Bioaccessibility of Phenolic Compounds from Mistletoe Infusions and Effect of In Vitro Digestion on Its Antioxidant and Pancreatic Lipase Inhibitory Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Mistletoe Infusions
2.3. Simulated Gastrointestinal In Vitro Digestion
2.4. Identification and Quantification of Phenolic Acids by UPLC-qToF-MS/MS
2.5. Bioaccessibility of Phenolic Acids from Mistletoe Infusions
2.6. Antioxidant Capacity Assays
2.6.1. Trolox Equivalent Antioxidant Capacity (TEAC)
2.6.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. Determination of Total Reducing Capacity and Flavonoid Content
2.8. Total Tannin Quantification
Condensed and Hydrolyzable Tannin Content
2.9. Pancreatic Lipase Inhibition
2.10. Statistical Analysis
3. Results
3.1. Effect of Simulated In Vitro Gastrointestinal Digestion on Mistletoe Infusions
3.1.1. Phenolic Compound Determination by UPLC-MS
3.1.2. Total Reducing Capacity and Total Flavonoid Content
3.1.3. Tannin Content
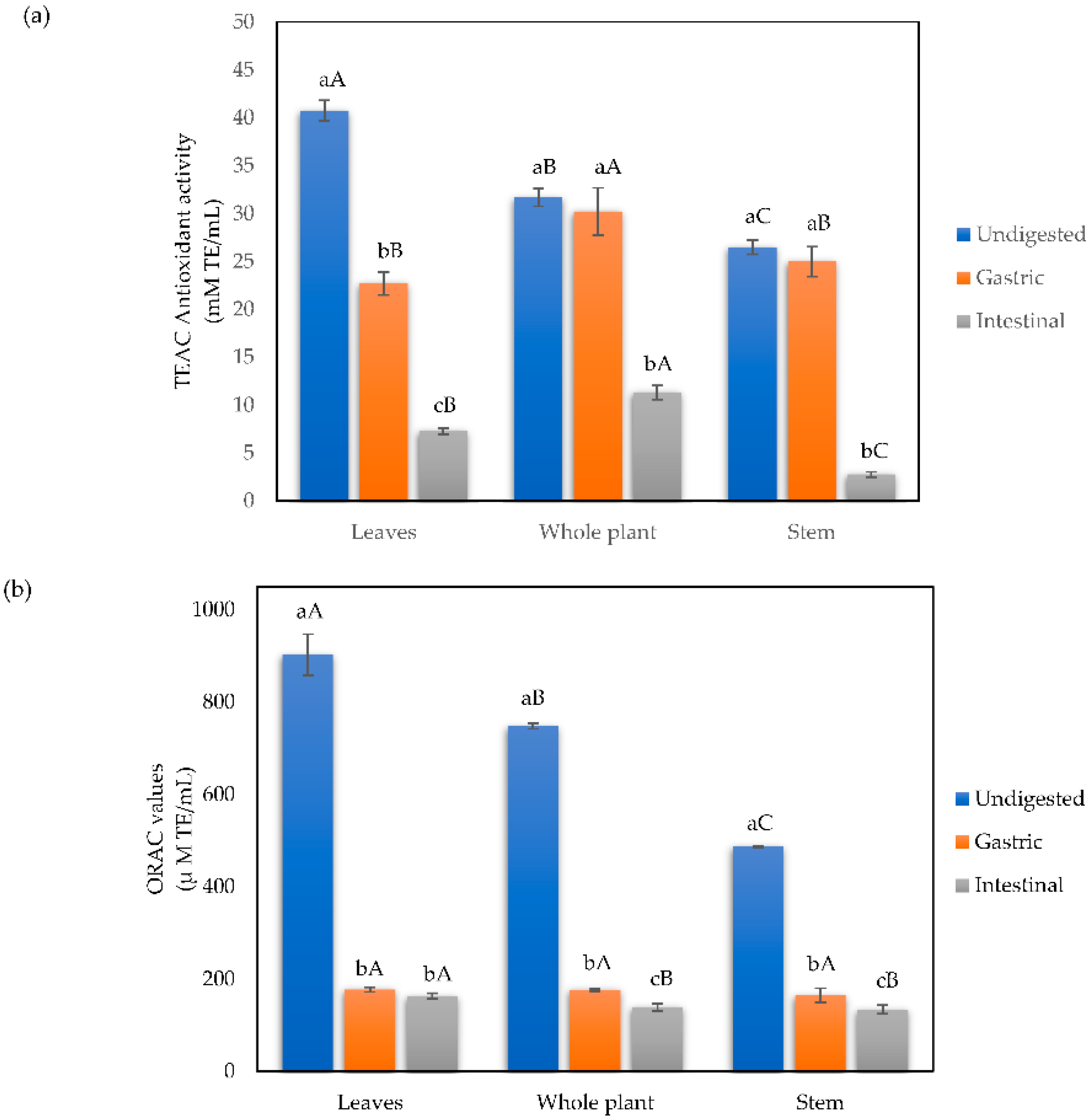
3.1.4. Antioxidant Capacity
3.1.5. Inhibition of Pancreatic Lipase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 25 April 2022).
- Thearle, M.; Aronne, L.J. Obesity and pharmacologic therapy. Endocrinol. Metab. Clin. N. Am. 2003, 32, 1005–1024. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freuding, M.; Keinki, C.; Kutschan, S.; Micke, O.; Buentzel, J.; Huebner, J. Mistletoe in oncological treatment: A systematic review. J. Cancer Res. Clin. Oncol. 2019, 145, 927–939. [Google Scholar] [CrossRef]
- Kienle, G.S.; Kiene, H. Review Article: Influence of Viscum album L. (European Mistletoe) Extracts on Quality of Life in Cancer Patients: A Systematic Review of Controlled Clinical Studies. Integr. Cancer Ther. 2010, 9, 142–157. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Juárez-Vázquez, M.d.C.; Domínguez, F.; González-Sánchez, I.; Estrada-Castillón, E.; López-Toledo, G.; Chávez, M.; Cerbón, M.A.; García-Carranca, A. The antitumoral effect of the American mistletoe Phoradendron serotinum (Raf.) M.C. Johnst. (Viscaceae) is associated with the release of immunity-related cytokines. J. Ethnopharmacol. 2012, 142, 857–864. [Google Scholar] [CrossRef]
