Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review
Abstract
1. Introduction
2. Methods
2.1. Searching Strategy
2.2. Study Selection and Inclusion, Exclusion Criteria
2.3. Data Extraction
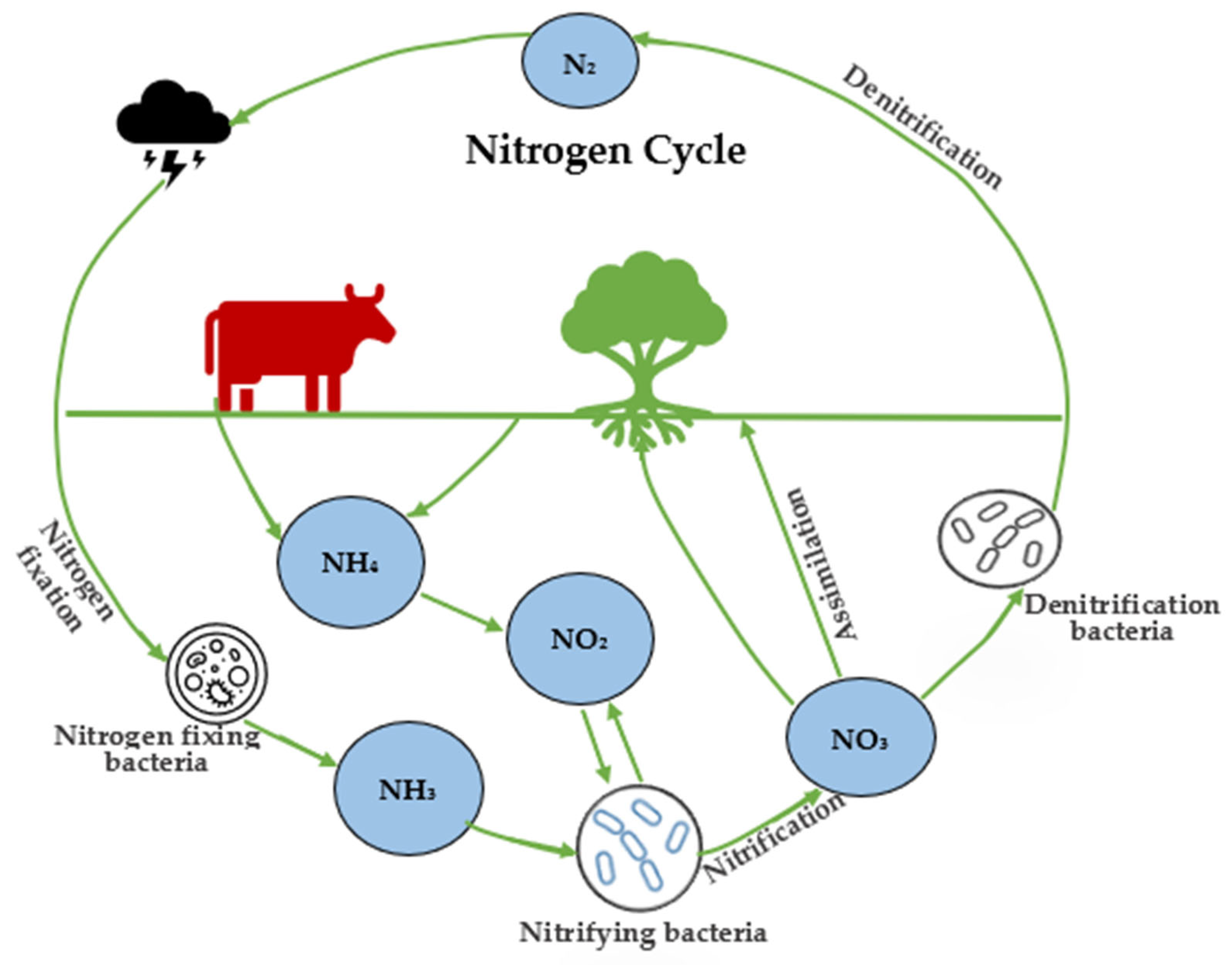
3. Sources of Nitrite
- (1)
- Nitrosomonas bacteria converts ammonia to nitrite2NH4++ 3O2 → 2NO2− + 4H+ + 2H2O
- (2)
4. Function of Nitrite in Cured Meats
4.1. Cured Color Development
- (1)
- NO2− + H+ ↔ HNO2
- (2)
- 2HNO2 ↔ N2O3 + H2O
- (3)
- N2O3 ↔ NO + NO2
4.2. Cured Flavour Development
- ✓
- Lipid oxidation suppression by nitrite;
- ✓
- Nitrite related flavor development.
