Pomegranate Husk Scald Browning during Storage: A Review on Factors Involved, Their Modes of Action, and Its Association to Postharvest Treatments
Abstract
:1. Introduction
2. Evidence for Husk Scald Incidence Mechanism
3. Hypothetical Model of Pomegranate Husk Scald Incidence
4. Factors Affecting Husk Scald Incidence
4.1. Pre-Harvest Factors
4.2. Postharvest Treatments for Husk Scald Control
4.2.1. Effect of CA
4.2.2. Effect of MAP
4.2.3. Film Wrapping
4.2.4. Effect of Intermittent Warming
4.2.5. Effect of Coating
4.2.6. Effect of 1-MCP
4.2.7. Exogenous Putrescine Treatment
| Reference | Key Findings | Treatment Description | Fruit Treatment |
|---|---|---|---|
| [13] | Reduced HS | 2% O2 + 0%CO2 | CA |
| [47] | The more O2 available the more HS occurs | 10% O2 + 5% CO2 | |
| 5% O2 + 5% CO2 | |||
| 5% O2 + 0% CO2 | |||
| [6] | The best combination controlling HS | 5% O2 + 15% CO2 | |
| [48] | The higher CO2 concentration The better HS control | 3% and 6% CO2 | |
| [10] | PPP film and storage at 5 °C the best for controlling HS | Unperforated polypropylene (UPP) film and Perforated polypropylene (PPP) film | MAP |
| [2] | Inhibited HS | Film wrapping in combination with 600 mgL−1 fludioxonil | |
| [78] | Decreased HS | Intermittent warming cycles of 1 day at 20 °C every 6 days | Intermittent warming |
| [19] | Delay HS incidence | Intermittent warming for 1 day at 15 °C every 5 days and 5% CO2 + 8% O2 | |
| [75] | Reduce severity of HS | Soy lecithin | Coating |
| [90] | Successfully controlled HS | Chitosan treatment 1% with MAP | |
| [18] | No effect on controlling HS | 1-MCP alone or in combination with diphenylamine (DPA) | 1-MCP |
| [99] | Peel browning index decreased in all treatments, up to 35% at 0.5 μL/L concentration | 1-MCP (0.25, 0.5, and 1 μL/L) | |
| [100] | 1-MCP partially prevents HS specially in air storage | 1-MCP combined with controlled atmosphere (2% O2 + 5% CO2) and air | |
| [105] | Effective for husk scald prevention for the first 3 months storage, afterwards not effective | 2 and 3 mmol/L before storage at 5 °C | Putrescine |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, Horticulture, Breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- D’Aquino, S.; Palma, A.; Schirra, M.; Continella, A.; Tribulato, E.; La Malfa, S. Influence of film wrapping and fludioxonil application on quality of pomegranate fruit. Postharvest Biol. Technol. 2010, 55, 121–128. [Google Scholar] [CrossRef]
- Elyatem, S.M.; Kader, A.A. Post-Harvest Physiology and Storage Behaviour of Pomegranate Fruits. Sci. Hortic. 1984, 24, 287–298. [Google Scholar] [CrossRef]
- Opara, L.U.; Al-Ani, M.R.; Al-Shuaibi, Y.S. Physico-chemical Properties, Vitamin C Content, and Antimicrobial Properties of Pomegranate Fruit (Punica granatum L.). Food Bioprocess Technol. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Erkan, M.; Dogan, A. Pomegranate. In Postharvest Physiological Disorders in Fruits and Vegetables; Freitas, S.T.D., Pareek, S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 529–550. [Google Scholar]
- Hess-Pierce, B.; Kader, A.A. Responses of ‘Wonderful’ pomegranates to controlled atmospheres. Acta Hort. 2003, 600, 751–757. [Google Scholar] [CrossRef]
- McGlasson, W.B.; Scott, K.J.; Mendoza, D.B. The refrigerated storage of tropical and subtropical products. Int. J. Refrig. 1979, 2, 199–206. [Google Scholar] [CrossRef]
- Hardenburg, R.E.; Watada, A.E.; Wang, C.Y. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; US Department of Agriculture: Washington, DC, USA, 1986; p. 130.
