J. Chemom. 1999, 13, 185–192. [Google Scholar] [CrossRef]
- Saeys, W.; Mouazen, A.M.; Ramon, H. Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. Biosyst. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Williams, P.C.; Norris, K.H. Variables Affecting Near-Infrared Spectroscopic Analysis. In Near Infrared Technology in the Agricultural and Food Industries; Williams, P.C., Norris, K., Eds.; American Association of Cereal Chemists Inc.: St. Paul, MN, USA, 2001; pp. 145–185. [Google Scholar]
- Cecchinato, A.; De Marchi, M.; Penasa, M.; Albera, A.; Bittane, G. Near-infrared reflectance spectroscopy predictions as indicator traits in breeding programs for enhanced beef quality. J. Anim. Sci. 2011, 89, 2687–2695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Marchi, M. On-line prediction of beef quality traits using near infrared spectroscopy. Meat Sci. 2013, 94, 455–460. [Google Scholar] [CrossRef]
- Chambaz, A.; Morel, I.; Scheeder, M.R.L.; Kreuzer, M.; Dufey, P.A. Characteristics of steers of six beef breeds fattened from eight months of age and slaughtered at a target level of intra-muscular fat. I. Growth performance and carcass quality. Arch. Tierzucht. 2001, 44, 395–411. [Google Scholar] [CrossRef]
- De Marchi, M.; Riovanto, R.; Penasa, M.; Cassandro, M. At-line prediction of fatty acid profile in chicken breast using near infrared reflectance spectroscopy. Meat Sci. 2012, 90, 653–657. [Google Scholar] [CrossRef]
- Prieto, N.; Andrés, S.; Giráldez, F.J.; Mantecón, A.R.; Lavín, P. Ability of near infrared reflectance spectroscopy (NIRS) to estimate physical parameters of adult steers (oxen) and young cattle meat samples. Meat Sci. 2008, 79, 692–699. [Google Scholar] [CrossRef]
- Moreno-Grande, A.; Rueda, V.; Ceular, A. Análisis cuantitativo del pH de canales de vacuno en matadero. Arch. Zootec. 1999, 4, 33–42. [Google Scholar]