4.3. Antioxidant Properties against Lipid and Protein Oxidation
4.4. Antimicrobial Effect
5. Health Concerns Associated with Nitrite in Meat
6. Potential Alternatives to Nitrite in Processed Meat and Their Effect on Color, Flavor, Antimicrobial and Antioxidant Properties
6.1. Plant Extracts
6.2. Organic Acids and Salts
6.3. High Hydrostatic Pressure
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarado, C.; McKee, S. Marination to improve functional properties and safety of poultry meat. J. Appl. Poult. Res. 2007, 16, 113–120. [Google Scholar] [CrossRef]
- Patarata, L.; Martins, S.; Silva, J.S.; Fraqueza, M.J. Red wine and garlic as a possible alternative to minimize the use of nitrite for controlling Clostridium sporogenes and Salmonella in a cured sausage: Safety and sensory implications. Foods 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Pegg, R.B.; Honikel, K.O. Principles of Curing. In Handbook of Fermented Meat and Poultry; Fidel, T., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 19–30. [Google Scholar]
- Pourreza, N.; Fat’hi, M.R.; Hatami, A. Indirect cloud point extraction and spectrophotometric determination of nitrite in water and meat products. Microchem. J. 2012, 104, 22–25. [Google Scholar] [CrossRef]
- Afkhami, A.; Masahi, S.; Bahram, M. Spectrophotometric determination of nitrite based on its reaction with p-nitroaniline in the presence of diphenylamine in micellar media. Bull. Korean Chem. Soc. 2004, 25, 1009–1011. [Google Scholar]
- Yildiz, G.; Oztekin, N.; Orbay, A.; Senkal, F. Voltammetric determination of nitrite in meat products using polyvinylimidazole modified carbon paste electrode. Food Chem. 2014, 152, 245–250. [Google Scholar] [CrossRef]
- Lopez, C.M.; Dallolio, G.; Bonilauri, P.; Rebecchi, A. Strategies for nitrite replacement in fermented sausages and effect of high pressure processing against Salmonella spp. and Listeria innocua. Foods 2021, 10, 2617. [Google Scholar] [CrossRef]
- Badea, M.; Amine, A.; Benzine, M.; Curulli, A.; Moscone, D.; Lupu, A.; Volpe, G.; Palleshi, G. Rapid and selective electrochemical determination of nitrite in cured meat in the presence of ascorbic acid. Microchim. Acta 2004, 147, 51–58. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Stringer, S.; Fernandez, M. A study on the toxigenesis by Clostridium botulinum in nitrate and nitrite-reduced dry fermented sausages. Int. J. Food Microbiol. 2016, 218, 66–70. [Google Scholar] [CrossRef]
- Patarata, L.; Carvalho, F.; Fraqueza, M.J. Nitrite-free implications on consumer acceptance and the behavior of pathogens in cured pork loins. Foods 2022, 11, 796. [Google Scholar] [CrossRef]
- Candan, T.; Bağdatlı, A. Natural applications for nitrite/nitrate reduction in meat products. Pamukkale Univ. J. Eng. Sci. 2018, 24, 1382–1387. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A. A green and efficient vortex-assisted liquid-phase microextraction based on supramolecular solvent for UV–VIS determination of nitrite in processed meat and chicken products. Food Chem. 2020, 332, 127395. [Google Scholar] [CrossRef] [PubMed]
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimikar, M.; Azar, H.H.; Mehrasbi, M.R.; Daneshamooz, S.; Raesi, M.; Jannat, B.; Afshari, A. Using natural antioxidants in meat and meat products as preservatives: A review. Adv. Anim. Vet. Sci. 2019, 7, 417–426. [Google Scholar] [CrossRef]
- Dos Santos, B.D.; da Silva, D.V.T.; Paschoalin, V.M.F. A narrative review on dietary strategies to provide nitric oxide as a non-drug cardiovascular disease therapy: Beetroot formulations—A smart nutritional intervention. Foods 2021, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Hunault, C.C.; van Velzen, A.G.; Sips, A.J.A.M.; Schothorst, R.C.; Meulenbelt, J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol. Lett. 2009, 190, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Balligand, J.-L. Regulation of cardiac function by nitric oxide. In Nitric Oxide. Handbook of Experimental Pharmacology; Mayer, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 207–234. [Google Scholar]
- Milkowski, A.; Garg, H.K.; Coughlin, J.R.; Bryan, N.S. Nutritional epidemiology in the context of nitric oxide biology: A risk–benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 2010, 22, 110–119. [Google Scholar] [CrossRef]
- Bryan, N.S.; van Grinsven, H. The role of nitrate in human health. Adv. Agron. 2013, 119, 153–182. [Google Scholar]
- Brender, J.D. Human health effects of exposure to nitrate, nitrite, and nitrogen dioxide. In Just Enough Nitrogen; Sutton, M.A., Ed.; Springer Natute: Cham, Swizerland, 2020; pp. 283–294. [Google Scholar]
- Oliveira, S.; Lopes, T.; Rangel, A.O. Spectrophotometric determination of nitrite and nitrate in cured meat by sequential injection analysis. J. Food Sci. 2004, 69, C690–C695. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Talla, L.B.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Onco. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar]
- Richi, E.B.; Baumer, B.; Conrad, B.; Darioli, R.; Schmid, A.; Keller, U. Health risks associated with meat consumption: A review of epidemiological studies. Int. J. Vitam. Nutr. Res. 2015, 85, 70–78. [Google Scholar] [CrossRef]
- Casoni, D.; Badiu, R.R.; Frenţiu, T. Spectrophotometric determination and assessment of potential health risk of nitrite from meat and processed meat products. Stud. UBB Chem. 2019, 2, 265–277. [Google Scholar] [CrossRef]
