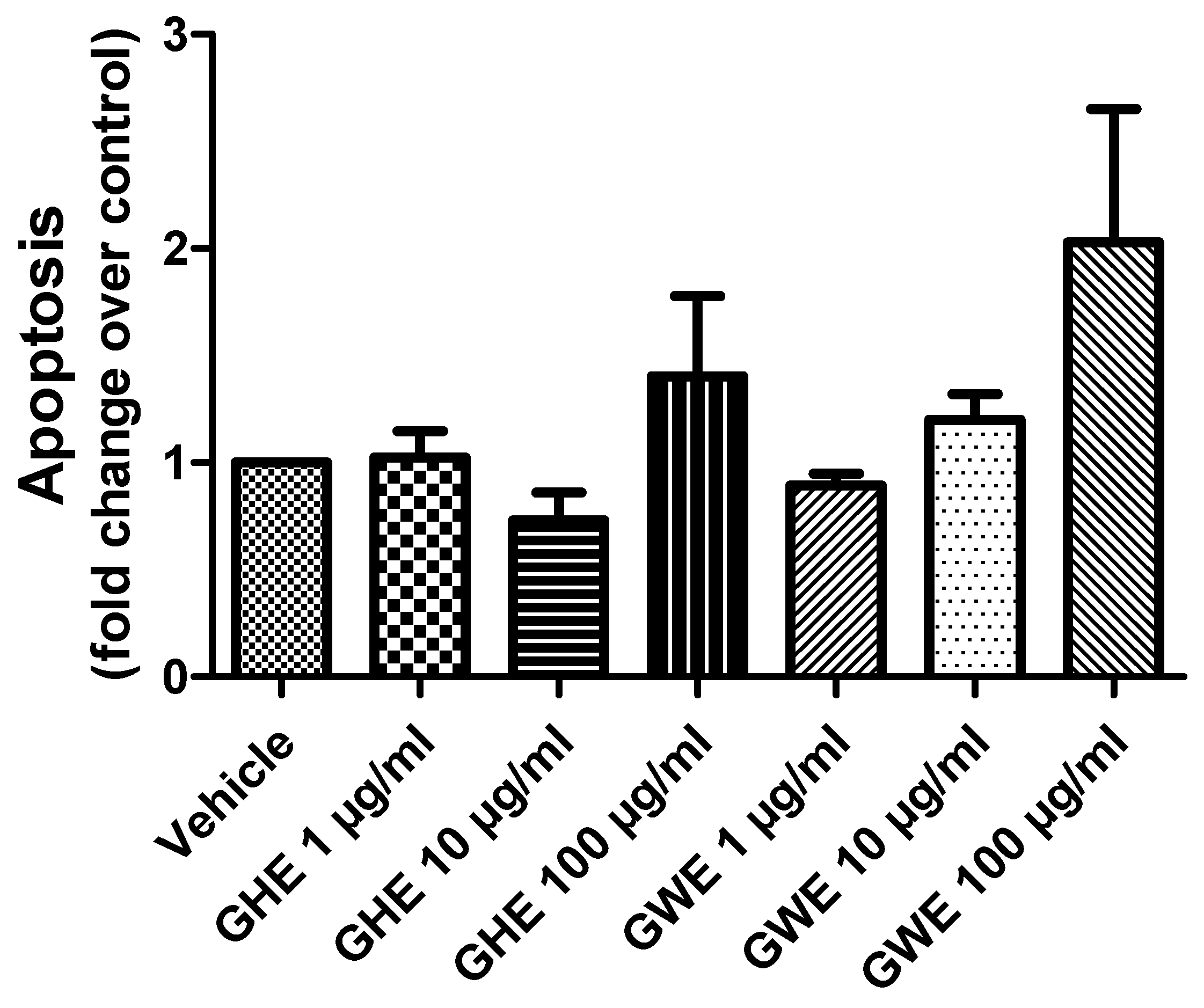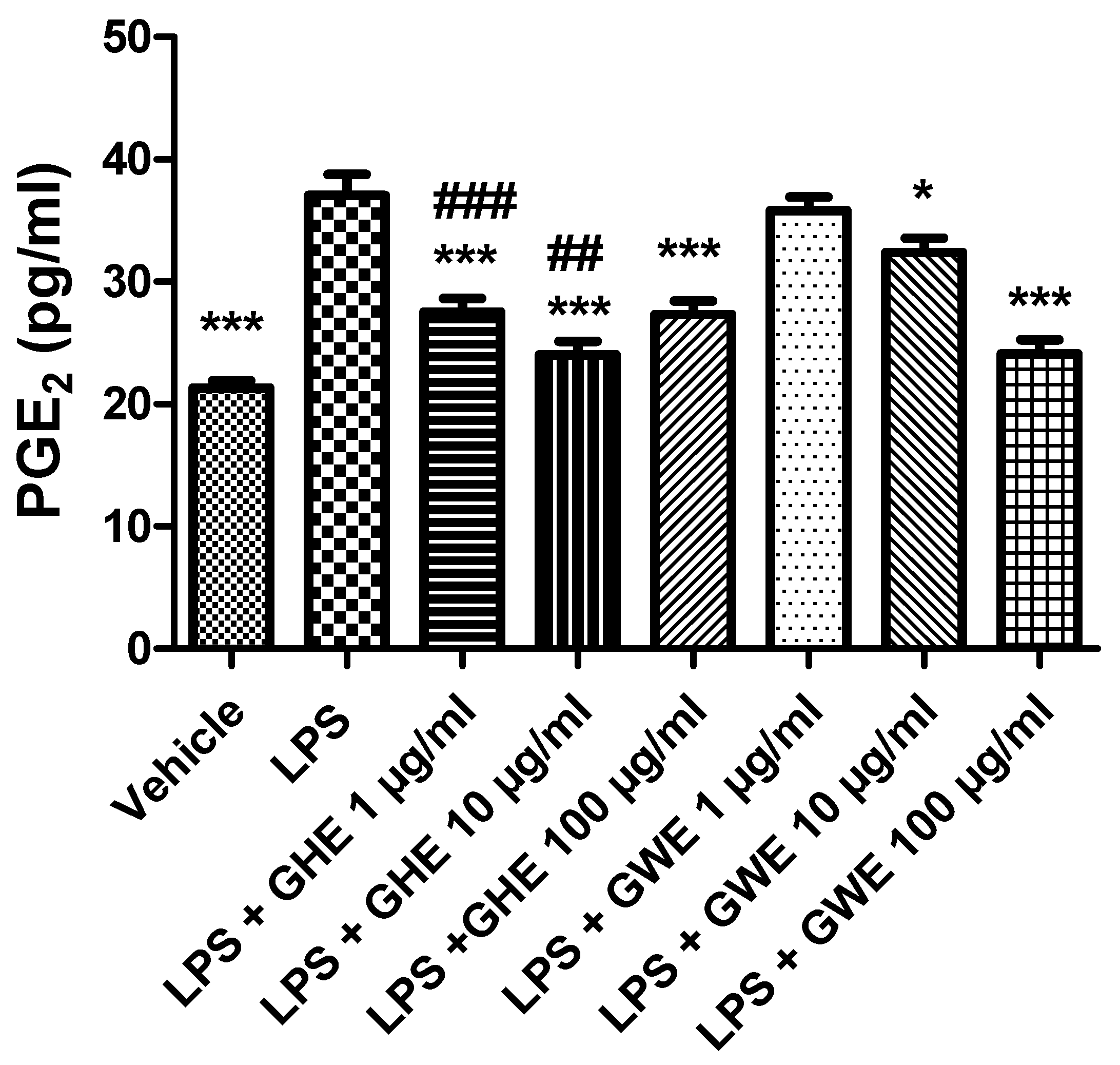Abstract
Inflammatory bowel diseases (IBDs) are chronic and multifactorial inflammatory conditions of the colonic mucosa (ulcerative colitis), characterized by increased and unbalanced immune response to external stimuli. Garlic and its bioactive constituents were reported to exert various biological effects, including anti-inflammatory, antioxidant and immunomodulatory activities. We aimed to evaluate the protective effects of a hydroalcoholic (GHE) and a water (GWE) extract from a Sicilian variety of garlic, known as Nubia red garlic, on an ex vivo experimental model of ulcerative colitis, involving isolated LPS-treated mouse colon specimens. Both extracts were able to counteract LPS-induced cyclooxygenase (COX)-2, tumor necrosis factor (TNF)-α, nuclear factor-kB (NF-kB), and interleukin (IL)-6 gene expression in mouse colon. Moreover, the same extracts inhibited prostaglandin (PG)E2, 8-iso-PGF2α, and increased the 5-hydroxyindoleacetic acid/serotonin ratio following treatment with LPS. In particular, GHE showed a better anti-inflammatory profile. The anti-inflammatory and antioxidant effects induced by both extracts could be related, at least partially, to their polyphenolic composition, with particular regards to catechin. Concluding, our results showed that GHE and GWE exhibited protective effects in colon, thus suggesting their potential use in the prevention and management of ulcerative colitis.