- Makule, E.; Dimoso, N.; Tassou, S.A. Precooling and Cold Storage Methods for Fruits and Vegetables in Sub-Saharan Africa & mdash; A Review. Horticulturae 2022, 8, 776. [Google Scholar]
- Artes, F.; Villaescusa, R.; Tudela, J.A. Modified Atmosphere Packaging of Pomegranate. J. Food Sci. 2000, 65, 1112–1116. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Opara, U.L.; Witthuhn, C.R. Modelling the respiration rates of pomegranate fruit and arils. Postharvest Biol. Technol. 2012, 64, 49–54. [Google Scholar] [CrossRef]
- Roy, S.K.; Waskar, D.P. Pomegranate. In Postharvest Physiology and Storage of Tropical and Subtropical Fruits; Mitra, S., Ed.; Cab International: Wallingford, UK, 1997. [Google Scholar]
- Ben-Arie, R.; Or, E. The development and control of husk scald on ‘Wonderful’ pomegranate fruit during storage. J. Am. Soc. Hort. Sci. Hort. 1986, 111, 395–399. [Google Scholar] [CrossRef]
- Erkan, M.; Kader, A.A. Pomegranate (Punica granatum L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead: New York, NY, USA, 2011; pp. 287–311. [Google Scholar]
- Arendse, E. Determining Optimum Storage Conditions for Pomegranate Fruit (cv. Wonderful). Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2014. [Google Scholar]
- Kader, A.A. Postharvest biology and technology of pomegranates. In Pomegranates: Ancient Roots to Modern Medicine; Seeram, N.P., Schulman, R.N., Heber, D., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 211–220. [Google Scholar]
- Baghel, R.S.; Keren-Keiserman, A.; Ginzberg, I. Metabolic changes in pomegranate fruit skin following cold storage promote chilling injury of the peel. Sci. Rep. 2021, 11, 9141. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Whitaker, B.D.; Hess-Pierce, B.M.; Kader, A.A. Development and control of scald on wonderful pomegranates during long-term storage. Postharvest Biol. Technol. 2006, 41, 234–243. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, R.G. Study on the mechanism of browning of pomegranate (Punica granatum L. cv. “Ganesh”) peel in different storage conditions. Agric. Sci. China 2008, 7, 65–73. [Google Scholar] [CrossRef]
- Zhanyuan, D.; William, J.B. Peroxidative Activity of Apple Peel in Relation to Development of Poststorage Disorders. HortScience 1995, 30, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, P. Postharvest Storage Procedures and Oxidative Stress. HortScience 2004, 39, 938–942. [Google Scholar] [CrossRef] [Green Version]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled Abiotic Stresses Revisited: From Homeostasis through Hormesis to Extreme Stresses and the Impact on Nutraceuticals and Quality during Pre- and Postharvest Applications in Horticultural Crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.; Dubey, R.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef]