- Hung, Y.; Verbeke, W.; de Kok, T.M. Stakeholder and consumer reactions towards innovative processed meat products: Insights from a qualitative study about nitrite reduction and phytochemical addition. Food Control 2016, 60, 690–698. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Sodium nitrite in processed meat and poultry meats: A review of curing and examining the risk/benefit of its use. Am. Meat Sci. Assoc. White Pap. Ser. 2011, 3, 1–14. [Google Scholar]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Shariati-Rad, M.; Irandoust, M.; Mohammadi, S. Spectrophotometric determination of nitrite in soil and water using cefixime and central composite design. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 190–195. [Google Scholar] [CrossRef]
- Lefevre, S.; Jensen, F.B.; Huong, D.T.T.; Wang, T.; Phuong, N.T.; Bayley, M. Effects of nitrite exposure on functional haemoglobin levels, bimodal respiration, and swimming performance in the facultative air-breathing fish Pangasianodon hypophthalmus. Aquat. Toxicol. 2011, 104, 86–93. [Google Scholar] [CrossRef]
- Bijad, M.; Maleh, H.K.; Farsi, M.; Shahidi, S.A. Simultaneous determination of amaranth and nitrite in foodstuffs via electrochemical sensor based on carbon paste electrode modified with CuO/SWCNTs and room temperature ionic liquid. Food Anal. Methods 2017, 10, 3773–3780. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J. Food Process. 2014, 2014, 918018. [Google Scholar] [CrossRef]
- Tsikas, D.; Mitscke, A.; Gutzki, F.M.; Engeli, S.; Jordan, J. Evidence by gas chromatography–mass spectrometry of ex vivo nitrite and nitrate formation from air nitrogen oxides in human plasma, serum, and urine samples. Anal. Biochem. 2010, 397, 126–128. [Google Scholar] [CrossRef]
- Yang, B.K.; Vivas, E.X.; Reiter, C.D.; Gladwin, M.T. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic. Res. 2003, 37, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Ward, B.B.; Arp, D.J.; Klotz, M.G. Nitrification; ASM Press: Washington, DC, USA, 2011. [Google Scholar]
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate or nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, D.K.; Bryan, N.S. Sodium nitrite: The “cure” for nitric oxide insufficiency. Meat Sci. 2012, 92, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 2012, 26, 259–266. [Google Scholar] [CrossRef]
- Ahmed, M.M.A.A. Spectrophotometric Determination of Nitrite Content in Cured Meats. Master’s Thesis, Sudan University of Science and Technology, Khartoum, Sudan, 2017. [Google Scholar]
- Delgado-Pando, G.; Fischer, E.; Allen, P.; Kerry, J.P.; Sullivan, M.G.O.; Hamill, R.M. Salt content and minimum acceptable levels in whole-muscle cured meat products. Meat Sci. 2018, 139, 179–186. [Google Scholar] [CrossRef]
- Skibsted, L.H. Nitric oxide and quality and safety of muscle based foods. Nitric Oxide 2011, 24, 176–183. [Google Scholar] [CrossRef]
- Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Green alternatives to nitrates and nitrites in meat-based products–A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2133–2148. [Google Scholar] [CrossRef]
- Sullivan, G.A. Naturally Cured Meats: Quality, Safety, and Chemistry. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2011. [Google Scholar]
- Feiner, G. Salami: Practical Science and Processing Technology; Academic Press: Cambridge, MA, USA, 2016; p. 230. [Google Scholar]
- Gray, J.I.; Macdonald, B.; Pearsopn, A.M.; Morton, I.D. Role of nitrite in cured meat flavor: A review. J. Food Prot. 1981, 44, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Lipid-derived flavors in meat products. In Meat Processing: Improving Quality; Kerry, J., Ed.; Woodhead Publishing: Sawston, Cambridge, 2002; pp. 105–121. [Google Scholar]
- Ramarathnam, N.; Rubin, L. The flavour of cured meat. In Flavor of Meat and Meat Products; Shahidi, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 174–198. [Google Scholar]
- Van Boekel, M. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- Villaverde, A.; Ventanas, J.; Estévez, M. Nitrite promotes protein carbonylation and Strecker aldehyde formation in experimental fermented sausages: Are both events connected? Meat Sci. 2014, 98, 665–672. [Google Scholar] [CrossRef]
- Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.D. Strategies to improve meat products’ quality. Foods 2020, 9, 1883. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gomez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, K.-I.; Kim, J.-Y.; Ahn, Y.K.; Rha, S.W.; Kim, Y.J.; Choi, Y.S.; Choi, S.W.; Jeon, D.W.; Min, P.K.; et al. Non-lipid effects of rosuvastatin–fenofibrate combination therapy in high-risk Asian patients with mixed hyperlipidemia. Atherosclerosis 2012, 221, 169–175. [Google Scholar] [CrossRef]
- Department of Environmental Conservation. Guidelines for Conducting Bird and Bat Studies at Commercial Wind Energy Projects; Department of Environmental Conservation: New York, NY, USA, 2016; p. 35. Available online: https://www.dec.ny.gov/docs/wildlife_pdf/windguide.pdf (accessed on 26 June 2022).
- Sebranek, J.G. Basic curing ingredients. In Ingredients in Meat Products; Tarte, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–23. [Google Scholar]
- Al-Shuibi, A.; Al-Abdullah, B. Substitution of nitrite by sorbate and the effect on properties of mortadella. Meat Sci. 2002, 62, 473–478. [Google Scholar] [CrossRef]
- Girouard, J.; Frenette, G.; Sullivan, R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int. J. Androl. 2011, 34, e475–e486. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Rysman, T.; Hecke, T.V.; Poucke, C.V.; Smet, S.D.; Royen, G.V. Protein oxidation and proteolysis during storage and in vitro digestion of pork and beef patties. Food Chem. 2016, 209, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, C.; Jia, X.; Guo, Y.; Lei, N.; Hackman, R.M.; Chen, L.; Zhou, G. Influence of sodium nitrite on protein oxidation and nitrosation of sausages subjected to processing and storage. Meat Sci. 2016, 116, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heiononen, M.; Baron, C.V.P.; Estevez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Pierson, M.D.; Smoot, L.A.; Robach, M.C. Nitrite, nitrite alternatives, and the control of Clostridium botulinum in cured meats. Crit. Rev. Food Sci. Nutr. 1983, 17, 141–187. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.; Cannon, J.; Montoya, D.; Dickson, J.; Lonergan, S.; Sebranek, J. Effects of high hydrostatic pressure and varying concentrations of sodium nitrite from traditional and vegetable-based sources on the growth of Listeria monocytogenes on ready-to-eat (RTE) sliced ham. Meat Sci. 2013, 94, 69–76. [Google Scholar] [CrossRef]
