Effects of Dietary Tannic Acid on Obesity and Gut Microbiota in C57BL/6J Mice Fed with High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Animals and Diets
2.3. Sample Collection
2.4. Serum and Liver Index Biochemical Analysis
2.5. Histological Examination
2.6. RT-qPCR Analysis
2.7. Western Blot Analysis
2.8. 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
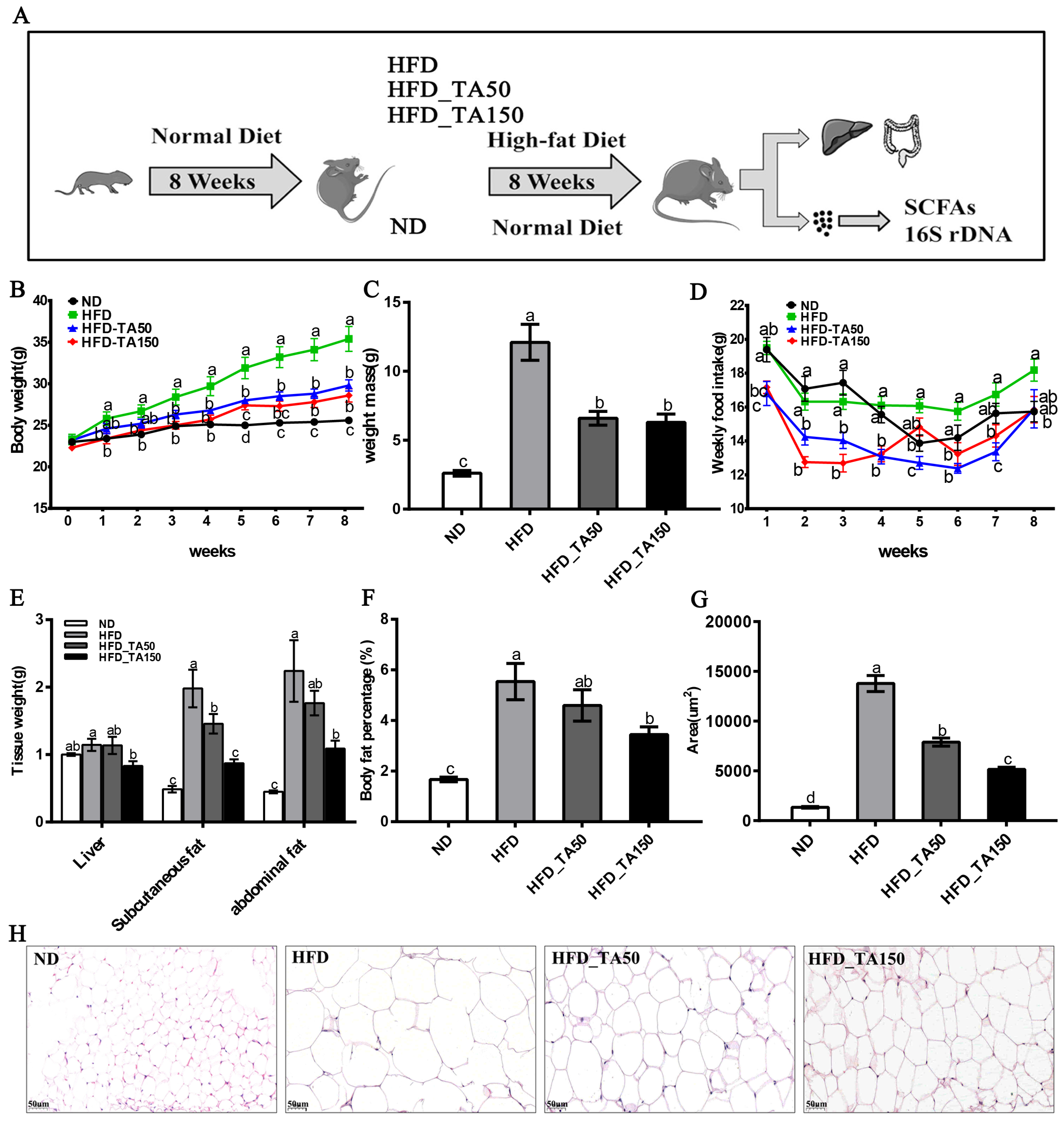
3.1. Dietary Tannic Acid Prevents Obesity in Mice with a High-Fat Diet
3.2. Dietary Tannic Acid Improves the Glucolipid Metabolism in Mice with a High-Fat Diet
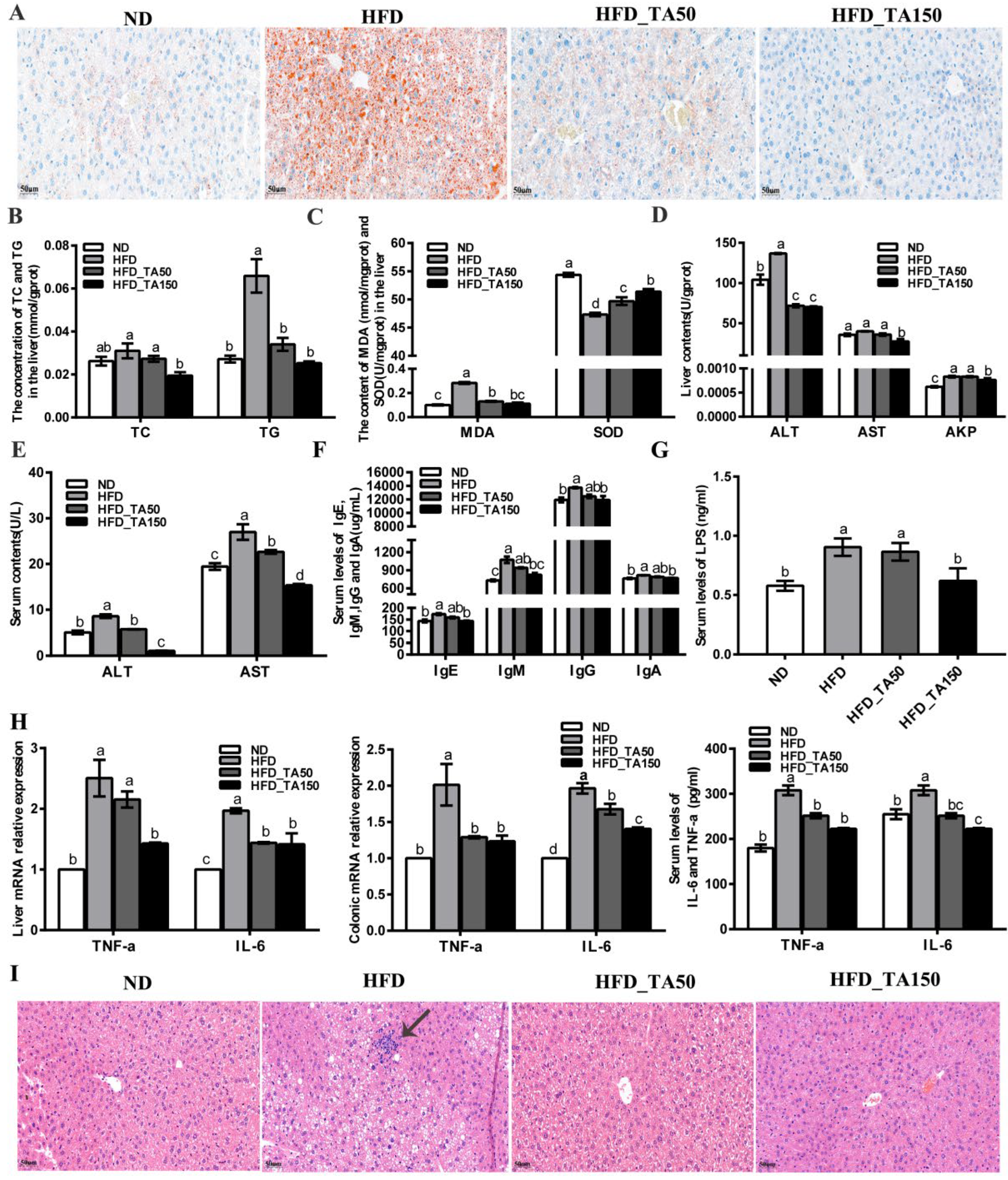
3.3. Dietary Tannic Acid Attenuates Liver Lipid Accumulation, Oxidative Stress, and Liver Toxicity in Mice with a High-Fat Diet
3.4. Dietary Tannic Acid Reduces Systemic Inflammation in Mice with a High-Fat Diet
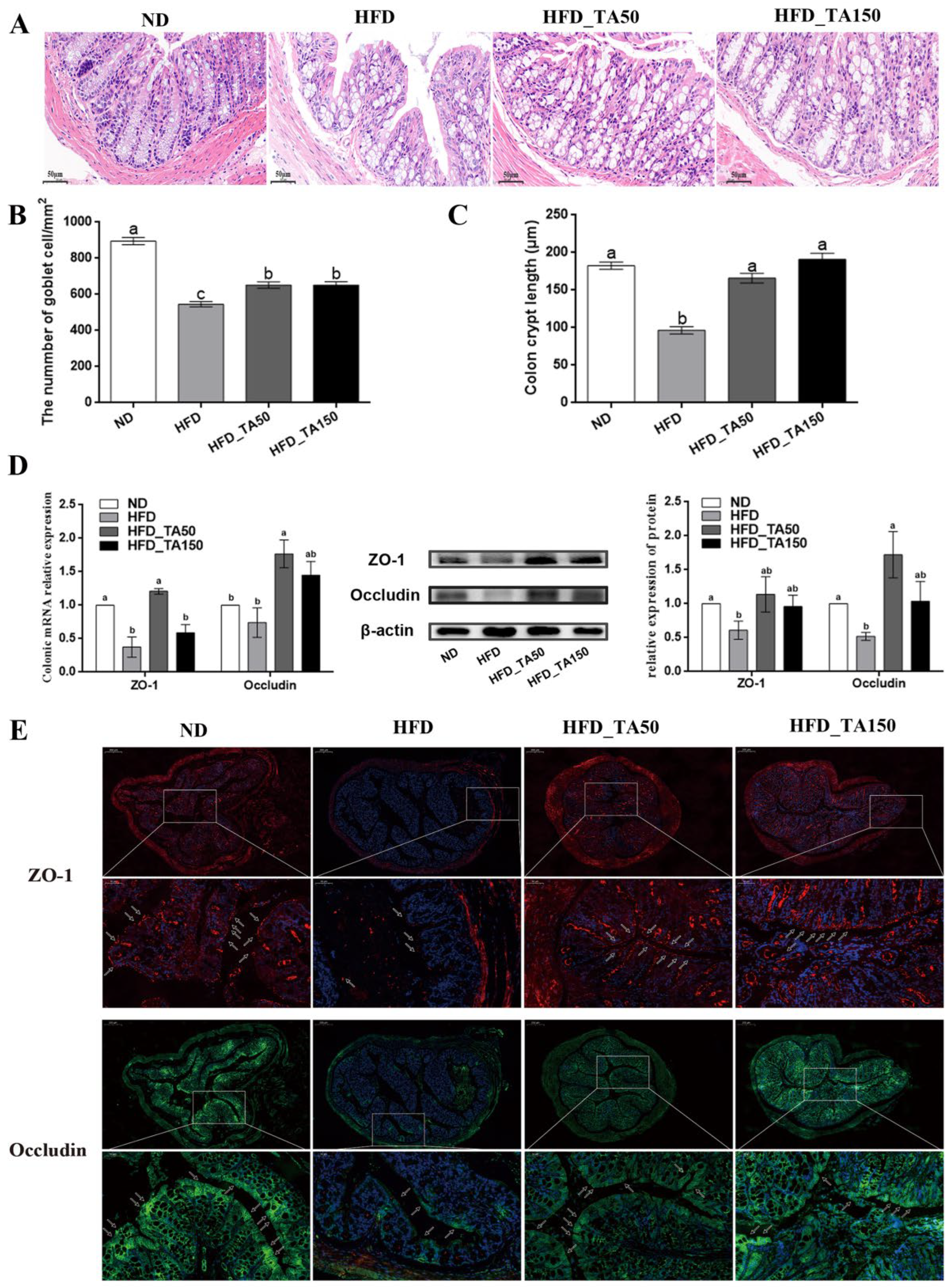
3.5. Dietary Tannic Acid Ameliorates the Gut Barrier Damage Caused by High-Fat Diet
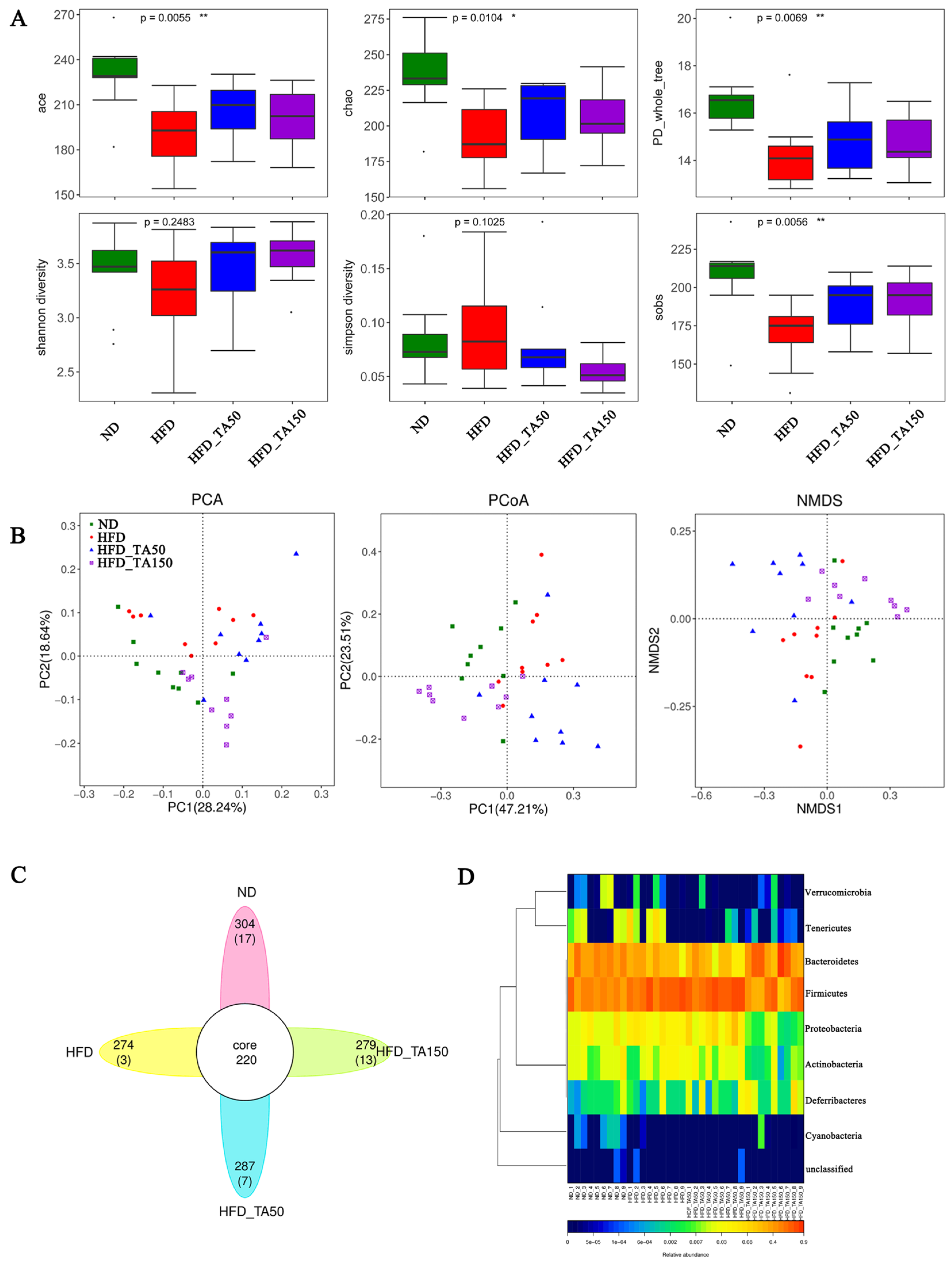
3.6. Dietary Tannic Acid Improves the Structure of Gut Microbiota in Mice with High-Fat Diet
3.7. Correlation between the Gut Microbiota and Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reilly, J.J. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 1496. [Google Scholar] [PubMed] [Green Version]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, P. Obesity and diabetes in the developing world-a growing challenge. N. Engl. J. Med. 2007, 356, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cases, J.; Romain, C.; Dallas, C.; Gerbi, A.; Cloarec, M. Regular