Micro-Encapsulated Microalgae Oil Supplementation Has No Systematic Effect on the Odor of Vanilla Shake-Test of an Electronic Nose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Shake Powder
2.2. Determination of the Product’s Fatty Acid Profile
2.3. Omega-3 Fatty Acid Enrichment Protocol
2.4. Aroma Analysis with the Electronic Nose
2.5. Microbiological Testing
2.6. Statistical Evaluation
3. Results
3.1. Fatty Acid Profile of the Enriched Shake
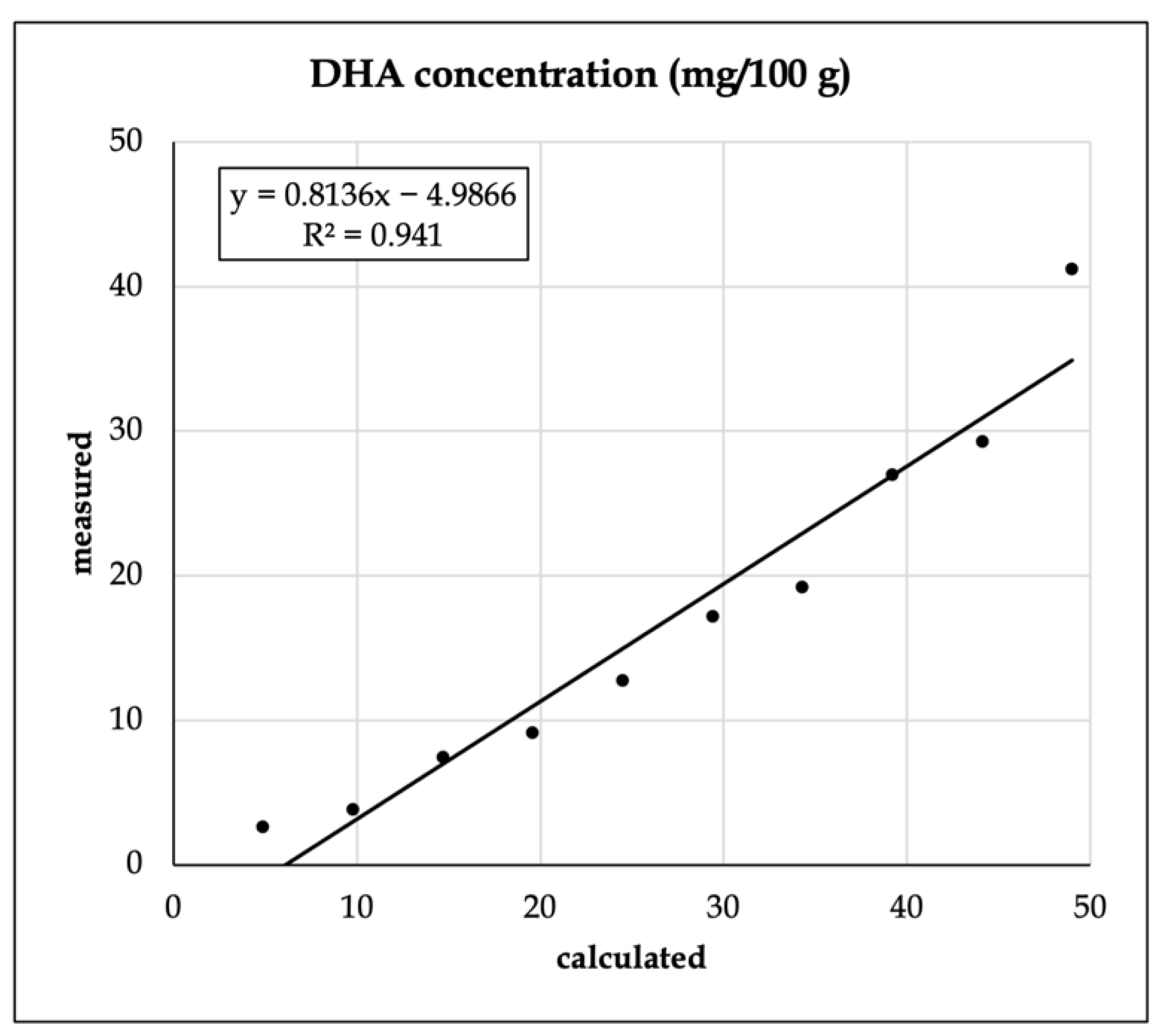
3.2. Fatty Acid Recovery from the Enriched Shake
3.3. Microbiology
3.4. Electronic Nose
4. Discussion
4.1. Original Fatty Acid Profile of the Shake Powder
4.2. Meeting the Dietetic Recommendations Regarding Omega-3 Fatty Acids for Humans
4.3. The Possibly Negative Side-Effects of Omega-3 Enrichment
4.4. E-Nose Odor Profiling
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Rahman, M.R.T.; van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Ozdal, T.; Yolci-Omeroglu, P.; Tamer, E.C. Role of Encapsulation in Functional Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Lakkis, J.M. Encapsulation and Controlled Release Technologies in Food Systems, 2nd ed.; Blackwell Publishing: Oxford, UK, 2016. [Google Scholar]
- García-Moreno, P.J.; Özdemir, N.; Stephansen, K.; Mateiu, R.V.; Echegoyen, Y.; Lagaron, J.M.; Chronakis, I.S.; Jacobsen, C. Development of carbohydrate-based nano-microstructures loaded with fish oil by using electrohydrodynamic processing. Food Hydrocoll. 2017, 69, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Bioactive Encapsulated Powders for Functional Foods—A Review of Methods and Current Limitations. Food Bioprocess Technol. 2015, 8, 1825–1837. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional spray-drying and future trends for the microencapsulation of fish oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Barrow, C.J.; Wang, B.; Adhikari, B.; Liu, H. Spray drying and encapsulation of omega-3 oils. In Food Enrichment with Omega-3 Fatty Acids; Jacobsen, C., Nielsen, N.S., Horn, A.F., Moltke Sørensen, A., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 194–225. [Google Scholar] [CrossRef]
- Anwar, S.H.; Weissbrodt, J.; Kunz, B. Microencapsulation of fish oil by spray granulation and fluid bed film coating. J. Food Sci. 2010, 75, E359–E371. [Google Scholar] [CrossRef]
- Busolo, M.A.; Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Electrospraying assisted by pressurized gas as an innovative high-throughput process for the microencapsulation and stabilization of docosahexaenoic acid-enriched fish oil in zein prolamine. Innov. Food Sci. Emerg. Technol. 2019, 51, 12–19. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Nano-and microdelivery systems for marine bioactive lipids. Mar. Drugs 2014, 12, 6014–6027. [Google Scholar] [CrossRef] [Green Version]
- Henry, C. Functional foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef] [Green Version]
- Lang, T. Functional foods. Br. Med. J. 2007, 334, 1015–1016. [Google Scholar] [CrossRef]