- Cui, H.; Gabriel, A.A.; Nakano, H. Antimicrobial efficacies of plant extracts and sodium nitrite against Clostridium botulinum. Food Control 2010, 21, 1030–1036. [Google Scholar] [CrossRef]
- Tompkin, R.B. Nitrite. In Antimicrobials in Food, 3rd ed.; Davidson, P.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 169–236. [Google Scholar]
- Allaker, R.P.; Mendez, L.S.; Hardie, J.M.; Benjamin, N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol. Immunol. 2001, 16, 253–256. [Google Scholar] [CrossRef]
- Tompkin, R.B.; Bedale, W.; Milkowski, A.; Glass, K.; Sindelar, J.J. Nitrite. In Antimicrobials in Food, 4th ed.; Davidson, P.M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 219–308. [Google Scholar]
- Majou, D.; Christieans, S. Mechanisms of the bactericidal effects of nitrate and nitrite in cured meats. Meat Sci. 2018, 145, 273–284. [Google Scholar] [CrossRef]
- Prolo, C.; Álvarez, M.N.; Radi, R. Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. Biofactors 2014, 40, 215–225. [Google Scholar] [CrossRef]
- McLean, S.; Bowman, L.A.H.; Sanguinetti, G.; Read, R.C.; Poole, R.K. Peroxynitrite toxicity in Escherichia coli K12 elicits expression of oxidative stress responses and protein nitration and nitrosylation. J. Biol. Chem. 2010, 285, 20724–20731. [Google Scholar] [CrossRef]
- Landry, A.P.; Duan, X.; Huang, H.; Dimg, H. Iron–sulfur proteins are the major source of protein-bound dinitrosyl iron complexes formed in Escherichia coli cells under nitric oxide stress. Free Radic. Biol. Med. 2011, 50, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-C.; Foster, N.F.; Riley, T.V. Susceptibility of Clostridium difficile to the food preservatives sodium nitrite, sodium nitrate and sodium metabisulphite. Anaerobe 2016, 37, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Scannell, A.G.; Schwarz, G.; Hill, C.; Ross, R.P.; Arendt, E.K. Pre-inoculation enrichment procedure enhances the performance of bacteriocinogenic Lactococcus lactis meat starter culture. Int. J. Food Microbiol. 2001, 64, 151–159. [Google Scholar] [CrossRef]
- Zanardi, E.; Dazzi, G.; Madarena, G.; Chizzolini, R. Comparative study on nitrite and nitrate ions determination. Ann. Fac. Med. Vet. Univ. Parma 2002, 22, 70–86. [Google Scholar]
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Pourazrang, H.; Moazzami, A.; Bazzaz, B.F. Inhibition of mutagenic N-nitroso compound formation in sausage samples by using L-ascorbic acid and α-tocopherol. Meat Sci. 2002, 62, 479–483. [Google Scholar] [CrossRef]
- Van den Brand, A.D.; Beukers, M.; Niekerk, M.; Van Donkersgoed, G.; Van Der Aa, M.; Van De Ven, B.; Bulder, A.; Van Der Voet, H.; Sprong, C.R. Assessment of the combined nitrate and nitrite exposure from food and drinking water: Application of uncertainty around the nitrate to nitrite conversion factor. Food Addit. Contam. Part A 2020, 37, 568–582. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Granby, K.; Duedahl-Olesen, L. Formation and mitigation of N-nitrosamines in nitrite preserved cooked sausages. Food Chem. 2015, 174, 516–526. [Google Scholar] [CrossRef]
- Alexander, D.D.; Weed, D.L.; Cushing, C.A.; Lowe, K.A. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur. J. Cancer Prev. 2011, 20, 293–307. [Google Scholar] [CrossRef]
- Sanchez-Echaniz, J.; Benito-Fernández, J.; Mintegui-Raso, S. Methemoglobinemia and consumption of vegetables in infants. Pediatrics 2001, 107, 1024–1028. [Google Scholar] [CrossRef]
- Demeyer, D.; Mertens, B.; Smet, S.D.; Ulens, M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2747–2766. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Solano, M.; Echandi, M.L.A. Comparación de métodos para el análisis de coliformes totales y fecales en muestras de agua mediante la técnica de Número Más Probable (NMP). UNED Res. J. 2011, 3, 41–43. [Google Scholar] [CrossRef][Green Version]
- Yurchenko, S.; Mölder, U. The occurrence of volatile N-nitrosamines in Estonian meat products. Food Chem. 2007, 100, 1713–1721. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Duedahl-Olesen, L.; Christensen, T.; Olesen, P.T.; Granby, K. Dietary exposure to volatile and non-volatile N-nitrosamines from processed meat products in Denmark. Food Chem. Toxicol. 2015, 80, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Stoica, M. Overview of sodium nitrite as a multifunctional meat-curing ingredient. Ann. Univ. Dunarea de Jos Galati Fascicle VI-Food Technol. 2019, 43, 155–167. [Google Scholar] [CrossRef]
- Iqbal, A. Effect of food on causation and prevention of gastric cancer. J. Cancer Prev. Curr. Res. 2017, 8, 10–15406. [Google Scholar] [CrossRef]
- Stepien, M.; Chajes, V.; Romieu, I. The role of diet in cancer: The epidemiologic link. Salud Publica Mex. 2016, 58, 261–273. [Google Scholar] [CrossRef]
- Loh, Y.H.; Jakszyn, P.; Luben, R.N.; Mulligan, A.A.; Mitrou, P.N.; Khaw, K.T. N-nitroso compounds and cancer incidence: The European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk study. Am. J. Clin. Nutr. 2011, 93, 1053–1061. [Google Scholar] [CrossRef]
- Keszei, A.P.; Goldbohm, R.A.; Schouten, L.J.; Jakszyn, P.; van den Brandt, P.A. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2013, 97, 135–146. [Google Scholar] [CrossRef]