- Wang, C.Y. Approaches to Reduce Chilling Injury of Fruits and Vegetables. Hortic. Rev. 2010, 15, 63–95. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, N.; Malhotra, S.; Dhawan, K.; Singh, R. Antioxidant systems in ripening tomato fruits. Biol. Plant. 2004, 48, 49–53. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Witthuhn, C.R. Modified atmosphere packaging of pomegranate fruit and arils. Food Bioprocess Technol. 2012, 5, 15–30. [Google Scholar] [CrossRef]
- Ioannou, I.; Ghoul, M. Prevention of enzymatic browning in fruit and vegetables. Eur. Sci. J. 2013, 9, 310–341. [Google Scholar]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic Browning of Fruit and Vegetables: A Review. Enzyme. Food Technol. 2018, 63–78. [Google Scholar] [CrossRef]
- Sun, Y.-q.; Tao, X.; Men, X.-m.; Xu, Z.-w.; Wang, T. In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. J. Integr. Agric. 2017, 16, 1808–1818. [Google Scholar] [CrossRef] [Green Version]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Dunford, H.; Stillman, J. On the function and mechanism of action of peroxidases. Coord. Chem. Rev. 1976, 19, 187–251. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.; Nicolas, J. Phenolic composition and browning susceptibility of various apple cultivars at maturity. J. Food Sci. 1992, 57, 958–962. [Google Scholar]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Fischer, U.A.; Jaksch, A.V.; Carle, R.; Kammerer, D.R. Determination of Lignans in Edible and Nonedible Parts of Pomegranate (Punica granatum L.) and Products Derived Therefrom, Particularly Focusing on the Quantitation of Isolariciresinol Using HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2012, 60, 283–292. [Google Scholar] [CrossRef]
- Landete, J. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Zhao, Y.; Yu, C. Composition of pomegranate peel polyphenols and its antioxidant activities. Sci. Agric. Sin. 2009, 42, 4035–4041. [Google Scholar]
- Cerdá, B.; Llorach, R.; Cerón, J.J.; Espín, J.C.; Tomás-Barberán, F.A. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur. J. Nutr. 2003, 42, 18–28. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Matityahu, I.; Glazer, I.; Holland, D.; Bar-Ya’akov, I.; Ben-Arie, R.; Amir, R. Total Antioxidative Capacity and Total Phenolic Levels in Pomegranate Husks Correlate to Several Postharvest Fruit Quality Parameters. Food Bioprocess Technol. 2013, 7, 1938–1949. [Google Scholar] [CrossRef]
- Whitaker, B. Oxidative Stress and Superficial Scald of Apple Fruit. HortScience 2004, 39, 933–937. [Google Scholar] [CrossRef]
- Gil, M.I.; García-Viguera, C.; Artés, F.; Tomás-Barberán, F.A. Changes in pomegranate juice pigmentation during ripening. J. Sci. Food Agric. 1995, 68, 77–81. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Vorster, A.; van Heerden, C.; Opara, U.L. Transcriptomic changes associated with husk scald incidence on pomegranate fruit peel during cold storage. Food Res. Int. 2020, 135, 109285. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Functional characterisation of lenticels, micro-cracks, wax patterns, peel tissue fractions and water loss of pomegranate fruit (cv. Wonderful) during storage. Postharvest Biol. Technol. 2021, 178, 111539. [Google Scholar] [CrossRef]