consumption of fiit-ns, a polyphenol extract from fruit and vegetables frequently consumed within the mediterranean diet, improves metabolic ageing of obese volunteers:a randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2015, 66, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, J.P.; Ke, W.X.; Wang, J.; Li, D.T.; Liu, R.L.; Jia, Y.; Wang, X.H.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radical. Bio. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Ota, Y.; Nishiyama, K.; Kunitake, H.; Yamasaki, Y.; Tari, H.; Araki, K.; Arakawa, T.; Yamasaki, M. Blueberry Leaf Polyphenols Prevent Body Fat Accumulation in Mice Fed High-fat, High-sucrose Diet. J. Oleo Sci. 2019, 68, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Shin, Y.; Jung, S.; Kim, Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr. Res. 2017, 42, 1368–1377. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- MasEk, T.; StarcEvic, K. Lipogenesis and lipid peroxidation in rat testes after long-term treatment with sucrose and tannic acid in drinking water. Andrologia 2017, 49, 12632. [Google Scholar] [CrossRef]
- Bonelli, F.; Turini, L.; Sarri, G.; Serra, A.; Buccioni, A.; Mele, M. Oral administration of chestnut tannins to reduce the duration of neonatal calf diarrhea. BMC Vet. Res. 2018, 14, 227–332. [Google Scholar] [CrossRef]
- Zielińska-Przyjemska, M.; Ignatowicz, E.; Krajka-Kuźniak, V.; Baer-Dubowska, W. Effect of tannic acid, resveratrol and its derivatives, on oxidative damage and apoptosis in human neutrophils. Food Chem. Toxicol. 2015, 84, 37–46. [Google Scholar] [CrossRef]