- Grosse, Y.; Baan, R.; Straif, K.; Secretan, B.; Ghissassi, F.E.; Cogliano, V. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. 2006, 7, 628–629. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol. Lett. 2011, 200, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, R.L.; Pierre, F.; Corpet, D.E. Processed meat and colorectal cancer: A review of epidemiologic and experimental evidence. Nutr. Cancer 2008, 60, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zeng, X.; Sun, Z.; Wu, A.; He, J.; Dang, Y.; Pan, D. Production of a safe cured meat with low residual nitrite using nitrite substitutes. Meat Sci. 2020, 162, 108027. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Qader, H.A. Flow-injection spectrophotometric determination of nitarate in wastewater samples using diazotization coupling reaction. App. Sci. Rep. 2013, 3, 125–131. [Google Scholar]
- Kroupova, H.; Machova, J.; Svobodova, Z. Nitrite influence on fish: A review. Vet. Med. Praha 2005, 50, 461. [Google Scholar] [CrossRef]
- Rehman, A.; Shehadeh, M.; Khirfan, D.; Jones, A. Severe acute haemolytic anaemia associated with severe methaemoglobinaemia in a G6PD-deficient man. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; de Kok, T.M.; Verbeke, W. Consumer attitude and purchase intention towards processed meat products with natural compounds and a reduced level of nitrite. Meat Sci. 2016, 121, 119–126. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.J.; Kyriancou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Riel, G.; Boulaaba, A.; Popp, J.; Klein, G. Effects of parsley extract powder as an alternative for the direct addition of sodium nitrite in the production of mortadella-type sausages–Impact on microbiological, physicochemical and sensory aspects. Meat Sci. 2017, 131, 166–175. [Google Scholar] [CrossRef]
- Horsch, A.; Sebranek, J.G.; Dickson, J.S.; Niebuhr, S.E.; Larson, E.M.; Lavieri, N.A.; Ruther, B.L.; Wilson, L.A. The effect of pH and nitrite concentration on the antimicrobial impact of celery juice concentrate compared with conventional sodium nitrite on Listeria monocytogenes. Meat Sci. 2014, 96, 400–407. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Jackson-Daavis, A.L.; Myer, K.L.; Lavieri, N.A. Beyond celery and starter culture: Advances in natural/organic curing processes in the United States. Meat Sci. 2012, 92, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-M.; Hwang, K.-E.; Lee, C.-W.; Kim, T.-K.; Park, Y.-S.; Han, S.G. Effect of Swiss chard (Beta vulgaris var. cicla) as nitrite replacement on color stability and shelf-life of cooked pork patties during refrigerated storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 418. [Google Scholar] [PubMed]
- Pyo, Y.-H.; Lee, T.-C.; Logendra, L.; Rosen, R.T. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004, 85, 19–26. [Google Scholar] [CrossRef]
- Khalehgi, A.; Kasaai, R.; Darani, K.K.; Rezaei, K. Combined use of black barberry (Berberis crataegina L.) extract and nitrite in cooked beef sausages during the refrigerated storage. J. Agric. Sci. Technol. 2016, 18, 601–614. [Google Scholar]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebert, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef]
- Hayes, J.; Canonico, I.; Allen, P. Effects of organic tomato pulp powder and nitrite level on the physicochemical, textural and sensory properties of pork luncheon roll. Meat Sci. 2013, 95, 755–762. [Google Scholar] [CrossRef]
- Tan, S.; Ke, Z.; Chai, D.; Miao, Y.; Luo, K.; Li, W. Lycopene, polyphenols and antioxidant activities of three characteristic tomato cultivars subjected to two drying methods. Food Chem. 2021, 338, 128062. [Google Scholar] [CrossRef]
- Francis, D.M.; Barringer, S.A.; Whitmoyer, R.E. Ultrastructural characterization of yellow shoulder disorder in a uniform ripening tomato genotype. HortScience 2000, 35, 1114–1117. [Google Scholar] [CrossRef]
- Eyiler, E.; Oztan, A. Production of frankfurters with tomato powder as a natural additive. LWT-Food Sci. Technol. 2011, 44, 307–311. [Google Scholar] [CrossRef]
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H.; Savadkoohi, S. Oxidation phenomena and color properties of grape pomace on nitrite-reduced meat emulsion systems. Meat Sci. 2016, 121, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Jayaprakasha, G.; Jena, B. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Chneg, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Aliyari, P.; Kazaj, B.; Barzegar, M.; Gavlighi, H.A. Production of functional sausage using pomegranate peel and pistachio green hull extracts as natural preservatives. J. Agric. Sci. Technol. 2020, 22, 159–172. [Google Scholar]
- Al-Gubory, K.H.; Blachier, F.; Faure, P.; Garrel, C. Pomegranate peel extract decreases small intestine lipid peroxidation by enhancing activities of major antioxidant enzymes. J. Sci. Food Agric. 2016, 96, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Yolmeh, M.; Najafi, M.B.H.; Farhoosh, R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM). Food Chem. 2014, 155, 319–324. [Google Scholar] [CrossRef]
- Van Cuong, T.; Thoa, N.T. Effects of partial replacement of nitrite by annatto (Bixa orellana, L.) seed powder on the properties of pork sausages. J. Sci. Technol. 2017, 55, 178–187. [Google Scholar]
- Karwowska, M.; Dolatowski, Z.J. Effect of acid whey and freeze-dried cranberries on lipid oxidation and fatty acid composition of nitrite-/nitrate-free fermented sausage made from deer meat. Asian-Aust. J. Anim. Sci. 2017, 30, 85. [Google Scholar] [CrossRef]
- Drosinos, E.H.; Mataragas, M.; Kampani, A.; Kritikos, D.; Mataxopoulos, I. Inhibitory effect of organic acid salts on spoilage flora in culture medium and cured cooked meat products under commercial manufacturing conditions. Meat Sci. 2006, 73, 75–81. [Google Scholar] [CrossRef]