- Giarola, V.; Krey, S.; von den Driesch, B.; Bartels, D. The Craterostigma plantagineum glycine-rich protein CpGRP1 interacts with a cell wall-associated protein kinase 1 (CpWAK1) and accumulates in leaf cell walls during dehydration. New Phytol. 2016, 210, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Artes, F.; Maren, J.G.; Martenez, J.A. Controlled atmosphere storage of pomegranate. Z. Lebensm.-Unters. Forsch. 1996, 203, 33–37. [Google Scholar] [CrossRef]
- Nerya, O.; Gizis, A.; Tsyilling, A.; Gemarasni, D.; Sharabi-Nov, A.; Ben-Arie, R. Controlled Atmosphere Storage of Pomegranate. Acta Hortic. 2006, 712, 655–660. [Google Scholar] [CrossRef]
- Low, P.S.; Merida, J.R. The oxidative burst in plant defense: Function and signal transduction. Physiol. Plant 1996, 96, 533–542. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Vranová, E.; Dat, J.F.; Inzé, D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001, 161, 405–414. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.; Pech, J.-C.; Koiwa, H. Signaling Molecules Involved in the Postharvest Stress Response of Plants. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2014; pp. 259–276. [Google Scholar] [CrossRef]
- Pareek, S. Postharvest Physiological Disorders in Fruit and Vegetables; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–14. [Google Scholar]
- Volz, R.K.; Alspach, P.A.; Fletcher, D.J.; Ferguson, I.B. Genetic variation in bitter pit and fruit calcium concentrations within a diverse apple germplasm collection. Euphytica 2006, 149, 1. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Amarante, C.V.T.d.; Labavitch, J.M.; Mitcham, E.J. Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol. Technol. 2010, 57, 6–13. [Google Scholar] [CrossRef]
- Juan Pablo, F.-T.; Javier, O.; Juan Antonio, M.; Antonio Luis, A.; Iban, E.; Pere, A.; Antonio José, M. Mapping Fruit Susceptibility to Postharvest Physiological Disorders and Decay Using a Collection of Near-isogenic Lines of Melon. J. Amer. Soc. Hort. Sci. 2007, 132, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.Y.; Shi, F.H.; Chang, N.J. Experiment of pomegranate storage. Deciduous Fruit Tree 1987, 3, 28–30. (In Chinese) [Google Scholar]
- Matityahu, I.; Marciano, P.; Holland, D.; Ben-Arie, R.; Amir, R. Differential effects of regular and controlled atmosphere storage on the quality of three cultivars of pomegranate (Punica granatum L.). Postharvest Biol. Technol. 2016, 115, 132–141. [Google Scholar] [CrossRef]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef]