- Zeng, L.R.; Zhang, L.; Li, K.X.; He, J.C.; Xin, H.W.; Wang, Q. Effect of gelatinization processing on the antioxidant, digestion, and physicochemical properties of wheat starch enhanced with tannic acid. LWT—Food Sci. Technol. 2020, 125, 109228. [Google Scholar] [CrossRef]
- He, J.C.; Zeng, L.R.; Gong, J.A.; He, Y.L.; Liu, X.; Zhang, L.; Xu, N.; Wang, Q. Effects of two contrasting dietary polysaccharides and tannic acid on the digestive and physicochemical properties of wheat starch. Food Sci. Nutr. 2021, 9, 5800–5808. [Google Scholar] [CrossRef]
- Liu, X.; Kim, J.K.; Li, Y.; Li, J.; Liu, F.; Chen, X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J. Nutr. 2005, 135, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Wu, D.; Tian, W.; Ma, X.F. Inhibitory effects of tannic acid on fatty acid synthase and 3T3-L1 preadipocyte. BBA-Mol. Cell Biol. L. 2013, 1831, 1260–1266. [Google Scholar] [CrossRef]
- Sanna, S.; Van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vila, A.V.; Vosa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Wodarczyk, M.; Liewska, K. Obesity as the 21st century’s major disease: The role of probiotics and prebiotics in prevention and treatment. Food Biosci. 2021, 42, 101115. [Google Scholar] [CrossRef]