- Lomiwes, D.; Reis, M.M.; Wiklund, E.; Young, O.A.; North, M. Near infrared spectroscopy as an on-line method to quantitatively determine glycogen and predict ultimate pH in pre Rigor bovine M. longissimus dorsi. Meat Sci. 2010, 86, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Mouresan, E.F.; González-Rodríguez, A.; Cañas-Álvarez, J.J.; Díaz, C.; Altarriba, J.; Baro, J.A.; Piedrafita, J.; Molina, A.; Toro, M.A.; Varona, L. On the haplotype diversity along the genome in Spanish beef cattle populations. Livest. Sci. 2017, 201, 30–33. [Google Scholar] [CrossRef]
- Lei, H.; Yang, T.; Mahmood, S.; Roy, B.; Li, C.; Plastow, G.S.; Bruce, H.L. A genome-wide association study of dark cutting in beef cattle. Meat Sci. 2017, 131, 204. [Google Scholar]
- Yanga, Y.; Zhuangb, H.; Yoonb, S.; Wanga, W.; Jianga, H.; Jia, B. Rapid classification of intact chicken breast fillets by predicting principal component score of quality traits with visible/near-Infrared spectroscopy. Food Chem. 2018, 244, 184–189. [Google Scholar] [CrossRef]
- Monroy, M.; Prasher, S.; Ngadi, M.O.; Wang, N.; Karimi, Y. Pork meat quality classification using visible/near-infrared spectroscopic data. Biosyst. Eng. 2010, 107, 271–276. [Google Scholar] [CrossRef]
- Murray, I.; Williams, P.C. Chemical Principles of Near-Infrared Technology. In Near Infrared Technology in the Agricultural and Food Industries; Williams, P.C., Norris, K., Eds.; American Association of Cereal Chemists Inc.: St. Paul, MN, USA, 1987; pp. 17–34. [Google Scholar]
- Page, J.K.; Wulf, D.M.; Schwotzer, T.R. A survey of beef muscle color and pH. J. Anim. Sci. 2001, 79, 678–687. [Google Scholar] [CrossRef]
- Kapper, C.; Klont, R.E.; Verdonk, J.M.; Urlings, H.A. Prediction of pork quality with near infrared spectroscopy (NIRS): 1. Feasibility and robustness of NIRS measurements at laboratory scale. Meat Sci. 2012, 91, 294–299. [Google Scholar] [CrossRef]