- Kang, S.N. Ethanol Extracts from Mistletoe (Viscum album L.) Act as Natural Antioxidants and Antimicrobial Agents in Uncooked Pork Patties during Refrigerated Storage. Asian-australas. J. Anim. Sci. 2016, 29, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Polhill, R.; Wiens, D. Mistletoes of Africa; Royal Botanic Gardens: Richmond, UK, 2000. [Google Scholar]
- Gelis, B.W.; Tovar, C.; Moody, J.B. Mistletoes of North American Conifers; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2002; pp. 1–123. [Google Scholar]
- López-Martínez, S.; Navarrete-Vázquez, G.; Estrada-Soto, S.; León-Rivera, I.; Rios, M.Y. Chemical constituents of the hemiparasitic plant Phoradendron brachystachyum DC Nutt (Viscaceae). Nat. Prod. Res. 2013, 27, 130–136. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Navarrete-Vazquez, G.; Hidalgo-Figueroa, S.; Hernández-Abreu, O.; Estrada-Soto, S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: Ex vivo and in silico studies. Fitoterapia 2012, 83, 1023–1029. [Google Scholar] [CrossRef]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; García-Jiménez, S.; Rios, M.Y.; Medina-Franco, J.L.; López-Vallejo, F.; Webster, S.P.; Binnie, M.; Ibarra-Barajas, M.; Ortiz-Andrade, R.; Estrada-Soto, S. Antihyperglycemic and sub-chronic antidiabetic actions of morolic and moronic acids, in vitro and in silico inhibition of 11β-HSD 1. Phytomedicine 2013, 20, 571–576. [Google Scholar] [CrossRef]
- Velderrain-Rodriguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sanchez, M.; Astiazaran-Garcia, H.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, B.; Xia, Y.; Li, H.; Shi, X.; Wang, J.; Deng, Z. Bioaccessibility and transformation pathways of phenolic compounds in processed mulberry (Morus alba L.) leaves after in vitro gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2019, 60, 103406. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Gámez-Meza, N.; Molina-Domínguez, C.C.; Hayano-Kanashiro, C.; Medina-Juárez, L.A. Simulated Gastrointestinal Digestion, Bioaccessibility and Antioxidant Capacity of Polyphenols from Red Chiltepin (Capsicum annuum L. Var. glabriusculum) Grown in Northwest Mexico. Plant Foods Hum. Nutr. 2018, 73, 116–121. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Bernal-Millán, M.d.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-Dried Microencapsulation of Oregano (Lippia graveolens) Polyphenols with Maltodextrin Enhances Their Stability during In Vitro Digestion. J. Chem. 2022, 2022, 8740141. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Picos-Salas, M.A.; Gutiérrez-Grijalva, E.P.; Valdez-Torres, B.; Angulo-Escalante, M.A.; López-Martínez, L.X.; Delgado-Vargas, F.; Heredia, J.B. Supercritical CO2 extraction of oregano (Lippia graveolens) phenolic compounds with antioxidant, α-amylase and α-glucosidase inhibitory capacity. J. Food Meas. Charact. 2021, 15, 3480–3490. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Osuna-Enciso, T.; León-Félix, J.; Heredia, J.B. Cellular antioxidant activity and in vitro intestinal permeability of phenolic compounds from four varieties of mango bark (Mangifera indica L.). J. Sci. Food Agric. 2019, 99, 3481–3489. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; McKey, D. Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.H.; Guillen Quispe, Y.N.; Gonzales Arce, P.H.; Lim, S.S. Investigation of the antioxidant and aldose reductase inhibitory activities of extracts from Peruvian tea plant infusions. Food Chem. 2017, 231, 222–230. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, C.M.; Anselmo-Moreira, F.; Teixeira-Costa, L.; Ceccantini, G.; Salminen, J.-P. Does Phoradendron perrottetii (mistletoe) alter polyphenols levels of Tapirira guianensis (host plant)? Plant Physiol. Biochem. 2019, 136, 222–229. [Google Scholar] [CrossRef]
- Hu, B.; Sakakibara, H.; Takebayashi, Y.; Peters, F.S.; Schumacher, J.; Eiblmeier, M.; Arab, L.; Kreuzwieser, J.; Polle, A.; Rennenberg, H. Mistletoe infestation mediates alteration of the phytohormone profile and anti-oxidative metabolism in bark and wood of its host Pinus sylvestris. Tree Physiol. 2017, 37, 676–691. [Google Scholar] [CrossRef]