- Bibiloni, R.; Waget, A.; Neyrinck, A.M.; Burcelin, R.; Delzenne, N.M.; Cani, P.D.; Knauf, C. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Knauf, C.; Delzenne, N. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Possemiers, S.; Wiele, T.V.D.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Ashwin, K.; Pattanaik, A.K.; Howarth, G.S. Polyphenolic bioactives as an emerging group of nutraceuticals for promotion of gut health: A review. Food Biosci. 2021, 44, 101376. [Google Scholar] [CrossRef]
- Chung, K.T.; Lu, Z.; Chou, M.W. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1999, 36, 1053–1060. [Google Scholar] [CrossRef]
- Reverón, I.; Rodríguez, H.; Campos, G.; Curiel, J.A.; Ascaso, C.; Carrascosa, A.V.; Prieto, A.; Rivas, B.D.L.; Muñoz, R.; Felipe, F.L.D. Tannic acid-dependent modulation of selected lactobacillus plantarum traits linked to gastrointestinal survival. PLoS ONE 2013, 8, e66473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.M.; Huang, H.J.; Hu, Y.P.; Huang, J.; Yang, H.S.; Wang, L.; Chen, S.; Chen, C.Q.; He, S.P. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J. Anim. Sci. 2020, 98, skaa112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Luo, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Huang, Z.; Luo, J.; Luo, Y.; Yan, H.; et al. Tannic acid extracted from gallnut prevents post-weaning diarrhea and improves intestinal health of weaned piglets. Anim. Nutr. 2021, 7, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Craig, P.M.; Moyes, C.D.; Lemoine, C. Sensing and responding to energetic stress: Evolution of the AMPK network. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 224, 156–159. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ashford, M.L. AMPK: Regulating energy balance at the cellular and whole body levels. Physiology 2014, 29, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Kepez, A.; Oto, A.; Dagdelen, S. Peroxisome Proliferator-Activated Receptor-γ. BioDrugs 2006, 20, 121–135. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Wang, S.; Guo, H.; Dong, S.; Wang, T.; Zhang, L.; Jiang, Z. Emodin ameliorates hepatic steatosis through endoplasmic reticulum-stress sterol regulatory element-binding protein 1c pathway in liquid fructose-feeding rats. Hepatol. Res. 2016, 46, 105–117. [Google Scholar] [CrossRef]
- Russell-Guzmán, J.A.; Karachon, L.; Gacitúa, T.A.; Freundlich, A.; Rodrigo, R. Role of exercise in the mechanisms ameliorating hepatic steatosis in non-alcoholic fatty liver disease. Sport Sci. Health 2018, 14, 463–473. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.H.; Yu, H.S.; Geun, P.H.; Kang, U.G.; Min, A.Y.; Sik, K.Y. The effect of clozapine on the AMPK-ACC-CPT1 pathway in the rat frontal cortex. Int. J. Neuropsychoph. 2011, 15, 907–917. [Google Scholar] [CrossRef] [Green Version]
- Cock, T.A.; Houten, S.M.; Auwerx, J. Peroxisome proliferator-activated receptort-γ: Too much of a good thing causes harm. EMBO Rep. 2004, 5, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Reinehr, T.; Roth, C.L. A new link between skeleton, obesity and insulin resistance: Relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int. J. Obes. 2010, 34, 852–858. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Jyoti, P.; Jain, S.K.; Bhattacharjee, J. Correlation of Adiponectin and Leptin with Insulin Resistance:A Pilot Study in Healthy North Indian Population. Ind. J. Clin. Biochem. 2011, 26, 193–196. [Google Scholar] [CrossRef] [Green Version]