- Prieto, N.; López-Campos, O.; Aalhus, J.L.; Dugan, M.E.R.; Juárez, M.; Uttaro, B. Use of near infrared spectroscopy for estimating meat chemical composition, quality traits and fatty acid content from cattle fed sunflower or flaxseed. Meat Sci. 2014, 98, 279–288. [Google Scholar] [CrossRef]
- Savoia, S.; Brugiapaglia, A.; Pauciullo, A.; Di Stasio, L.; Schiavon, S.; Bittante, G.; Albera, A. Characterization of beef production systems and their effect on carcass and meat quality traits of Piemontese young bulls. Meat Sci. 2019, 153, 75–85. [Google Scholar] [CrossRef]
- De Marchi, M.; Penasa, M.; Cecchinato, A.; Bittante, G. The relevance of different near infrared technologies and sample treatments for predicting meat quality traits in commercial beef cuts. Meat Sci. 2013, 93, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sahar, A.; Allen, P.; Sweeney, T.; Cafferky, J.; Downey, G.; Cromie, A.; Hamill, R.M. Online prediction of physico-chemical quality attributes of beef using visible-near-infrared spectroscopy and chemometrics. Foods 2019, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lyon, B.G.; Windham, W.R.; Realini, C.E.; Pringle, T.D.D.; Duckett, S. Prediction of color, texture, and sensory characteristics of beef steaks by visible and near infrared reflectance spectroscopy. A feasibility study. Meat Sci. 2003, 65, 1107–1115. [Google Scholar] [CrossRef]
- Elmasry, G.; Sun, D.W.; Allen, P. Near-infrared hyperspectral imaging for predicting colour, pH and tenderness of fresh beef. J. Food Eng. 2012, 110, 127–140. [Google Scholar] [CrossRef]
- De Marchi, M.; Berzaghi, P.; Boukha, A.; Mirisola, M.; Gallo, L. Use of near infrared spectroscopy for assessment of beef quality traits. Ital. J. Anim. Sci. 2007, 6, 421–423. [Google Scholar] [CrossRef]