- Shwartz, E.; Glazer, I.; Bar-Ya’akov, I.; Matityahu, I.; Bar-Ilan, I.; Holland, D.; Amir, R. Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chem. 2009, 115, 965–973. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Dang, Z.; Xu, J.; Ren, X. New insights on phenolic compound metabolism in pomegranate fruit during storage. Sci. Hortic. 2021, 285, 110138. [Google Scholar] [CrossRef]
- Deirdre, M.H.; Maria, I.G.; Adel, A.K. Effect of Carbon Dioxide on Anthocyanins, Phenylalanine Ammonia Lyase and Glucosyltransferase in the Arils of Stored Pomegranates. J. Am. Soc. Hortic. Sci. Jashs 1998, 123, 136–140. [Google Scholar] [CrossRef]
- Zauberman, G.; Ronen, R.; Akerman, M.; Weksler, A.; Rot, I.; Fuchs, Y. Post-harvest retention of the red colour of litchi fruit pericarp. Sci. Hortic. 1991, 47, 89–97. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Li, J. Browning control, shelf life extension and quality maintenance of frozen litchi fruit by hydrochloric acid. J. Food Eng. 2004, 63, 147–151. [Google Scholar] [CrossRef]
- Moire, L.; Schmutz, A.; Buchala, A.; Yan, B.; Stark, R.E.; Ryser, U. Glycerol Is a Suberin Monomer. New Experimental Evidence for an Old Hypothesis1. Plant Physiol. 1999, 119, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Sudhakar Rao, D.V.; Krishnamurthy, S. Effects of shrink film wrapping and storage temperature on the shelf life and quality of pomegranate fruits cv. Ganesh. Postharvest Biol. Technol. 2001, 22, 61–69. [Google Scholar] [CrossRef]
- Porat, R.; Kosto, I.; Daus, A. Bulk storage of “Wonderful” pomegranate fruit using modified atmosphere bags. Isr. J. Plant Sci. 2016, 63, 45. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. Changes in antioxidant activity and postharvest quality of sweet pomegranates cv. Hicrannar under modified atmosphere packaging. Postharvest Biol. Technol. 2014, 92, 29–36. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. Changes in phenolic compounds and antioxidant activity of sour–sweet pomegranates cv. ‘Hicaznar’ during long-term storage under modified atmosphere packaging. Postharvest Biol. Technol. 2015, 109, 30–39. [Google Scholar] [CrossRef]
- Candir, E.; Özdemir, A.E.; Aksoy, M.C. Effects of modified atmosphere packaging on the storage and shelf life of Hicaznarpomegranate fruits. Turk. J. Agric. For. 2019, 43, 241–253. [Google Scholar] [CrossRef]
- Ali, S.; Sattar Khan, A.; Ullah Malik, A.; Anjum, M.A.; Nawaz, A.; Shoaib Shah, H.M. Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shaheen, T.; Shahid, M. Pre-storage methionine treatment inhibits postharvest enzymatic browning of cold stored ‘Gola’ litchi fruit. Postharvest Biol. Technol. 2018, 140, 100–106. [Google Scholar] [CrossRef]
- Song, L.; Chen, H.; Gao, H.; Fang, X.; Mu, H.; Yuan, Y.; Yang, Q.; Jiang, Y. Combined modified atmosphere packaging and low temperature storage delay lignification and improve the defense response of minimally processed water bamboo shoot. Chem. Cent. J. 2013, 7, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.; Zhang, X.-L.; Luo, H.-H.; Zhou, J.-J.; Gong, Y.-H.; Li, W.-J.; Shi, Z.-W.; He, Q.; Wu, Q.; Li, L. An intracellular laccase is responsible for epicatechin-mediated anthocyanin degradation in litchi fruit pericarp. Plant Physiol. 2015, 169, 2391–2408. [Google Scholar] [CrossRef]