- Sidari, R.; Tofalo, R. A Comprehensive Overview on Microalgal-Fortified/Based Food and Beverages. Food Rev. Int. 2019, 35, 778–805. [Google Scholar] [CrossRef]
- Gallaher, J.J.; Hollender, R.; Peterson, D.G.; Roberts, R.F.; Coupland, J.N. Effect of composition and antioxidants on the oxidative stability of fluid milk supplemented with an algae oil emulsion. Int. Dairy J. 2005, 15, 333–341. [Google Scholar] [CrossRef]
- Chee, C.P.; Djordjevic, D.; Faraji, H.; Decker, E.A.; Hollender, R.; McClements, D.J.; Peterson, D.G.; Roberts, R.F.; Coupland, J.N. Sensory Properties of Vanilla and Strawberry Flavored Ice Cream Supplemented with Omega-3 Fatty Acids. Milchwissenschaft 2007, 62, 66–69. [Google Scholar]
- Valencia, I.; Ansorena, D.; Astiasarán, I. Development of dry fermented sausages rich in docosahexaenoic acid with oil from the microalgae Schizochytrium sp.: Influence on nutritional properties, sensorial quality and oxidation stability. Food Chem. 2007, 104, 1087–1096. [Google Scholar] [CrossRef]
- Chee, C.P.; Gallaher, J.J.; Djordjevic, D.; Faraji, H.; McClements, D.J.; Decker, E.A.; Hollender, R.; Peterson, D.G.; Roberts, R.F.; Coupland, J.N. Chemical and sensory analysis of strawberry flavoured yogurt supplemented with an algae oil emulsion. J. Dairy Res. 2005, 72, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Agustini, T.W.; Ma’ruf, W.F.; Widayat; Suzery, M.; Hadiyanto; Benjakul, S. Application of Spirulina platensis on ice cream and soft cheese with respect to their nutritional and sensory perspectives. J. Teknol. 2016, 2, 245–251. [Google Scholar] [CrossRef] [Green Version]
- de Medeiros, V.P.B.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Mohebi-Nejad, A.; Bikdeli, B. Omega-3 supplements and cardiovascular diseases. Tanaffos 2014, 13, 6–14. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Wang, J.L.; Dong, X.Y.; Wei, F.; Zhong, J.; Liu, B.; Yao, M.H.; Yang, M.; Zheng, C.; Quek, S.Y.; Chen, H. Preparation and characterization of novel lipid carriers containing microalgae oil for food applications. J. Food Sci. 2014, 79, E169-77. [Google Scholar] [CrossRef]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Mortazavian, A.M.; Rezaei, K.; Sohrabvandi, S. Application of advanced instrumental methods for yogurt analysis. Crit. Rev. Food Sci. Nutr. 2009, 49, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, H.G.; Kovacs, Z.; Toth, T.; Bazar, G. Trends in artificial aroma sensing by means of electronic nose technologies to advance dairy production—A review. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.L.; Boilot, P.; Gardner, J.W.; Gongora, M.A. Patter Analysis for Electronic Noses. In Handbook of Machine Olfaction; Pearce, T.C., Schiffman, S.S., Nagle, H.T., Gardner, J.W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Scott, S.M.; James, D.; Ali, Z. Data analysis for electronic nose systems. Microchim. Acta 2006, 156, 183–207. [Google Scholar] [CrossRef]