- Anselmo-Moreira, F.; Teixeira-Costa, L.; Ceccantini, G.; Furlan, C.M. Mistletoe effects on the host tree Tapirira guianensis: Insights from primary and secondary metabolites. Chemoecology 2019, 29, 11–24. [Google Scholar] [CrossRef]
- García-García, J.D.; Anguiano-Cabello, J.C.; Arredondo-Valdés, R.; Candido del Toro, C.A.; Martínez-Hernández, J.L.; Segura-Ceniceros, E.P.; Govea-Salas, M.; González-Chávez, M.L.; Ramos-González, R.; Esparza-González, S.C.; et al. Phytochemical Characterization of Phoradendron bollanum and Viscum album subs. austriacum as Mexican Mistletoe Plants with Antimicrobial Activity. Plants 2021, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, L.; Roxo, M.; Cavero, R.Y.; Calvo, M.I.; Ansorena, D.; Astiasarán, I.; Wink, M. Bioaccessibility and biological activity of Melissa officinalis, Lavandula latifolia and Origanum vulgare extracts: Influence of an in vitro gastrointestinal digestion. J. Funct. Foods 2018, 44, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Wang, X.; Dai, T.; McClements, D.J.; Liu, J. Impact of in vitro simulated digestion on the potential health benefits of proanthocyanidins from Choerospondias axillaris peels. Food Res. Int. 2015, 78, 378–387. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Gupta, G.; Kazmi, I.; Afzal, M.; Rahman, M.; Saleem, S.; Ashraf, M.S.; Khusroo, M.J.; Nazeer, K.; Ahmed, S.; Mujeeb, M.; et al. Sedative, antiepileptic and antipsychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J. Ethnopharmacol. 2012, 141, 810–816. [Google Scholar] [CrossRef]
- Iloki-Assanga, S.B.; Lewis-Lujan, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef]
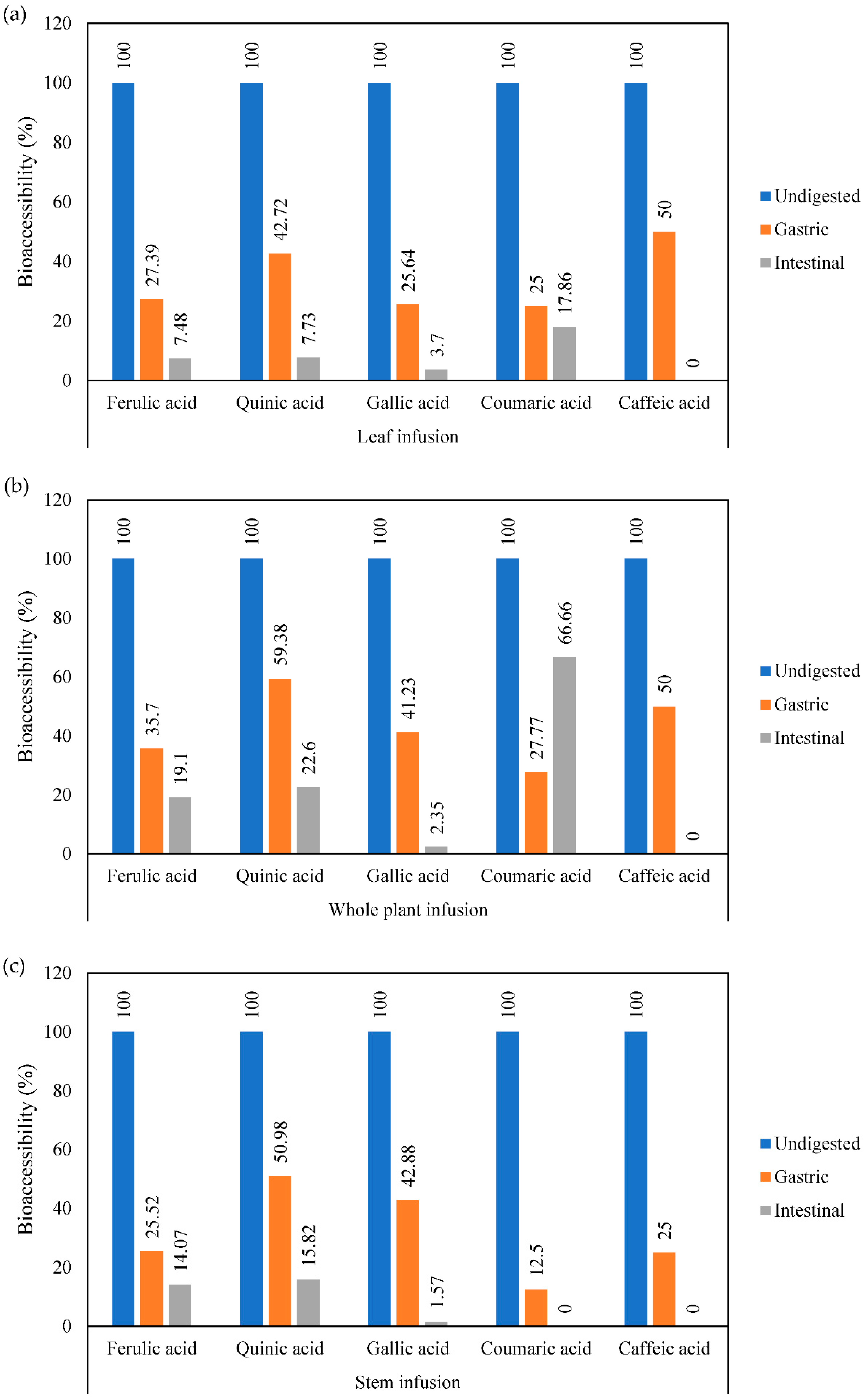



| Part | Compound | Phenolic Content Before and After In Vitro Gastrointestinal Digestion (μg/mL) | ||
|---|---|---|---|---|
| Undigested | Gastric | Intestinal | ||
| Leaves | Ferulic acid | 58.12 ± 1.40 a,AB | 15.92 ± 0.33 b,B | 4.35 ± 0.09 c,C |
| Quinic acid | 54.98 ± 1.92 a,B | 23.49 ± 0.13 b,B | 4.25 ± 0.07 c,C | |