- Mcclure, B.N. The Effect of Lactate on Nitrite in A Cured Meat System. Master’s Thesis, Iowa State University, Ames, IA, USA, 2009; p. 68. [Google Scholar]
- Doores, S. Organic acids. In Antimicrobials in Food, 3rd ed.; Davidson, P.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 91–140. [Google Scholar]
- Vijayakumar, J.; Aravindan, R.; Viruthagiri, T. Recent trends in the production, purification and application of lactic acid. Chem. Biochem. Eng. Q. 2008, 22, 245–264. [Google Scholar]
- McClure, B.N.; Sebranek, J.G.; Kim, Y.H.; Sullivan, G.A. The effects of lactate on nitrosylmyoglobin formation from nitrite and metmyoglobin in a cured meat system. Food Chem. 2011, 129, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keeton, J.T.; Smith, S.B.; Berghman, L.R.; Savell, J.W. Role of lactate dehydrogenase in metmyoglobin reduction and color stability of different bovine muscles. Meat Sci. 2009, 83, 376–382. [Google Scholar] [CrossRef]
- Serdengecti, N.; Yildirim, I.; Gokoglu, N. Effects of sodium lactate, sodium acetate and sodium diacetate on microbiological quality of vacuum-packed beef during refrigerated storage. J. Food Saf. 2006, 26, 62–71. [Google Scholar] [CrossRef]
- Tan, W.; Shelef, L. Effects of sodium chloride and lactates on chemical and microbiological changes in refrigerated and frozen fresh ground pork. Meat Sci. 2002, 62, 27–32. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Merx, M.W.; Sjiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Nat. Acad. Sci. USA 2008, 105, 10256–10261. [Google Scholar] [CrossRef]
- Kim, Y.; Keeton, J.T.; Smith, S.B.; Maxim, J.E.; Yang, H.S.; Savell, J.W. Evaluation of antioxidant capacity and colour stability of calcium lactate enhancement on fresh beef under highly oxidising conditions. Food Chem. 2009, 115, 272–278. [Google Scholar] [CrossRef]
- Sedghi, H.; Mohammadi, T.; Shariati, M.A.; Atarod, S.; Branch, Q. The Effect of Substituting Sodium Nitrite, with Sodium Acetate on Microbial, Physiochemical and Sensory Characteristics of Meat Products. Available online: https://www.semanticscholar.org/paper/The-effect-of-substituting-sodium-nitrite%2C-with-on-Sedghi-Mohammadi/a689e9e16ff3660a1b90adc57a3d1cf1fc865db5 (accessed on 26 June 2022).
- Vasavada, M.; Carpenter, C.E.; Cornforth, D.P.; Ghorpade, V. Sodium levulinate and sodium lactate effects on microbial growth and stability of fresh pork and turkey sausages. J. Muscle Foods 2003, 14, 119–129. [Google Scholar] [CrossRef]
- Carpenter, C.; Broadbent, J.R. External concentration of organic acid anions and pH: Key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. J. Food Sci. 2009, 74, R12–R15. [Google Scholar] [CrossRef]
- Bradley, E.M.; Williams, J.B.; Schilling, M.W.; Coggins, P.C.; Crist, C.; Yodre, S.; Campano, S.G. Effects of sodium lactate and acetic acid derivatives on the quality and sensory characteristics of hot-boned pork sausage patties. Meat Sci. 2011, 88, 145–150. [Google Scholar] [CrossRef]
- Aran, N. The effect of calcium and sodium lactates on growth from spores of Bacillus cereus and Clostridium perfringens in a ‘sous-vide’ beef goulash under temperature abuse. Int. J. Food Microbiol. 2001, 63, 117–123. [Google Scholar] [CrossRef]
- Juneja, V.K. Delayed Clostridium perfringens growth from a spore inocula by sodium lactate in sous-vide chicken products. Food Microbiol. 2006, 23, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.; Thippareddi, H. Control of Clostridium perfringens in a model roast beef by salts of organic acids during chilling. J. Food Saf. 2004, 24, 95–108. [Google Scholar] [CrossRef]
- Sabah, J.; Juneja, V.; Fung, D. Effect of spices and organic acids on the growth of Clostridium perfringens during cooling of cooked ground beef. J. Food Prot. 2004, 67, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Bingöl, E.B.; Bostan, K. Effect of sodium lactate on the microbiological quality and shelf life of sausages. Turk. J. Vet. Anim. Sci. 2007, 31, 333–339. [Google Scholar]
- Sallam, K.I.; Samejima, K. Microbiological and chemical quality of ground beef treated with sodium lactate and sodium chloride during refrigerated storage. LWT-Food Sci. Technol. 2004, 37, 865–871. [Google Scholar] [CrossRef]
- Cegielska-Radziejewska, R.; Pikul, J. Sodium lactate addition on the quality and shelf life of refrigerated sliced poultry sausage packaged in air or nitrogen atmosphere. J. Food Prot. 2004, 67, 601–606. [Google Scholar] [CrossRef]
- Quilo, S.; Pohlman, F.W.; Brown, A.H.; Crandall, P.G.; Dias-Morse, P.N.; Baublits, R.T.; Aparico, J.L. Effects of potassium lactate, sodium metasilicate, peroxyacetic acid, and acidified sodium chlorite on physical, chemical, and sensory properties of ground beef patties. Meat Sci. 2009, 82, 44–52. [Google Scholar] [CrossRef]
- De Gonzalez, M.N.; Keeton, J.; Ringer, L. Sensory and physicochemical characteristics of frankfurters containing lactate with antimicrobial surface treatments. J. Food Sci. 2004, 69, S221–S228. [Google Scholar] [CrossRef]
- Thippareddi, H.; Juneja, V.K.; Phebus, R.K.; Marsden, J.L.; Kastner, C.L. Control of Clostridium perfringens germination and outgrowth by buffered sodium citrate during chilling of roast beef and injected pork. J. Food Prot. 2003, 66, 376–381. [Google Scholar] [CrossRef]
- Juneja, V.; Thippareddi, H. Inhibitory effects of organic acid salts on growth of Clostridium perfringens from spore inocula during chilling of marinated ground turkey breast. Int. J. Food Microbiol. 2004, 93, 155–163. [Google Scholar] [CrossRef]
- Food and Drug Administration, Substances generally recognized as safe. Code of Federal Regulations, Office of the Federal Register, 2003.