- Gardener, J.; Bartlett, P. Electronic Noses, Principles and Application; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Ampuero, S.; Bosset, J.O. The electronic nose applied to dairy products: A review. Sens. Actuators B Chem. 2003, 94, 1–12. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification and Structural Analysis of Lipids, 3rd ed.; Oily Press: Bridgwater, UK, 2003. [Google Scholar]
- Roszkos, R.; Bazar, G.; Tóth, T.; Kovacs, Z.; Febel, H.; Mezes, M. Effect of n-3 polyunsaturated fatty acid feeding on the fatty acid profile and odor of milk in danbred sows. J. Appl. Anim. Res. 2021, 49, 447–459. [Google Scholar] [CrossRef]
- MSZ ISO 21527-2; Microbiology of Food and Animal Fedding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 2: Colony Count Technique in Products with Water Activity Less than of Equal to 0.95. Hungarian Standards Institution: Budapest, Hungary, 2013.
- Kovacs, Z.; Bodor, Z.; Zinia Zaukuu, J.L.; Kaszab, T.; Bazar, G.; Tóth, T.; Mohácsi-Farkas, C. Electronic nose for monitoring odor changes of Lactobacillus species during milk fermentation and rapid selection of probiotic candidates. Foods 2020, 9, 1539. [Google Scholar] [CrossRef]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. Multivariate Calibration and Classification; NIR Publictions: Chichester, UK, 2002. [Google Scholar]
- Hewlings, S. Coconuts and Health: Different Chain Lengths of Saturated Fats Require Different Consideration. J. Cardiovasc. Dev. Dis. 2020, 7, 59. [Google Scholar] [CrossRef]
- Gunstone, F.D. Vegetable Oils in Food Technology Composition, Properties and Uses, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition: Rome, Italy, 2010; Volume 91. [Google Scholar]
- EFSA. European Food Standard Agency Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids. EFSA J. 2010, 8, 1–107. [Google Scholar]
- ISSFAL. Report of the Sub-Committee on Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults. 2014. Available online: https://www.issfal.org/assets/globalrecommendationssummary19nov2014landscape_-3-.pdf%0A%0A (accessed on 5 August 2022).
- Simopoulos, A.P.; Leaf, A.; Salem, N. Workshop Statement on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Summary of the NATO Advanced Research Workshop on Dietary w3 and w6 Fatty Acids: Biological Effects and Nutritional Essentiality. J. Nutr. 1989, 119, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Cetin, I.; Brenna, J.T. Perinatal Lipid Intake Working Group, Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Association of Perinatal Medicine; European Society for Clinical Nutrition Metabolism; European Society for Paediatric Gastroenterology. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Let, M.B.; Jacobsen, C.; Meyer, A.S. Lipid oxidation in milk, yoghurt, and salad dressing enriched with neat fish oil or pre-emulsified fish oil. J. Agric. Food Chem. 2007, 55, 7802–7809. [Google Scholar] [CrossRef]
- Lafarga, T.; Mayre, E.; Echeverria, G.; Viñas, I.; Villaró, S.; Acién-Fernández, F.G.; Castellari, M.; Aguiló-Aguayo, I. Potential of the microalgae Nannochloropsis and Tetraselmis for being used as innovative ingredients in baked goods. Lwt 2019, 115, 108439. [Google Scholar] [CrossRef]