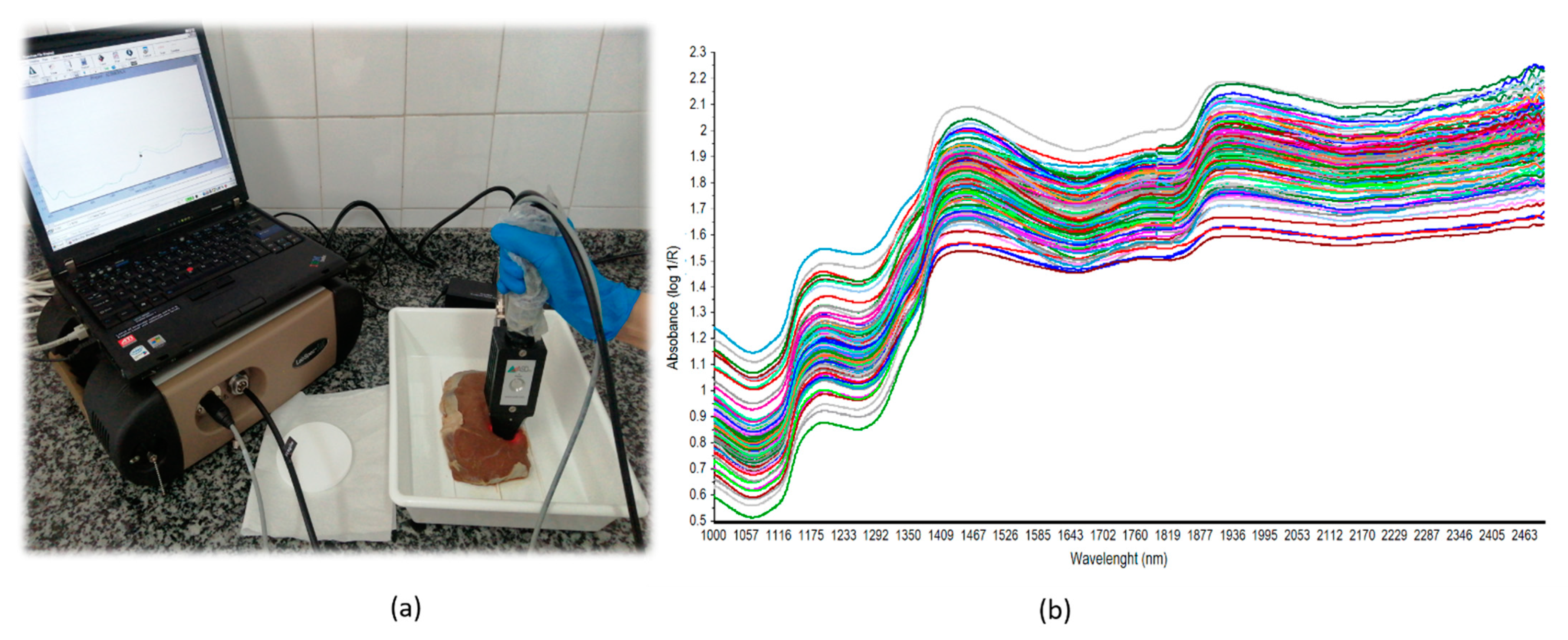
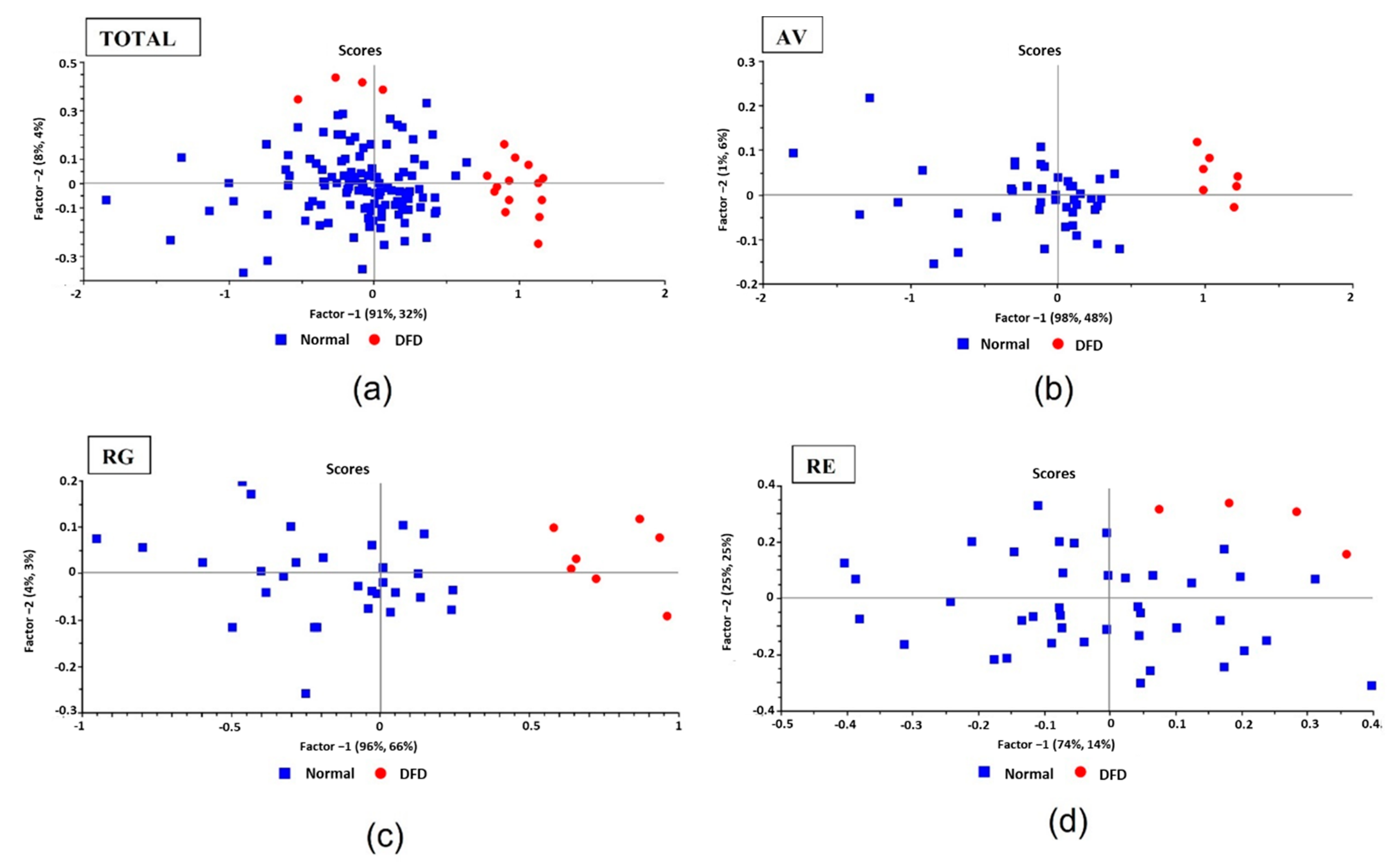
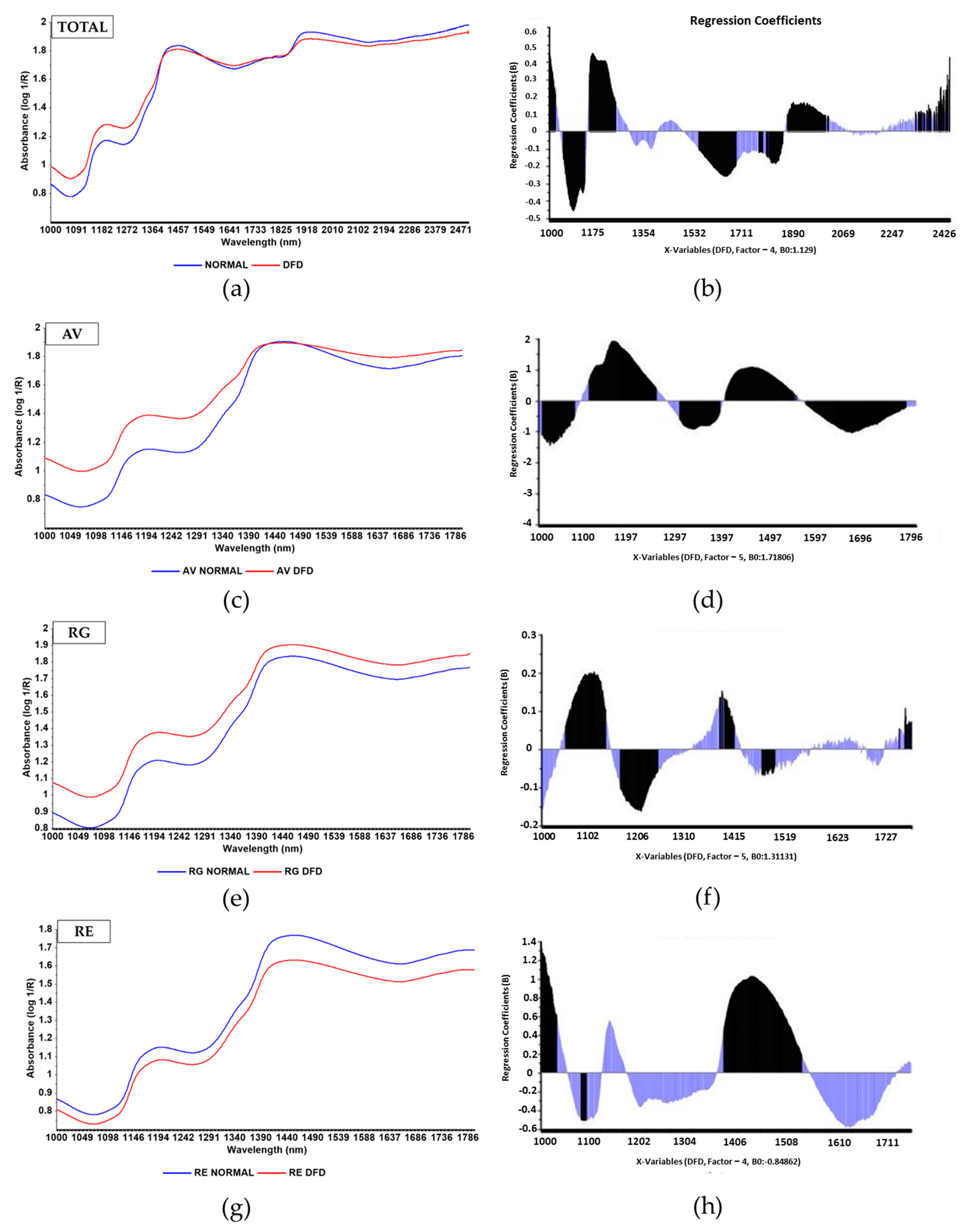
| Variable | PM Time | N | Max | Min | Median | Mean | SD | CV |
|---|---|---|---|---|---|---|---|---|
| pH24 | 24 h | 129 | 6.91 | 5.31 | 5.55 | 5.68 | 0.30 | 5.31 |
| Drip loss | 24 h | 129 | 3.39 | 0.64 | 1.58 | 1.62 | 0.57 | 35.02 |
| CIE-L* | 60 min | 117 | 50.77 | 23.85 | 38.95 | 38.47 | 4.66 | 12.13 |
| CIE-a* | 60 min | 117 | 27.02 | 4.63 | 12.80 | 14.77 | 5.79 | 39.19 |
| CIE-b* | 60 min | 117 | 21.14 | 3.27 | 11.54 | 11.24 | 2.99 | 26.62 |
| Hue | 60 min | 117 | 58.06 | 21.74 | 40.76 | 38.73 | 11.71 | 30.24 |
| Chroma | 60 min | 117 | 32.43 | 6.19 | 18.43 | 19.33 | 5.91 | 30.58 |
| Calibration | Cross-Validation | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample Set | N | Pretreatment | Range (nm) | LVs | R2c | R2cv | SE | SP |
| Total | 129 | Absorbance (log1/R) | 1000–2500 | 4 | 0.471 | 0.414 | 85.84 | 5.56 |
| AV | 50 | Absorbance (log1/R) | 1000–1800 | 6 | 0.879 | 0.8101 | 97.67 | 100 |
| RG | 37 | Absorbance (log1/R) | 1000–1800 | 5 | 0.856 | 0.811 | 93.33 | 71.43 |
| RE | 42 | Absorbance (log1/R) | 1000–1800 | 4 | 0.648 | 0.525 | 95 | 50 |
| Normal | DFD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Sets | n | Pretreatment | Range (nm) | PCs | SE | SP | PCs | SE | SP |
| Total | 129 | Abs (log1/R) | 1000–2500 | 2 | 94.6 | 55.6 | 2 | 100 | 19.8 |
| AV | 50 | Abs (log1/R) | 1000–1800 | 2 | 93.02 | 71.42 | 2 | 100 | 100 |
| RG | 37 | Abs (log1/R) | 1000–1800 | 2 | 96.6 | 42.8 | 2 | 100 | 100 |
| RE | 42 | Abs (log1/R) | 1000–1800 | 2 | 97.5 | 50 | 2 | 100 | 90 |
| Calibration | Cross-Validation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | PM Time | Math Treatment | Range (nm) | N | LVs | RMSEC | R2c | RMSECV | R2cv | RER | RPD |