| Gallic acid | 9.71 ± 0.48 a,C | 2.49 ± 0.34 b,C | 0.36 ± 0.01 c,A | |
| Coumaric acid | 0.28 ± 0.00031 a,A | 0.07 ± 0.0015 b,A | 0.05 ± 0.0015 c,B | |
| Caffeic acid | 0.02 ± 0.0006 a,B | 0.01 ± 0.0006 b,A | 0.00 ± 0.00 c,A | |
| Whole plant | Ferulic acid | 55.15 ± 1.17 a,B | 19.69 ± 1.61 b,A | 10.53 ± 0.40 c,A |
| Quinic acid | 52.61 ± 0.49 a,B | 31.24 ± 1.03 b,A | 11.89 ± 0.30 c,A | |
| Gallic acid | 15.28 ± 0.88 a,B | 6.30 ± 0.41 b,B | 0.36 ± 0.01 c,A | |
| Coumaric acid | 0.18 ± 0.0020 a,B | 0.05 ± 0.0015 c,B | 0.12 ± 0.0010 b,A | |
| Caffeic acid | 0.02 ± 0.0006 a,B | 0.01 ± 0.0006 b,A | 0.00 ± 0.00 c,A | |
| Stem | Ferulic acid | 60.77 ± 0.98 a,A | 15.51 ± 1.20 b,B | 8.55 ± 0.37 c,B |
| Quinic acid | 60.24 ± 2.47 a,A | 30.71 ± 0.79 b,A | 9.53 ± 0.15 c,B | |
| Gallic acid | 22.90 ± 1.05 a,A | 9.82 ± 0.31 b,A | 0.36 ± 0.01 c,A | |
| Coumaric acid | 0.08 ± 0.0017 a,C | 0.01 ± 0.0006 b,C | 0.00 ± 0.00 c,C | |
| Caffeic acid | 0.04 ± 0.0006 a,A | 0.01 ± 0.0006 b,A | 0.00 ± 0.00 c,A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Grijalva, E.P.; Zamudio-Sosa, V.E.; Contreras-Angulo, L.A.; Leyva-López, N.; Heredia, J.B. Bioaccessibility of Phenolic Compounds from Mistletoe Infusions and Effect of In Vitro Digestion on Its Antioxidant and Pancreatic Lipase Inhibitory Activity. Foods 2022, 11, 3319. https://doi.org/10.3390/foods11213319
Gutiérrez-Grijalva EP, Zamudio-Sosa VE, Contreras-Angulo LA, Leyva-López N, Heredia JB. Bioaccessibility of Phenolic Compounds from Mistletoe Infusions and Effect of In Vitro Digestion on Its Antioxidant and Pancreatic Lipase Inhibitory Activity. Foods. 2022; 11(21):3319. https://doi.org/10.3390/foods11213319
Chicago/Turabian StyleGutiérrez-Grijalva, Erick Paul, Victor Eduardo Zamudio-Sosa, Laura Aracely Contreras-Angulo, Nayely Leyva-López, and J. Basilio Heredia. 2022. "Bioaccessibility of Phenolic Compounds from Mistletoe Infusions and Effect of In Vitro Digestion on Its Antioxidant and Pancreatic Lipase Inhibitory Activity" Foods 11, no. 21: 3319. https://doi.org/10.3390/foods11213319