- Islam, M.; Chen, J.; Doyle, M.P.; Chinnan, M. Effect of selected generally recognized as safe preservative sprays on growth of Listeria monocytogenes on chicken luncheon meat. J. Food Prot. 2002, 65, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Sofos, J.N.; Kain, M.L.; Scanga, J.A.; Belk, K.E.; Smith, G.C. Organic acids and their salts as dipping solutions to control Listeria monocytogenes inoculated following processing of sliced pork bologna stored at 4 °C in vacuum packages. J. Food Prot. 2001, 64, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.; Preston, D.; Veesenmeyer, J. Inhibition of Listeria monocytogenes in turkey and pork-beef bologna by combinations of sorbate, benzoate, and propionate. J. Food Prot. 2007, 70, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Chen, J.; Doyle, M.P.; Chinnan, M. Control of Listeria monocytogenes on turkey frankfurters by generally-recognized-as-safe preservatives. J. Food Prot. 2002, 65, 1411–1416. [Google Scholar] [CrossRef]
- Mulvey, L.; Everis, L.; Leeks, D.; Hughes, H.; Wood, A. Alternatives to Nitrates and Nitrites in Organic Meat Products; Campden BRI Group: London, UK, 2010; p. 91. [Google Scholar]
- San Martin, M.; Barbosa-Cánovas, G.; Swanson, B. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645. [Google Scholar] [CrossRef]
- Patterson, M.F.; Linton, M.; Doona, C.J. Introduction to High Pressure Processing of Foods. In High Pressure Processing of Foods; Doona, C.J., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 1–14. [Google Scholar]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stubler, A.S.; de Lamballerie, M.; Hertel, C.; Bruggemann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hi, C.; Sleator, R.D. High-pressure processing–effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef]
- Yordanov, D.; Angelova, G. High pressure processing for foods preserving. Biotechnol. Biotechnol. Equip. 2010, 24, 1940–1945. [Google Scholar] [CrossRef]
- Wilson, D.R.; Dabrowski, L.; Stringer, S.; Moezelaar, R.; Brocklehurst, T.F. High pressure in combination with elevated temperature as a method for the sterilisation of food. Trends Food Sci. Technol. 2008, 19, 289–299. [Google Scholar] [CrossRef]
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT-Food Sci. Technol. 2011, 44, 1251–1260. [Google Scholar] [CrossRef]
- Chotyakul, N.; Boonnoon, N. High pressure food processing: An alternative technology to reduce food additives used in processed meat products. Panyapiwat J. 2016, 8, 327–341. [Google Scholar]
- Hugas, M.; Garriga, M.; Monfort, J. New mild technologies in meat processing: High pressure as a model technology. Meat Sci. 2002, 62, 359–371. [Google Scholar] [CrossRef]
- Ros-Polski, V.; Koutchma, T.; Xue, J.; Defelice, C.; Balamurugan, S. Effects of high hydrostatic pressure processing parameters and NaCl concentration on the physical properties, texture and quality of white chicken meat. Innov. Food Sci. Emerg. Technol. 2015, 30, 31–42. [Google Scholar] [CrossRef]
- Campus, M. High pressure processing of meat, meat products and seafood. Food Eng. Rev. 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Guardia, M.D.; Garriga, M. Assessment of high hydrostatic pressure and starter culture on the quality properties of low-acid fermented sausages. Meat Sci. 2007, 76, 46–53. [Google Scholar] [CrossRef][Green Version]
- Simonin, H.; Duranton, F.; de Lamballerie, M. New insights into the high-pressure processing of meat and meat products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- Rubio, B.; Martinez, B.; Garcia-Cachan, D.; Rovira, J.; Jaime, I. The effects of high pressure treatment and of storage periods on the quality of vacuum-packed “salchichón” made of raw material enriched in monounsaturated and polyunsaturated fatty acids. Innov. Food Sci. Emerg. Technol. 2007, 8, 180–187. [Google Scholar] [CrossRef]
- Alfaia, A.; Alfaia, C.M.; Patarata, L.; Fernandes, M.J.; Fernandes, M.H.; Elias, M.; Ribeiro, M.H.; Fraqueza, M.J. Binomial effects of high isostatic pressure and time on the microbiological, sensory characteristics and lipid composition stability of vacuum packed dry fermented sausages “chouriço”. Innov. Food Sci. Emerg. Technol. 2015, 32, 37–44. [Google Scholar] [CrossRef][Green Version]
- Gill, A.O.; Ramaswamy, H.S. Application of high pressure processing to kill Escherichia coli O157 in ready-to-eat meats. J. Food Prot. 2008, 71, 2182–2189. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Gaudette, N.; Johnston, S. The impact of high hydrostatic pressure on the functionality and consumer acceptability of reduced sodium naturally cured wieners. Meat Sci. 2017, 129, 127–134. [Google Scholar] [CrossRef]
- Pietrzak, D.; Fonberg-Broczek, M.; Mucka, A.; Windyga, B. Effects of high pressure treatment on the quality of cooked pork ham prepared with different levels of curing ingredients. High Press. Res. 2007, 27, 27–31. [Google Scholar] [CrossRef]
- Vercammen, A.; Vanoirbeek, K.G.A.; Lurquin, I.; Steen, L.; Goemaere, O.; Szczepaniak, S.; Paelinck, H.; Hendrickx, M.E.G.; Michiels, C.W. Shelf-life extension of cooked ham model product by high hydrostatic pressure and natural preservatives. Innov. Food Sci. Emerg. Technol. 2011, 12, 407–415. [Google Scholar] [CrossRef]
- Myers, K.; Montoya, D.; Cannon, J.; Dickson, J.; Sebranek, J. The effect of high hydrostatic pressure, sodium nitrite and salt concentration on the growth of Listeria monocytogenes on RTE ham and turkey. Meat Sci. 2013, 93, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Slongo, A.P.; Rosenthai, A.; Camargo, L.M.Q.; Deliza, R.; Mathias, S.P.; de Aragio, G.M.F. Modeling the growth of lactic acid bacteria in sliced ham processed by high hydrostatic pressure. LWT-Food Sci. Technol. 2009, 42, 303–306. [Google Scholar] [CrossRef]