1. Introduction
Garlic (Allium sativum L.) is an herbaceous plant, belonging to the Amarillidaceae family, that is used all over the world as traditional medicine and spice [1]. Garlic contains a number of biologically active compounds, including phenolic compounds [2], saponins [3], polysaccharides [4], as well as organosulfur compounds [1] that contribute to its countless pharmacological properties. In particular, the health-promoting properties induced by garlic were suggested to be related to its bioactive compounds, including phenolic compounds, whose content in this plant is relatively high [5]. However, the content of phenolic compounds in garlic was reported to be dependent on environmental, genetic and agronomic factors [6]. Preclinical and clinical studies demonstrated the anti-inflammatory, antioxidant, antiatherosclerotic, anticancer, immunomodulatory, antidiabetic, antiobesity, neuroprotective, as well as digestive system protective activities of both garlic and its phenolic constituents [1]. Interestingly, raw garlic showed higher antioxidant activity compared to cooked garlic [7], suggesting that the antioxidant property of garlic could be modified by the processing methods. Moreover, in vitro and in vivo studies showed that garlic could suppress inflammation mainly through inhibition of various inflammatory biomarkers, including nitric oxide (NO), tumor necrosis factor (TNF)-α, and interleukin (IL)-1 [1].
The beneficial effects induced by garlic were also be related to its organosulfur compounds, including diallyl thiosulfonate (allicin), diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), E/Z-ajoene, S-allyl-cysteine (SAC), as well as S-allyl-cysteine sulfoxide (alliin) [1]. In particular, allicin was reported to be among the most important bioactive constituents exerting antioxidant effects [8].
Inflammatory bowel diseases (IBDs) are chronic and multifactorial inflammatory conditions of the colonic mucosa (ulcerative colitis), characterized by increased and unbalanced immune response to external stimuli [9,10,11]. In this context, antioxidant/anti-inflammatory herbal extracts were found to contrast IBD-related symptoms [12,13] by reducing various pro-inflammatory and oxidative biomarkers, such as reactive oxygen/nitrogen (ROS/RNS) species, prostaglandins, and cytokines.
Interestingly, garlic was suggested to exert protective effects against ulcerative colitis [14]. In addition, garlic oil suppressed endotoxin-induced neutrophil infiltration in small intestine of rats [15]. Moreover, Balaha and collaborators (2016) [16] showed that garlic oil (GO) was able to inhibit ulcerative colitis induced by dextran sulfate sodium in rats. This effect was suggested to be related to antioxidant, anti-inflammatory as well as to immunomodulatory effects of GO. Accordingly, more recently, GO was reported to decrease inflammation and cellular damage in a rat model of colitis induced by acetic acid [17].
On the basis of these findings, in this study, we aimed to evaluate the potential protective effects of a hydroalcoholic extract (GHE) and a water extract (GWE) of garlic on isolated mouse colon specimens treated ex vivo with E. coli lipopolysaccharide (LPS), which represents an experimental model of ulcerative colitis [18]. In particular, our research focused on hydroalcoholic and water extracts from a Sicilian variety of garlic (Nubia red garlic), known for the intense red color of the robes of its bulbils [19]. This variant is a protected denomination of origin product (DOP), which is appreciated all over the world. We reported here in, to the best of our knowledge, the first potential beneficial effects of Nubia red garlic on colon inflammation.
2. Materials and Methods
2.1. Preparation of Garlic Extracts
Garlic cloves were supplied as dried powder by il Grappolo S.r.l. (Soliera, Modena, Italy). Plant sample (1 g) was mixed with either a solution of ethanol–water (20:80, v/v) or water (final concentration = 1 g/mL), as previously reported [20,21,22]. The supernatant was filtered and then dried (freeze-drying). The dry residue, a yellow sugary solid, was stored at 4 °C, until chemical analyses were performed.