- D’Aquino, S.; Schirra, M.; Gentile, A.; Tribulato, E.; La Malfa, S.; Palma, A. Postharvest Lecithin Application Improves Storability of ‘Primosole’ Pomegranates. Acta Hortic. 2012, 934, 733–739. [Google Scholar] [CrossRef]
- Biswas, P.; East, A.R.; Hewett, E.W.; Heyes, J.A. Intermittent Warming in Alleviating Chilling Injury—A Potential Technique with Commercial Constraint. Food Bioprocess Technol. 2016, 9, 1–15. [Google Scholar] [CrossRef]
- Artés, F.; Tudela, J.A.; Villaescusa, R. Thermal postharvest treatments for improving pomegranate quality and shelf life. Postharvest Biol. Technol. 2000, 18, 245–251. [Google Scholar] [CrossRef]
- Taghipour, L.; Rahemi, M.; Assar, P.; Ramezanian, A.; Mirdehghan, S.H. Alleviating Chilling Injury in Stored Pomegranate Using a Single Intermittent Warming Cycle: Fatty Acid and Polyamine Modifications. Int. J. Food Sci. 2021, 2021, 2931353. [Google Scholar] [CrossRef]
- Artés, F.; Tudela, J.A.; Gil, M.I. Improving the keeping quality of pomegranate fruit by intermittent warming. Z. Lebensm.-Forsch. A 1998, 207, 316–321. [Google Scholar] [CrossRef]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Sharma, H.P.; Chaudhary, V.; Kumar, M. Importance of edible coating on fruits and vegetables: A review. J. Pharmacogn. Phytochem. 2019, 8, 4104–4110. [Google Scholar]
- Gennadios, A.; McHugh, T.H.; Krochta, J.M. Edible Coatings and Films. In Edible Coatings and Films to Improve Food Quality; Technomic Publ. Co.: Lancaster, PA, USA, 1994; p. 201. [Google Scholar]
- Cisneros-Zevallos, L.; Krochta, J.M. Internal Modified Atmospheres of Coated Fresh Fruits and Vegetables: Understanding Relative Humidity Effects. J. Food Sci. 2002, 67, 2792–2797. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Whey Protein Coatings for Fresh Fruits and Relative Humidity Effects. J. Food Sci. 2003, 68, 176–181. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of Coating Thickness on Viscosity of Coating Solution Applied to Fruits and Vegetables by Dipping Method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Khan, M.U.; Ehtasham Akram, M.; Pateiro, M.; Lorenzo, J.M.; Maan, A.A. Improvement of the Performance of Chitosan—Aloe vera Coatings by Adding Beeswax on Postharvest Quality of Mango Fruit. Foods 2021, 10, 2240. [Google Scholar] [CrossRef]
- Lu, J.; Li, T.; Ma, L.; Li, S.; Jiang, W.; Qin, W.; Li, S.; Li, Q.; Zhang, Z.; Wu, H. Optimization of heat-sealing properties for antimicrobial soybean protein isolate film incorporating diatomite/thymol complex and its application on blueberry packaging. Food Packag. Shelf Life 2021, 29, 100690. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Q.; Liang, X.; Fang, S. Fabrication of colloidal stable gliadin-casein nanoparticles for the encapsulation of natamycin: Molecular interactions and antifungal application on cherry tomato. Food Chem. 2022, 391, 133288. [Google Scholar] [CrossRef]