- Aymerich, T.; Jofre, A.; Garriga, M.; HUgas, M. Inhibition of Listeria monocytogenes and Salmonella by natural antimicrobials and high hydrostatic pressure in sliced cooked ham. J. Food Prot. 2005, 68, 173–177. [Google Scholar] [CrossRef]
- Morales, P.; Calzada, J.; Nunez, M. Effect of high-pressure treatment on the survival of Listeria monocytogenes Scott A in sliced vacuum-packaged Iberian and Serrano cured hams. J. Food Prot. 2006, 69, 2539–2543. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Grebol, N.; Garriga, M. Efficiency of high hydrostatic pressure at 600 MPa against food-borne microorganisms by challenge tests on convenience meat products. LWT-Food Sci. Technol. 2009, 42, 924–928. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Gui, M.; Zheng, H.; Dai, R.; Li, P. Combined effect of high hydrostatic pressure and enterocin LM-2 on the refrigerated shelf life of ready-to-eat sliced vacuum-packed cooked ham. Food Control 2012, 24, 64–71. [Google Scholar] [CrossRef]
- Garriga, M.; Aymerich, T. Advanced decontamination technologies: High hydrostatic pressure on meat products. In Safety of Meat and Processed Meat; Fidel, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 183–208. [Google Scholar]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. High-pressure processing and antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Food Microbiol. 2008, 25, 177–182. [Google Scholar] [CrossRef]
- Lavieri, N.A.; SEbranek, J.P.; Cordray, J.S.; Dickson, J.S.; Horsch, A.M.; Jung, S.; Manu, D.K.; Strecher, B.F.B.; Mendonca, A.F. Effects of different nitrite concentrations from a vegetable source with and without high hydrostatic pressure on the recovery of Listeria monocytogenes on ready-to-eat restructured ham. J. Food Prot. 2014, 77, 781–787. [Google Scholar] [CrossRef]
- Hayman, M.M.; Baxter, I.; O’Riordan, P.J.; Stewart, C.M. Effects of high-pressure processing on the safety, quality, and shelf life of ready-to-eat meats. J. Food Prot. 2004, 67, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Jonas, G.; Csehi, B.; Palotas, P.; Toth, A.; Kenesei, G.; Huszar, K.P.; Friedrich, L. Combined effects of high hydrostatic pressure and sodium nitrite on color, water holding capacity and texture of frankfurter. J. Phys. Conf. Ser. 2017, 950, 042006. [Google Scholar] [CrossRef]



| Additives | Meat Products | Effects | Reference |
|---|---|---|---|
| Parsley extract powder (PEP) | Mortadella type sausages | L. monocytogenes reduction, reduced residual nitrite level | [102] |
| Celery juice concentrate or powder | Ham slices | Control lipid oxidation, color development | [103] |
| Spray-dried Swiss chard powder | Cured pork loins | TBARS reduction | [105] |
| Barberry extract | Cooked beef sausage | Color development, potential antioxidant properties, negative interaction observed between nitrite and extract | [107] |
| Red wine or red wine + garlic | Chouriços cold-dried, smoked sausages | Color development, strong cured flavor, inhibitory properties against Salmonella | [2] |
| Beet root powder | Turkish fermented sausage | TBARS reduction | [108] |
| Tomato pulp powder | Pork luncheon roll, frankfurters | Control lipid oxidation | [110,113] |
| Pomegranate peel extract | Beef sausage | TBARS reduction, hydroperoxides reduction | [117] |
| Cranberry powder | Fermented sausage | Control lipid oxidation reduced growth of L. monocytogenes. | [121] |
| Annatto seed powder | Cooked sausages | Color development, TBARS reduction, control of bacterial growth | [120] |
| Treatment | Products | Effects | Reference |
|---|---|---|---|
| HHP at 600 Mpa | Sliced dry cured ham | L. monocytogenes inhibition after 120 days at 4 °C. | [160] |
| HHP at 400 Mpa | Vacuum-packed sliced cured ham | Extended the shelf life of products | [173] |
| HHP at 400 Mpa | Cooked sausages | Reduction of Enterobacteriaceae and Enterococci | [164] |
| HHP at 400 MPa + potassium lactate | Cooked ham (sliced) | Listeria monocytogenes and Salmonella spp. inhibition for 12 weeks | [174] |
| HHP at 450 MPa | Iberian ham | Listeria monocytogenes population reduction after 60 days | [175] |
| HHP at 800 MPa | Pork meat | Lipid oxidation retardation | [166] |
| HP at 600 MPa | Dry-cured ham, marinated beef loin and cooked ham | Salmonella enterica, L. monocytogenes, S. aureus below the detection levelduring 4 months of storage | [176] |
| HHP at 400 MPa + enterocin | Sliced ham | Suppress the development of Salmonella Enteritidis and L. monocytogenes | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. https://doi.org/10.3390/foods11213355
Shakil MH, Trisha AT, Rahman M, Talukdar S, Kobun R, Huda N, Zzaman W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods. 2022; 11(21):3355. https://doi.org/10.3390/foods11213355
Chicago/Turabian StyleShakil, Mynul Hasan, Anuva Talukder Trisha, Mizanur Rahman, Suvro Talukdar, Rovina Kobun, Nurul Huda, and Wahidu Zzaman. 2022. "Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review" Foods 11, no. 21: 3355. https://doi.org/10.3390/foods11213355
APA StyleShakil, M. H., Trisha, A. T., Rahman, M., Talukdar, S., Kobun, R., Huda, N., & Zzaman, W. (2022). Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods, 11(21), 3355. https://doi.org/10.3390/foods11213355