- Holman, R.T. Autoxidation of fats and related substances. Prog. Chem. Fats Other Lipids 1954, 2, 51–98. [Google Scholar] [CrossRef]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of marine omega-3 supplements and human health. Biomed Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.; Costello, M.; Drake, M.A.; Bodyfelt, F. The Sensory Evaluation of Dairy Products, 2nd ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Murage, M.W.; Muge, E.K.; Mbatia, B.N.; Mwaniki, M.W. Sensory Evaluation of Omega-3-Rich Nile Perch Fish Oil-Fortified Yogurt. Int. J. Food Sci. 2021, 2021, 8838043. [Google Scholar] [CrossRef]
- Robertson, R.C.; Mateo, M.R.G.; O’Grady, M.N.; Guihéneuf, F.; Stengel, D.B.; Ross, R.P.; Fitzgerald, G.F.; Kerry, J.P.; Stanton, C. An assessment of the techno-functional and sensory properties of yoghurt fortified with a lipid extract from the microalga Pavlova lutheri. Innov. Food Sci. Emerg. Technol. 2016, 37, 237–246. [Google Scholar] [CrossRef]
- Jia, H.-X.; Chen, W.-L.; Qi, X.-Y.; Su, M.-Y. CyTA-Journal of Food The stability of milk-based infant formulas during accelerated storage the stability of milk-based infant formulas during accelerated storage. CyTA-J. Food 2019, 17, 96–104. [Google Scholar] [CrossRef]
- Pérez-Silva, A.; Odoux, E.; Brat, P.; Ribeyre, F.; Rodriguez-Jimenes, G.; Robles-Olvera, V.; García-Alvarado, M.A.; Günata, Z. GC-MS and GC-olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chem. 2006, 99, 728–735. [Google Scholar] [CrossRef]
- Galetto, W.G.; Hoffman, P.G. Some benzyl ethers present in the extract of vanilla (Vanilla planifolia). J. Agric. Food Chem. 1978, 26, 195–197. [Google Scholar] [CrossRef]
- Fenaroli, G.; Bellanca, N.; Furia, T.E. Fenaroli’s Handbook of Flavor Ingredients, 2nd ed.; CRC Press, Inc.: Cleveland, OH, USA, 1975. [Google Scholar]
- Furia, T. Handbook of Food Additives, 2nd ed.; N.W. 24th Street, CRC Press, Inc.: Boca Raton, FL, USA, 1980. [Google Scholar]
- Jimenez-Alvarez, D.; Giuffrida, F.; Golay, P.; Cotting, C.; Destaillats, F.; Dionisi, F.; Keely, B. Profiles of volatile compounds in milk containing fish oil analyzed by HS-SPME-GC/MS. Eur. J. Lipid Sci. Technol. 2008, 110, 277–283. [Google Scholar] [CrossRef]
- Chen, C.; Husny, J.; Rabe, S. Predicting fishiness off-flavour and identifying compounds of lipid oxidation in dairy powders by SPME-GC/MS and machine learning. Int. Dairy J. 2018, 77, 19–28. [Google Scholar] [CrossRef]
- Kolanowski, W.; Jaworska, D.; Weißbrodt, J. Importance of instrumental and sensory analysis in the assessment of oxidative deterioration of omega-3 long-chain polyunsaturated fatty acid-rich foods. J. Sci. Food Agric. 2007, 87, 181–191. [Google Scholar] [CrossRef]
- Let, M.B.; Jacobsen, C.; Meyer, A.S. Sensory stability and oxidation of fish oil enriched milk is affected by milk storage temperature and oil quality. Int. Dairy J. 2005, 15, 173–182. [Google Scholar] [CrossRef]