2.2. High Performance Liquid Chromatography (HPLC)–Diode Array (DAD)–Mass Spectrometry (MS) Analysis
Selected phenolic compounds contained in the extracts were identified and quantified by HPLC–DAD–MS analysis. The HPLC apparatus consisted of a two PU-2080 PLUS chromatographic pump, a DG-2080-54 line degasser, a mix-2080-32 mixer, UV, diode array (DAD) and detectors, a mass spectrometer (MS) detector (expression compact mass spectrometer (CMS), Advion, Ithaca, NY, USA), an AS-2057 PLUS autosampler, and a CO-2060 PLUS column thermostat (all from Jasco, Tokyo, Japan). Integration was conducted through ChromNAV2 Chromatography software. The separation was performed on an Infinity lab Poroshell 120-SB reverse phase column (C18, 150 × 4.6 mm i.d., 2.7 µm) (Agilent, Santa Clara, CA, USA). Column temperature was set at 30 °C. The separation was conducted within 60 min of the chromatographic run, starting from the following separation conditions: 97% water with 0.1% formic acid, 3% methanol with 0.1% formic acid, as previously described [23]. Quantitative determination of phenolic compounds was conducted through a DAD detector. Qualitative analysis of GHE and GWE was performed by an MS detector in the positive and negative ion modes. MS signal identification was performed by comparison with a standard solution and MS spectra available in the MassBank Europe database (https://massbank.eu/MassBank/ (accessed on 11 November 2021). All HPLC grade solvents were purchased from Merck Science Life S.r.l. (Milan, Italy). Each analysis was performed in triplicate. The detailed protocol is enclosed as supplementary materials.
2.3. Headspace Solid-Phase Microextraction-Gas Chromatography–Mass Spectrometry (HS–SPME–GC–MS) Analysis
The 4 mL vials were loaded with 50 mg of the garlic dry residue. According to the extraction parameters optimized in our previous screening study performed on the garlic powder [15], the sample was allowed to equilibrate at 80 °C for 20 min and then the SPME fiber DVB-CAR-PDMS (Sigma Aldrich, now Merck KGaA, Darmstadt, Germany) was exposed to the head space of the vial for 20 min at 80 °C.
After sampling, fiber was withdrawn in the needle and exposed into the GC inlet at 260 °C for 0.5 min. The desorbed analytes were introduced in a gas chromatograph (6850, Agilent Technologies, Santa Clara, CA, USA) coupled with a mass spectrometer (5975, Agilent Technologies, Santa Clara, CA, USA), equipped with the non-polar capillary column HP-5MS (30 m × 0.25 mm inner diameter, and film thickness 0.25 µm). The gas-chromatographic parameters were set as follows: inlet temperature, 260 °C; injection mode, splitless (splitter valve was opened after 0.2 min and split ratio = 20/1); flow rate of the helium carrier gas (99.995% purity), 1.0 mL/min; oven temperature starting from 40 °C, after 5 min raised to 200 °C at 5 °C/min, and kept at this final temperature for 60 min. Mass spectrometry parameters were set as follows: EI energy, 70 eV; source temperature, 230 °C; quadrupole temperature, 150 °C; and mass scan was carried out over the 50–350 m/z range. The analyses were performed in triplicate.
Two analytical criteria were used to allow the identification of the eluted compounds, namely the comparison between the EI experimental spectra and those collected in the commercial (FFNSC 3) and free access databases (NIST 11, Flavor2) and the Kovats index (KI) measured using a mixture of n-alkanes (C7–C40) with the same chromatographic conditions, and then compared with values reported in the FFNSC 3 and NIST 11 databases. A manual integration of chromatographic peaks with a S/N ratio above 3 was performed without any further modification.
2.4. Positive-Ion Direct Infusion–Electrospray Ionization–Mass Spectrometry (DI–ESI–MS) Analysis
The dry residue of garlic was dissolved in H2O:MeOH (7:3) to a final concentration of 20 μg/mL and directly infused at 10 μL min−1 in the ESI source of a LTQ XL linear ion trap (Thermo Fisher Scientific, Waltman, MA, USA). The source parameters were set as follows: source voltage = 4.5 kV; capillary voltage = 15 V; capillary temperature = 300 °C; tube lens voltage 89.9 V, sheath gas flow rate 10 (arbitrary units). Each spectrum, acquired over the 170–2000 m/z range, was from averaging 10 full scans, each one consisting of 5 micro scans. The major peaks were isolated and submitted to MS tandem experiments to allow the compound identification by comparing their relevant MS/MS spectra with those reported in literature or collected in a free access database (https://massbank.eu/MassBank/Search (accessed on 19 May 2022). The precursor ion isolation width was 1–2 Da and the normalized collision energy was set to the value needed to reduce the intensity of the precursor ion to approximately 10%.
2.5. Colorimetric Analysis
Colorimetric analysis of garlic powder (GP) sample and GWE was performed by X-Rite MetaVueTM® (Prato, Italy) as previously described [15]. GP sample and GWE analysis was conducted at the time of delivery (t°) and after 12 months (t12m) of storage in the darkness at room temperature (25 ± 2 °C). The detailed description of colorimetric analysis is reported in the Supplementary Materials section.