| pH24 | 24 h | SG 1,4,4,1 SNV-DE | 1000–2500 | 128 | 4 | 0.194 | 0.581 | 0.239 | 0.377 | 6.695 | 1.263 |
| Drip loss | 24 h | Abs (log 1/R) | 1000–2500 | 111 | 4 | 0.352 | 0.415 | 0.374 | 0.349 | 4.972 | 1.238 |
| CIE-L* | 60 min | SNV-DE | 1000–2500 | 103 | 6 | 2.185 | 0.765 | 2.51 | 0.695 | 9.058 | 1.805 |
| CIE-a* | 60 min | SNV-DE | 1000–2500 | 102 | 8 | 1.998 | 0.878 | 2.508 | 0.818 | 8.929 | 2.296 |
| CIE- b* | 60 min | SG 1,4,4,1 SNV | 1000–2500 | 101 | 4 | 1.23 | 0.767 | 1.437 | 0.685 | 9.063 | 1.775 |
| Hue | 60 min | SG 1,4,4,1 SNV | 1000–1800 | 103 | 7 | 3.28 | 0.924 | 4.06 | 0.887 | 8.947 | 2.967 |
| Chroma | 60 min | SG 1,4,4,1 SNV | 1000–1800 | 104 | 6 | 2.098 | 0.867 | 2.43 | 0.825 | 10.800 | 2.387 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejerina, D.; Oliván, M.; García-Torres, S.; Franco, D.; Sierra, V. Use of Near-Infrared Spectroscopy to Discriminate DFD Beef and Predict Meat Quality Traits in Autochthonous Breeds. Foods 2022, 11, 3274. https://doi.org/10.3390/foods11203274
Tejerina D, Oliván M, García-Torres S, Franco D, Sierra V. Use of Near-Infrared Spectroscopy to Discriminate DFD Beef and Predict Meat Quality Traits in Autochthonous Breeds. Foods. 2022; 11(20):3274. https://doi.org/10.3390/foods11203274
Chicago/Turabian StyleTejerina, David, Mamen Oliván, Susana García-Torres, Daniel Franco, and Verónica Sierra. 2022. "Use of Near-Infrared Spectroscopy to Discriminate DFD Beef and Predict Meat Quality Traits in Autochthonous Breeds" Foods 11, no. 20: 3274. https://doi.org/10.3390/foods11203274
APA StyleTejerina, D., Oliván, M., García-Torres, S., Franco, D., & Sierra, V. (2022). Use of Near-Infrared Spectroscopy to Discriminate DFD Beef and Predict Meat Quality Traits in Autochthonous Breeds. Foods, 11(20), 3274. https://doi.org/10.3390/foods11203274