| Ingredients | % |
|---|---|
| milk whey concentrate | 69.2 |
| hydrolyzed collagen | 10 |
| vegetable fat (coconut fat) | 8 |
| branched chain amino acids | 4 |
| freeze dried gelatin | 4 |
| Na-carboxy-methyl-cellulose | 3 |
| vitamin premix | 1 |
| vanilla aroma | 0.3 |
| beta-carotene | 0.3 |
| Sucralose | 0.2 |
| Fatty Acid | mg FA/g Sample | Weight % of FA |
|---|---|---|
| C4:0 | 0.24 | 0.05 |
| C8:0 | 2.88 | 0.65 |
| C10:0 | 3.38 | 0.77 |
| C12:0 | 0.72 | 0.16 |
| C14:0 | 24.2 | 5.50 |
| C14:1n5 | 0.28 | 0.06 |
| C15:0 | 1.81 | 0.41 |
| C16:0 | 85.2 | 19.3 |
| C16:1n7 | 0.61 | 0.14 |
| C17:0 | 0.46 | 0.10 |
| C18:0 | 5.60 | 1.27 |
| C18:1n9c | 48.1 | 10.9 |
| C18:2n6c | 5.72 | 1.30 |
| C18:3n3 | 0.56 | 0.13 |
| C20:0 | 0.74 | 0.17 |
| C20:3n6 | 1.99 | 0.45 |
| C20:4n6 | 3.19 | 0.72 |
| C20:3n3 | 0.08 | 0.02 |
| C22:0 | 0.72 | 0.16 |
| C20:5n3 (EPA) | 7.37 | 1.67 |
| C24:0 | 0.68 | 0.15 |
| C22:6n3 (DHA) | 245.8 | 55.8 |
| Total FA mg/g sample | 440.3 | |
| n3 | 253.8 | 57.6 |
| n5 | 0.28 | 0.06 |
| n6 | 10.9 | 2.48 |
| n7 | 0.61 | 0.14 |
| n9 | 48.1 | 10.9 |
| n6/n3 | 0.04 | |
| saturated | 126.6 | 28.8 |
| monounsaturated | 49.0 | 11.1 |
| polyunsaturated | 264.7 | 60.1 |
| EPA + DHA mg in 100 g | 253.17 | 57.5 |
| Fatty Acid | mg FA/g Sample | Weight % of FA |
|---|---|---|
| C4:0 | 0.02 | 0.02 |
| C6:0 | 0.03 | 0.03 |
| C8:0 | 2.86 | 3.07 |
| C10:0 | 4.32 | 4.64 |
| C11:0 | 0.03 | 0.03 |
| C12:0 | 28.6 | 30.7 |
| C13:0 | 0.04 | 0.04 |
| C14:0 | 15.3 | 16.4 |
| C14:1n5 | 0.23 | 0.25 |
| C15:0 | 0.41 | 0.44 |
| C16:0 | 17.2 | 18.5 |
| C16:1n7 | 0.40 | 0.43 |
| C17:0 | 0.21 | 0.23 |
| C17:1n7 | 0.06 | 0.06 |
| C18:0 | 5.99 | 6.44 |
| C18:1n9t | 0.05 | 0.05 |
| C18:1n7t | 0.03 | 0.03 |
| C18:1n9c | 12.1 | 13.0 |
| C18:2n6t | 0.07 | 0.08 |
| C18:2n6c | 4.12 | 4.43 |
| CLA(9c,11t) | 0.18 | 0.19 |
| CLA(10t,12c) | 0.02 | 0.02 |
| C18:3n3 | 0.21 | 0.23 |
| C20:0 | 0.14 | 0.15 |
| C20:1n9 | 0.05 | 0.05 |
| C20:2n6 | 0.01 | 0.01 |
| C20:3n6 | 0.12 | 0.13 |
| C20:4n6 | 0.09 | 0.10 |
| C22:0 | 0.08 | 0.09 |
| C20:5n-3 (EPA) | 0.04 | 0.04 |
| C24:0 | 0.06 | 0.06 |
| Total FA mg/g sample | 93.1 | |
| n3 | 0.25 | 0.27 |
| n5 | 0.23 | 0.25 |
| n6 | 4.61 | 4.95 |
| n7 | 0.49 | 0.53 |
| n9 | 12.2 | 13.1 |
| n6/n3 | 18.4 | |
| saturated | 75.3 | 80.9 |
| monounsaturated | 12.9 | 13.9 |
| polyunsaturated | 4.86 | 5.20 |
| No. | Shake Powder | S17-P100 | Total Mass | Calc. DHA | Calc. EPA | Calc. EPA + DHA |
|---|---|---|---|---|---|---|
| mg in 10 g | ||||||
| 1 | 9980 | 20 | 10,000 | 4.9 | 1.5 | 6.4 |
| 2 | 9960 | 40 | 10,000 | 9.8 | 3.0 | 12.8 |
| 3 | 9940 | 60 | 10,000 | 14.7 | 4.4 | 19.1 |
| 4 | 9920 | 80 | 10,000 | 19.6 | 5.9 | 25.5 |
| 5 | 9900 | 100 | 10,000 | 24.5 | 7.4 | 31.9 |
| 6 | 9880 | 120 | 10,000 | 29.4 | 8.9 | 38.3 |
| 7 | 9860 | 140 | 10,000 | 34.3 | 10.4 | 44.7 |
| 8 | 9840 | 160 | 10,000 | 39.2 | 11.8 | 51.0 |
| 9 | 9820 | 180 | 10,000 | 44.1 | 13.3 | 57.4 |
| 10 | 9800 | 200 | 10,000 | 49.0 | 14.8 | 63.8 |