- Ozgen, M.; Palta, J.P. Use of Lysophosphatidylethanolamine (Lpe), A Natural Lipid, to Accelerate Ripening and Enhance Shelf Life of Cranberry Fruit. Acta Hortic. 2003, 628, 141–146. [Google Scholar] [CrossRef]
- Hoa, T.T.; Ducamp, M.-N. Effects of different coatings on biochemical changes of ‘cat Hoa loc’ mangoes in storage. Postharvest Biol. Technol. 2008, 48, 150–152. [Google Scholar] [CrossRef]
- Candir, E.; Ozdemir, A.E.; Aksoy, M.C. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Sci. Hortic. 2018, 235, 235–243. [Google Scholar] [CrossRef]
- Ghaouth, A.; Arul, J.; Ponnampalam, R.; Boulet, M. Chitosan Coating Effect on Storability and Quality of Fresh Strawberries. J. Food Sci. 1991, 56, 1618–1620. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn.) fruit. Postharvest Biol. Technol. 1997, 12, 195–202. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Blankenship, S. Development of apple superficial scald, soft scald, core flush, and greasiness is reduced by MCP. J. Agric. Food Chem. 1999, 47, 3063–3068. [Google Scholar] [CrossRef]
- Zohar, S.; Amnon, L.; Susan, L. Effect of Heat or 1-Methylcyclopropene on Antioxidative Enzyme Activities and Antioxidants in Apples in Relation to Superficial Scald Development. J. Am. Soc. Hortic. Sci. Jashs 2003, 128, 761–766. [Google Scholar] [CrossRef]
- Balogh, A.; Koncz, T.; Tisza, V.; Kiss, E.; Heszky, L. The Effect of 1-MCP on the Expression of Several Ripening-related Genes in Strawberries. HortScience 2005, 40, 2088–2090. [Google Scholar] [CrossRef]
- Cai, C.; Chen, K.; Xu, W.; Zhang, W.; Li, X.; Ferguson, I. Effect of 1-MCP on postharvest quality of Loquat fruit. Postharvest Biol. Technol. 2006, 40, 155–162. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; Zheng, Y. Effect of 1-methylcyclopene on senescence and quality maintenance of green bell pepper fruit during storage at 20 °C. Postharvest Biol. Technol. 2012, 70, 1–6. [Google Scholar] [CrossRef]
- Donald, J.H. Suppression of Ethylene Responses Through Application of 1-Methylcyclopropene: A Powerful Tool for Elucidating Ripening and Senescence Mechanisms in Climacteric and Nonclimacteric Fruits and Vegetables. HortScience Horts 2008, 43, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.H.; Zhang, Y.H.; Li, L.L.; Li, Y.X. Effects of 1-MCP on peel browning of pomegranates. Acta Hortic. 2008, 774, 275–282. [Google Scholar] [CrossRef]
- Gamrasni, D.; Gadban, H.; Tsvilling, A.; Goldberg, T.; Neria, O.; Ben-Arie, R.; Wolff, T.; Stern, Y. 1-Mcp Improves The Quality of Stored ‘Wonderful’ Pomegranates. Acta Hortic. 2015, 1079, 229–234. [Google Scholar] [CrossRef]
- Watkins, C.B.; Barden, C.L.; Bramlage, W.J. Relationships between Alpha-Farnesene, Ethylene Production and Superficial Scald Development of Apples. Acta Hortic. 1993, 343, 155–160. [Google Scholar] [CrossRef]