- Memon, S.; Afroz, M.N. Association of leptin with obesity and insulin resistance. Cureus 2020, 12, e12178. [Google Scholar]
- Panic, A.; Stanimirovic, J.; Sudar-Milovanovic, E.; Isenovic, E.R. Oxidative stress in obesity and insulin resistance. Explor. Med. 2022, 3, 58–70. [Google Scholar] [CrossRef]
- Hoehn, K.L.; Salmon, A.B.; Hohnen-Behrens, C.; Turner, N.; Hoy, A.J.; Maghzal, G.J.; Stocker, R.; Remmen, H.V.; Kraegen, E.W.; Cooney, G.J. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 17787–17792. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Dong, C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int. Immunol. 2016, 28, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A Mixture of trans-Galactooligosaccharides Reduces Markers of Metabolic Syndrome and Modulates the Fecal Microbiota and Immune Function of Overweight Adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, O.F.; Claassen, E. The mechanistic link between health and gut microbiota diversity. Sci. Rep. 2018, 8, 2183. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Song, Y.J.; Shen, H.T.; Liu, T.T.; Pan, B.J.; De Alwis, S.; Zhang, W.Y.; Luo, X.G.; Li, Z.Y.; Wang, N.; Ma, W.J.; et al. Effects of three different mannans on obesity and gut microbiota in high-fat diet-fed C57BL/6J mice. Food Funct. 2021, 12, 4606. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y.; Ai, C.; Wang, L.; Wen, C.; Yang, J.; Song, S.; Liu, X. Sulfated polysaccharide from sea cucumber and its depolymerized derivative prevent obesity in association with modification of gut microbiota in high-fat diet-fed mice. Mol. Nutr. Food Res. 2018, 62, e1800446. [Google Scholar] [CrossRef]
- Wang, S.; Lv, Z.; Zhao, W.; Wang, L.; He, N. Collagen peptide from walleye pollock skin attenuated obesity and modulated gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2020, 74, 104194. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Zeng, L.; He, Y.; Liu, X.; Zhang, T.; Wang, Q. Effects of Dietary Tannic Acid on Obesity and Gut Microbiota in C57BL/6J Mice Fed with High-Fat Diet. Foods 2022, 11, 3325. https://doi.org/10.3390/foods11213325
Fang J, Zeng L, He Y, Liu X, Zhang T, Wang Q. Effects of Dietary Tannic Acid on Obesity and Gut Microbiota in C57BL/6J Mice Fed with High-Fat Diet. Foods. 2022; 11(21):3325. https://doi.org/10.3390/foods11213325
Chicago/Turabian StyleFang, Jiangmin, Lirong Zeng, Yalun He, Xiong Liu, Tongcun Zhang, and Qiong Wang. 2022. "Effects of Dietary Tannic Acid on Obesity and Gut Microbiota in C57BL/6J Mice Fed with High-Fat Diet" Foods 11, no. 21: 3325. https://doi.org/10.3390/foods11213325
APA StyleFang, J., Zeng, L., He, Y., Liu, X., Zhang, T., & Wang, Q. (2022). Effects of Dietary Tannic Acid on Obesity and Gut Microbiota in C57BL/6J Mice Fed with High-Fat Diet. Foods, 11(21), 3325. https://doi.org/10.3390/foods11213325