| Sample No. | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid | mg/g | % | mg/g | % | mg/g | % | mg/g | % | mg/g | % |
| C4:0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 |
| C6:0 | 0.00 | 0.12 | 0.14 | 0.08 | 0.10 | 0.12 | 0.13 | 0.27 | 0.29 | |
| C8:0 | 1.07 | 1.36 | 3.20 | 3.72 | 2.92 | 3.48 | 3.28 | 3.85 | 3.95 | 4.22 |
| C10:0 | 3.03 | 3.85 | 3.93 | 4.56 | 3.71 | 4.43 | 3.85 | 4.51 | 4.20 | 4.48 |
| C11:0 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 |
| C12:0 | 24.6 | 31.3 | 27.6 | 32.0 | 25.5 | 30.5 | 24.7 | 29.0 | 26.6 | 28.4 |
| C13:0 | 0.03 | 0.04 | 0.03 | 0.04 | 0.04 | 0.05 | 0.05 | 0.06 | 0.05 | 0.05 |
| C14:0 | 13.2 | 16.8 | 14.1 | 16.4 | 13.6 | 16.2 | 13.6 | 16.0 | 14.6 | 15.5 |
| C14:1n5 | 0.18 | 0.22 | 0.15 | 0.17 | 0.15 | 0.17 | 0.18 | 0.21 | 0.20 | 0.22 |
| C15:0 | 0.26 | 0.33 | 0.32 | 0.37 | 0.37 | 0.44 | 0.40 | 0.47 | 0.40 | 0.43 |
| C16:0 | 16.2 | 20.5 | 16.6 | 19.3 | 16.9 | 20.2 | 17.5 | 20.5 | 17.4 | 18.5 |
| C16:1n7 | 0.27 | 0.35 | 0.33 | 0.38 | 0.33 | 0.39 | 0.30 | 0.35 | 0.43 | 0.46 |
| C17:0 | 0.14 | 0.17 | 0.17 | 0.20 | 0.19 | 0.23 | 0.21 | 0.24 | 0.21 | 0.22 |
| C17:1n7 | 0.05 | 0.06 | 0.04 | 0.05 | 0.04 | 0.05 | 0.05 | 0.06 | 0.06 | 0.07 |
| C18:0 | 5.28 | 6.71 | 5.30 | 6.15 | 5.65 | 6.74 | 5.66 | 6.64 | 5.94 | 6.34 |
| C18:1n9t | 0.02 | 0.02 | 0.05 | 0.05 | 0.04 | 0.05 | 0.04 | 0.04 | 0.03 | 0.04 |
| C18:1n7t | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 |
| C18:1n9c | 10.1 | 12.8 | 9.82 | 11.4 | 9.41 | 11.2 | 10.0 | 11.8 | 12.9 | 13.7 |
| C18:2n6t | 0.04 | 0.06 | 0.06 | 0.07 | 0.05 | 0.06 | 0.05 | 0.06 | 0.09 | 0.09 |
| C18:2n6c | 3.24 | 4.11 | 3.23 | 3.75 | 3.14 | 3.74 | 3.40 | 3.99 | 4.03 | 4.31 |
| CLA(9c, 11t) | 0.12 | 0.15 | 0.13 | 0.15 | 0.15 | 0.18 | 0.13 | 0.15 | 0.15 | 0.16 |
| CLA(10t, 12c) | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| C18:3n3 | 0.14 | 0.18 | 0.15 | 0.17 | 0.15 | 0.18 | 0.18 | 0.21 | 0.20 | 0.21 |
| C20:0 | 0.09 | 0.11 | 0.11 | 0.13 | 0.11 | 0.13 | 0.11 | 0.13 | 0.13 | 0.13 |
| C20:1n9 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 |
| C20:2n6 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 |
| C20:3n6 | 0.08 | 0.10 | 0.07 | 0.08 | 0.09 | 0.10 | 0.10 | 0.12 | 0.10 | 0.11 |
| C20:4n6 | 0.06 | 0.08 | 0.08 | 0.09 | 0.07 | 0.08 | 0.08 | 0.09 | 0.10 | 0.10 |
| C22:0 | 0.05 | 0.07 | 0.07 | 0.08 | 0.07 | 0.09 | 0.08 | 0.09 | 0.08 | 0.09 |
| C20:5n3 (EPA) | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.04 | 0.05 | 0.05 | 0.06 | 0.06 |
| C24:0 | 0.02 | 0.03 | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| C22:5n3 | 0.07 | 0.08 | 0.08 | 0.09 | 0.07 | 0.08 | 0.09 | 0.10 | 0.15 | 0.16 |
| C22:6n3 (DHA) | 0.26 | 0.33 | 0.28 | 0.32 | 0.74 | 0.88 | 0.91 | 1.07 | 1.27 | 1.36 |
| Total FA mg/g sample | 78.7 | 86.1 | 83.8 | 85.3 | 93.7 | |||||
| n3 | 0.48 | 0.61 | 0.52 | 0.61 | 1.00 | 1.19 | 1.23 | 1.44 | 1.68 | 1.79 |
| n5 | 0.18 | 0.22 | 0.15 | 0.17 | 0.15 | 0.17 | 0.18 | 0.21 | 0.20 | 0.22 |
| n6 | 3.56 | 4.53 | 3.58 | 4.16 | 3.51 | 4.19 | 3.78 | 4.43 | 4.50 | 4.80 |