- Watkins, C.B.; Nock, J.F.; Whitaker, B.D. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol. Technol. 2000, 19, 17–32. [Google Scholar] [CrossRef]
- Zanella, A. Control of apple superficial scald and ripening—A comparison between 1-methylcyclopropene and diphenylamine postharvest treatments, initial low oxygen stress and ultra low oxygen storage. Postharvest Biol. Technol. 2003, 27, 69–78. [Google Scholar] [CrossRef]
- Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory Effects of Exogenously Applied Polyamines on Postharvest Physiology, Antioxidant System and Shelf Life of Fruits: A Review. Int. J. Mol. Sci. 2017, 18, 1789. [Google Scholar] [CrossRef] [Green Version]
- Fawole, O.A.; Atukuri, J.; Arendse, E.; Opara, U.O. Postharvest physiological responses of pomegranate fruit (cv. Wonderful) to exogenous putrescine treatment and effects on physico-chemical and phytochemical properties. Food Sci. Hum. Wellness 2020, 9, 146–161. [Google Scholar] [CrossRef]
- Gomes, M.H.; Vieira, T.; Fundo, J.F.; Almeida, D.P. Polyphenoloxidase activity and browning in fresh-cut ‘Rocha’pear as affected by pH, phenolic substrates, and antibrowning additives. Postharvest Biol. Technol. 2014, 91, 32–38. [Google Scholar] [CrossRef]
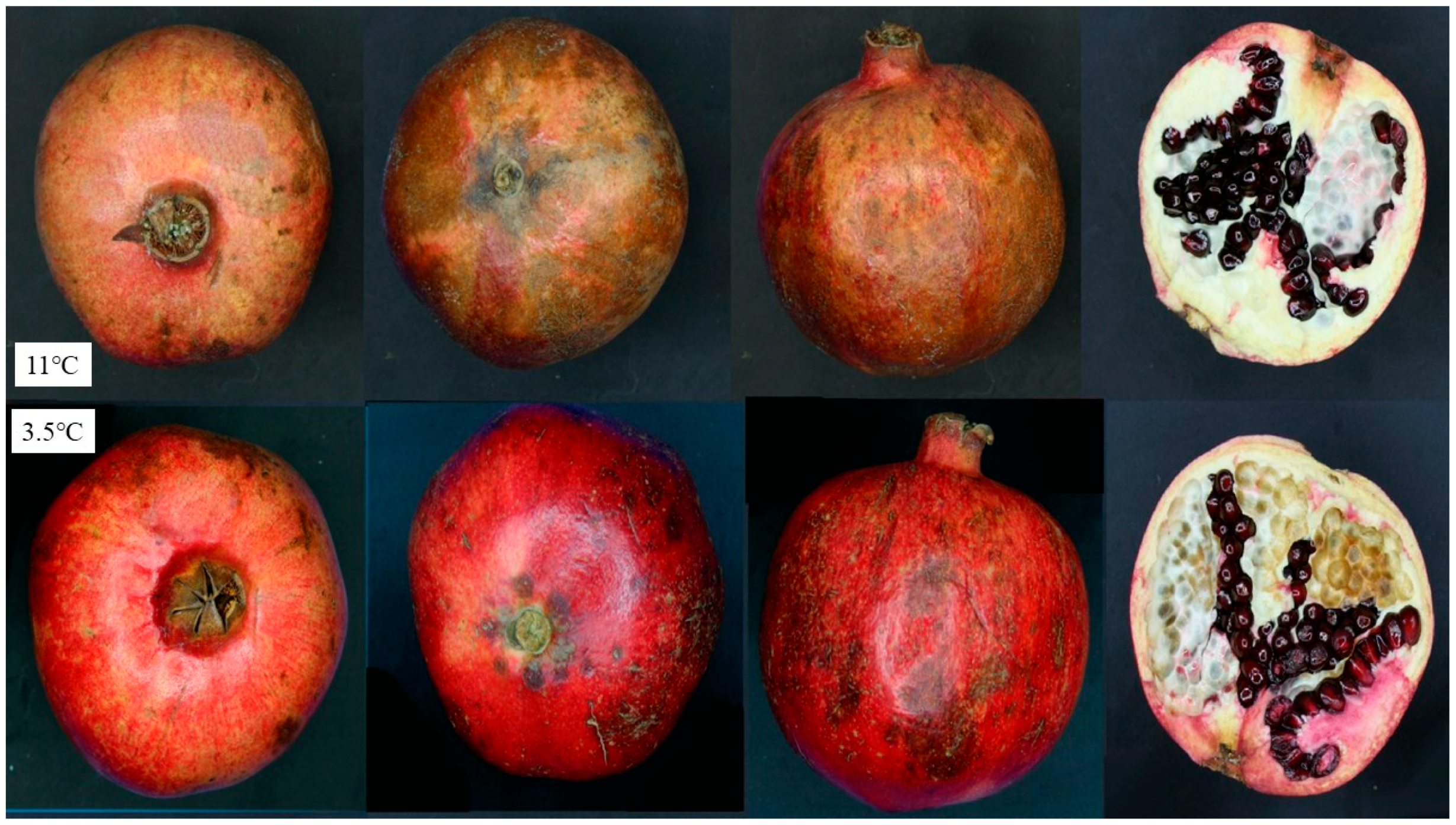
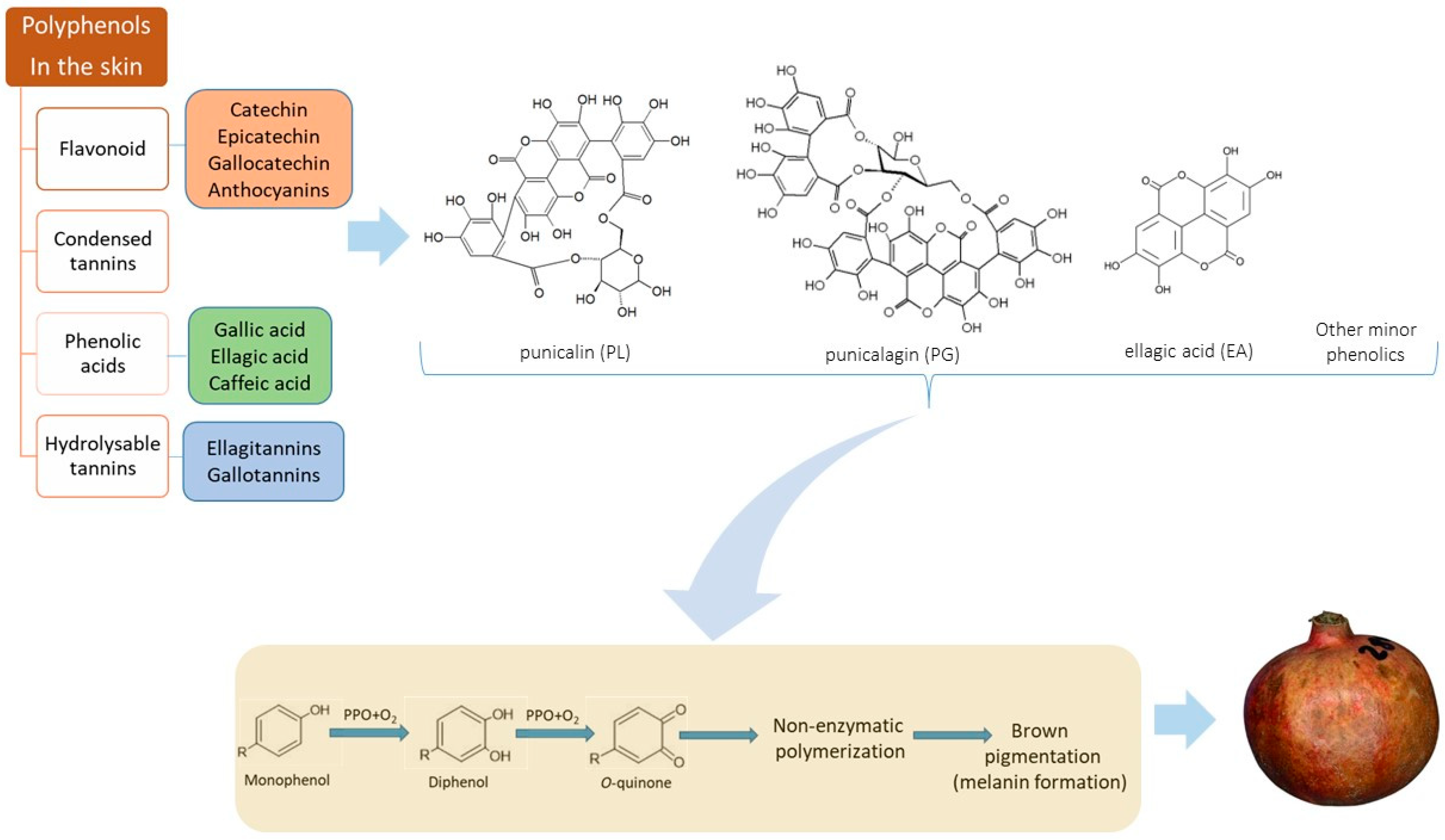
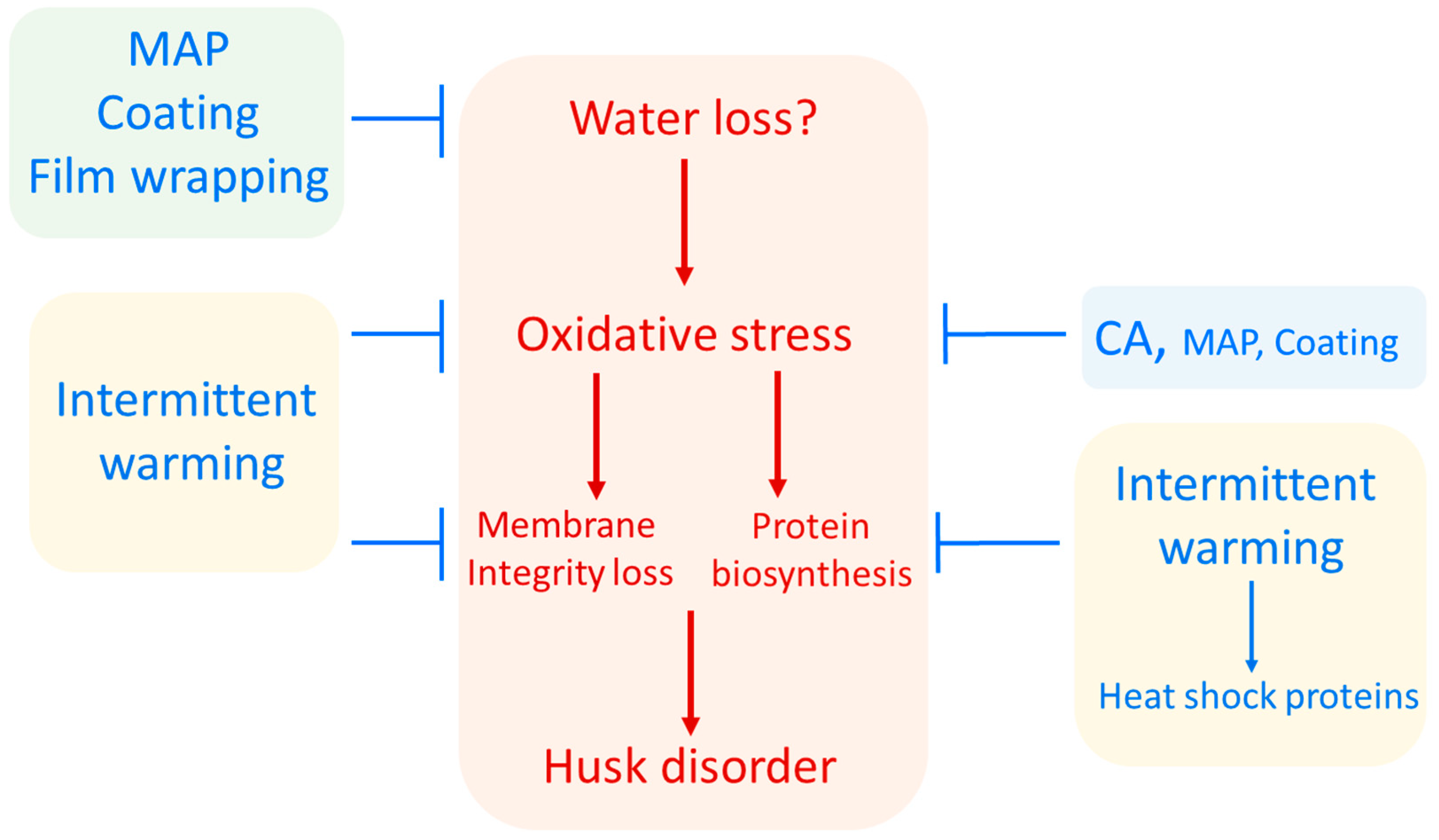
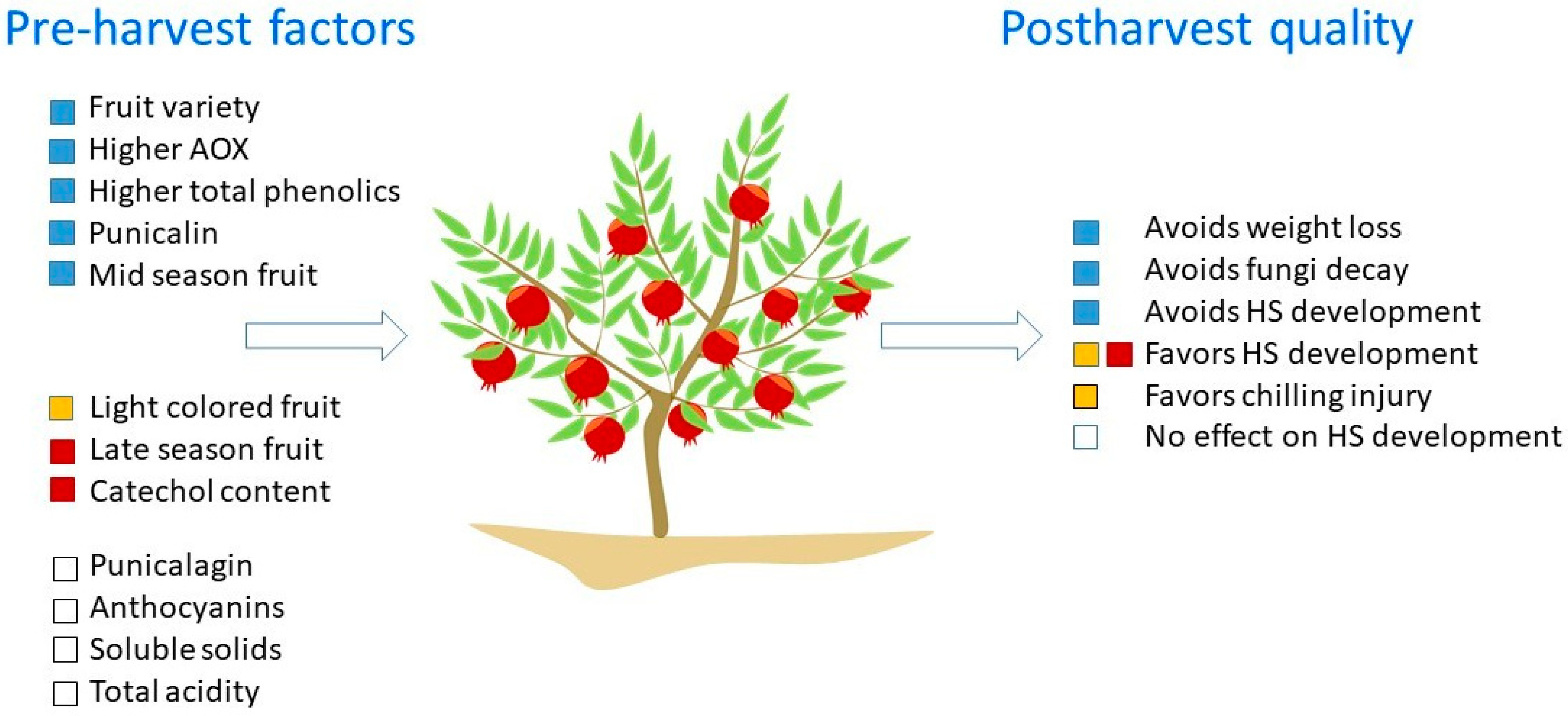
| Husk Scald (HS) | Chilling Injury (CI) |
|---|---|
| Long-term storage > 10–12 weeks | Short-term storage (starting from 6–8 weeks) |
| Storage between 6–10 °C or above | Storage < 5 °C |
| Loss of red pigment and brownish appearance on the stem end that spreads towards the blossom end and peel hardening | Dark brown region scatters in whole fruit skin, pitting |
| No injury to the arils or locular septa | Affects internal parts and arils |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maghoumi, M.; Amodio, M.L.; Fatchurrahman, D.; Cisneros-Zevallos, L.; Colelli, G. Pomegranate Husk Scald Browning during Storage: A Review on Factors Involved, Their Modes of Action, and Its Association to Postharvest Treatments. Foods 2022, 11, 3365. https://doi.org/10.3390/foods11213365
Maghoumi M, Amodio ML, Fatchurrahman D, Cisneros-Zevallos L, Colelli G. Pomegranate Husk Scald Browning during Storage: A Review on Factors Involved, Their Modes of Action, and Its Association to Postharvest Treatments. Foods. 2022; 11(21):3365. https://doi.org/10.3390/foods11213365
Chicago/Turabian StyleMaghoumi, Mahshad, Maria Luisa Amodio, Danial Fatchurrahman, Luis Cisneros-Zevallos, and Giancarlo Colelli. 2022. "Pomegranate Husk Scald Browning during Storage: A Review on Factors Involved, Their Modes of Action, and Its Association to Postharvest Treatments" Foods 11, no. 21: 3365. https://doi.org/10.3390/foods11213365