| n7 | 0.33 | 0.42 | 0.38 | 0.44 | 0.39 | 0.47 | 0.36 | 0.42 | 0.51 | 0.54 |
| n9 | 10.1 | 12.9 | 9.9 | 11.5 | 9.5 | 11.3 | 10.1 | 11.9 | 12.9 | 13.8 |
| n6/n3 | 7.44 | 6.84 | 3.52 | 3.08 | 2.68 | |||||
| saturated | 64.0 | 81.4 | 71.6 | 83.1 | 69.3 | 82.6 | 69.7 | 81.6 | 73.9 | 78.8 |
| monounsaturated | 10.6 | 13.5 | 10.4 | 12.1 | 10.0 | 12.0 | 10.7 | 12.5 | 13.7 | 14.6 |
| polyunsaturated | 4.04 | 5.13 | 4.10 | 4.76 | 4.51 | 5.38 | 5.00 | 5.86 | 6.18 | 6.59 |
| EPA + DHA mg in 100 g | 27.0 | 0.34 | 29.5 | 0.34 | 77.4 | 0.92 | 95.8 | 1.12 | 132.8 | 1.42 |
| Sample no. | 6 | 7 | 8 | 9 | 10 | |||||
| Fatty acid | mg/g | % | mg/g | % | mg/g | % | mg/g | % | mg/g | % |
| C4:0 | 0.04 | 0.05 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.04 | 0.05 | 0.05 |
| C6:0 | 0.40 | 0.44 | 0.04 | 0.05 | 0.07 | 0.08 | 0.37 | 0.37 | 0.40 | 0.41 |
| C8:0 | 4.08 | 4.40 | 2.77 | 3.10 | 2.57 | 2.98 | 4.18 | 4.27 | 3.98 | 4.13 |
| C10:0 | 4.13 | 4.44 | 3.82 | 4.28 | 3.43 | 3.99 | 4.26 | 4.35 | 4.06 | 4.21 |
| C11:0 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| C12:0 | 25.3 | 27.2 | 25.1 | 28.0 | 21.8 | 25.4 | 26.4 | 27.0 | 25.2 | 26.2 |
| C13:0 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.05 |
| C14:0 | 13.9 | 15.0 | 14.0 | 15.7 | 12.5 | 14.5 | 14.7 | 15.0 | 13.9 | 14.4 |
| C14:1n5 | 0.22 | 0.23 | 0.21 | 0.23 | 0.27 | 0.31 | 0.25 | 0.26 | 0.22 | 0.23 |
| C15:0 | 0.41 | 0.45 | 0.42 | 0.47 | 0.43 | 0.50 | 0.43 | 0.44 | 0.44 | 0.46 |
| C16:0 | 17.3 | 18.6 | 17.6 | 19.7 | 17.1 | 19.8 | 17.8 | 18.1 | 17.8 | 18.5 |
| C16:1n7 | 0.46 | 0.50 | 0.40 | 0.45 | 0.49 | 0.57 | 0.50 | 0.51 | 0.52 | 0.54 |
| C17:0 | 0.22 | 0.24 | 0.21 | 0.24 | 0.22 | 0.25 | 0.22 | 0.22 | 0.20 | 0.20 |
| C17:1n7 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.07 | 0.08 |
| C18:0 | 5.85 | 6.30 | 6.02 | 6.74 | 5.68 | 6.60 | 5.69 | 5.81 | 5.57 | 5.78 |
| C18:1n9t | 0.05 | 0.05 | 0.04 | 0.05 | 0.03 | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 |
| C18:1n7t | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | 0.02 |
| C18:1n9c | 13.3 | 14.3 | 11.6 | 13.0 | 13.2 | 15.4 | 14.1 | 14.4 | 13.9 | 14.4 |
| C18:2n6t | 0.09 | 0.10 | 0.08 | 0.09 | 0.09 | 0.10 | 0.07 | 0.08 | 0.08 | 0.08 |
| C18:2n6c | 4.14 | 4.46 | 3.77 | 4.22 | 3.94 | 4.57 | 4.34 | 4.43 | 4.08 | 4.23 |
| CLA(9c, 11t) | 0.17 | 0.19 | 0.16 | 0.18 | 0.20 | 0.23 | 0.19 | 0.20 | 0.24 | 0.24 |
| CLA(10t, 12c) | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| C18:3n3 | 0.21 | 0.22 | 0.19 | 0.21 | 0.24 | 0.27 | 0.24 | 0.24 | 0.24 | 0.25 |
| C20:0 | 0.14 | 0.15 | 0.13 | 0.15 | 0.13 | 0.15 | 0.12 | 0.12 | 0.13 | 0.14 |
| C20:1n9 | 0.05 | 0.05 | 0.04 | 0.05 | 0.05 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| C20:2n6 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 |
| C20:3n6 | 0.14 | 0.15 | 0.13 | 0.15 | 0.14 | 0.17 | 0.16 | 0.16 | 0.16 | 0.17 |
| C20:4n6 | 0.11 | 0.11 | 0.11 | 0.13 | 0.13 | 0.15 | 0.14 | 0.14 | 0.16 | 0.16 |
| C22:0 | 0.10 | 0.11 | 0.09 | 0.10 | 0.08 | 0.10 | 0.09 | 0.09 | 0.10 | 0.10 |
| C20:5n3 (EPA) | 0.07 | 0.08 | 0.08 | 0.08 | 0.12 | 0.14 | 0.13 | 0.13 | 0.16 | 0.16 |
| C24:0 | 0.05 | 0.06 | 0.05 | 0.06 | 0.06 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| C22:5n3 | 0.16 | 0.17 | 0.14 | 0.16 | 0.18 | 0.21 | 0.21 | 0.22 | 0.24 | 0.24 |
| C22:6n3 (DHA) | 1.72 | 1.85 | 1.92 | 2.14 | 2.70 | 3.13 | 2.92 | 2.99 | 4.12 | 4.28 |
| Total FA mg/g sample | 92.9 | 89.3 | 86.1 | 97.9 | 96.4 | |||||
| n3 | 2.16 | 2.32 | 2.32 | 2.60 | 3.23 | 3.75 | 3.50 | 3.57 | 4.76 | 4.93 |
| n5 | 0.22 | 0.23 | 0.21 | 0.23 | 0.27 | 0.31 | 0.25 | 0.26 | 0.22 | 0.23 |
| n6 | 4.67 | 5.02 | 4.29 | 4.80 | 4.52 | 5.25 | 4.93 | 5.04 | 4.74 | 4.92 |
| n7 | 0.55 | 0.60 | 0.48 | 0.54 | 0.58 | 0.68 | 0.60 | 0.61 | 0.60 | 0.63 |
| n9 | 13.4 | 14.4 | 11.7 | 13.1 | 13.3 | 15.5 | 14.2 | 14.5 | 14.0 | 14.5 |
| n6/n3 | 2.16 | 1.85 | 1.40 | 1.41 | 1.00 | |||||
| saturated | 71.9 | 77.4 | 70.4 | 78.8 | 64.2 | 74.5 | 74.4 | 76.0 | 72.1 | 74.8 |
| monounsaturated | 14.2 | 15.3 | 12.4 | 13.8 | 14.2 | 16.5 | 15.1 | 15.4 | 14.8 | 15.4 |
| polyunsaturated | 6.82 | 7.34 | 6.61 | 7.40 | 7.75 | 9.01 | 8.43 | 8.61 | 9.49 | 9.85 |
| EPA + DHA mg in 100 g | 179.1 | 1.93 | 199.2 | 2.23 | 281.3 | 3.27 | 305.0 | 3.12 | 428.0 | 4.44 |
| Retention Index | Compound 1 * | Compound 2 * | Compound 3 * |
|---|---|---|---|
| 547-1-A | tert-buthylmethylether | 1-propanol | 2-propanol |
| 612-1-A | ethylacetate | acetic acid | – |
| 756-1-A | ethyl isobutyrate | Pyrrole | – |
| 802-1-A | ethyl butyrate | propyl propanoate | – |
| 996-1-A | ethyl hexanoate | butyl butanoate | – |
| 1017-1-A | alpha-terpinene | 1,4-cinelole | acetylpyrazine |
| 1086-1-A | pentyl butanoate | benzyl butanoate | – |
| 778-2-A | pentanal | propyl acetate | pentan-2-one |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakubu, H.G.; Ali, O.; Ilyés, I.; Vigyázó, D.; Bóta, B.; Bazar, G.; Tóth, T.; Szabó, A. Micro-Encapsulated Microalgae Oil Supplementation Has No Systematic Effect on the Odor of Vanilla Shake-Test of an Electronic Nose. Foods 2022, 11, 3452. https://doi.org/10.3390/foods11213452
Yakubu HG, Ali O, Ilyés I, Vigyázó D, Bóta B, Bazar G, Tóth T, Szabó A. Micro-Encapsulated Microalgae Oil Supplementation Has No Systematic Effect on the Odor of Vanilla Shake-Test of an Electronic Nose. Foods. 2022; 11(21):3452. https://doi.org/10.3390/foods11213452
Chicago/Turabian StyleYakubu, Haruna Gado, Omeralfaroug Ali, Imre Ilyés, Dorottya Vigyázó, Brigitta Bóta, George Bazar, Tamás Tóth, and András Szabó. 2022. "Micro-Encapsulated Microalgae Oil Supplementation Has No Systematic Effect on the Odor of Vanilla Shake-Test of an Electronic Nose" Foods 11, no. 21: 3452. https://doi.org/10.3390/foods11213452
APA StyleYakubu, H. G., Ali, O., Ilyés, I., Vigyázó, D., Bóta, B., Bazar, G., Tóth, T., & Szabó, A. (2022). Micro-Encapsulated Microalgae Oil Supplementation Has No Systematic Effect on the Odor of Vanilla Shake-Test of an Electronic Nose. Foods, 11(21), 3452. https://doi.org/10.3390/foods11213452