2.6. HPLC–DAD Analysis
GWE was weighed, dissolved in water and filtered before injection into a HPLC Perkin Elmer apparatus (Series 200 LC pump, Series 200 DAD and Series 200 autosampler, Milan, Italy). Chromatography was conducted on RP-18 column (3 µ) using a linear gradient consisting of acetonitrile and acidified water (5% formic acid), from 100% aqueous phase to 85% in 15 min, 85 to 55% in 30′ and 55 to 40% in 20′, at a flow rate of 0.8 mL/min, at 254 nm. Alliin was quantified as previously described [22] (y = 6.35x + 50.34; R2 = 0.9987, in the range between 2 and 400 µg/mL, LOD 0.6 µg/g and LOQ 2.0 µg/g extract in dry weight). Each analysis was performed in quadruplicate. The detailed protocol related to HPLC-DAD analysis is described in the Supplementary Materials section.
2.7. Cell Lines
Colorectal cancer cell line SW480 was cultured in RPMI1640 (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), 1% Pen/Strep and 1% L-glutamine, and maintained in a humidified incubator at 37 °C, 5% CO2.
2.8. Cell Viability Assay
Evaluation of cell viability was performed by MTT assay [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (Sigma, St. Louis, MO, USA) as previously reported [24]. SW480 cell line was seeded in 96-well plates (5 × 103 cells/well) and was treated the following day with GHE (1, 10, and 100 μg/mL), GWE (1, 10, and 100 μg/mL) or vehicle (culture medium). Each analysis was performed in triplicate.
2.9. Apoptosis Assay
To evaluate apoptosis, BD Pharmingen™ APC Annexin V antibody (BD, Becton-Dickinson Biosciences, San Jose, CA, USA) and propidium iodide (PI) (Sigma, St. Louis, MO, USA) were used according to the manufacturer’s instructions, essentially as previously described [25]. Each analysis was performed in triplicate
2.10. Ex Vivo Studies
Adult C57/BL6 male mice (3-month-old, weight 20–25 g) were housed in Plexiglas cages (2–4 animals per cage; 55 cm × 33 cm × 19 cm) and maintained under standard laboratory conditions. Experimentation procedures were in agreement with the European Community ethical regulations (EU Directive no. 26/2014) on the care of animals for scientific research and approved by local ethical committee (‘G. d’Annunzio’ University, Chieti, Italy) and Italian Health Ministry (Project no. 885/2018-PR).
After collection, isolated colon specimens were maintained in a humidified incubator with 5% CO2 at 37 °C for 4 h (incubation period) in RPMI buffer with added bacterial LPS (10 μg/mL) [26,27] and treated with either GHE or GWE (1, 10, and 100 μg/mL). Prostaglandin (PG) E2 and 8-iso-PGF2α levels (pg/mg wet tissue) were measured by radioimmunoassay (RIA) in tissue supernatants [28,29]. Each analysis was performed in triplicate.
Extraction of total RNA from colon specimens was performed using TRI reagent (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s instructions. Reverse transcription was performed using High Capacity cDNA Reverse Transcription Kit (ThermoFischer Scientific, Waltman, MA, USA). Gene expression of cyclooxygenase (COX)-2, TNF-α, nuclear factor-kB (NF-kB), IL-6, and nuclear factor erythroid 2-related factor 2 (Nrf2) was measured by quantitative real-time PCR using TaqMan probe-based chemistry [30,31]. The real-time PCR was performed in triplicate. Relative quantification of gene expression was conducted through the comparative 2−ΔΔCt method [32]. The detailed description of real-time PCR is reported in the Supplementary Materials section.
Extraction of serotonin (5-HT), and 5-hydroxyindolacetic acid (5HIIA) was performed from individual colon specimens homogenized in perchloric acid solution (50 mM). Analysis of colon 5-HT, and 5HIIA levels was performed through high performance liquid chromatography coupled to electrochemical detection consisting of ESA Coulochem III detector equipped with ESA 5014B analytical cell [33,34]. Each analysis was performed in triplicate.
2.11. Statistical Analysis
Analysis of the data was performed by using the software GraphPad Prism version 6.0 (Graphpad Software Inc., San Diego, CA, USA). Means ± SEM were assessed for each experimental group and analyzed by one-way analysis of variance (ANOVA), followed by the Newman–Keuls multiple comparison post hoc test. As for quantification of the investigated phenolic compounds detected in GHE and GWE, analysis of the data was conducted by unpaired t test (two-tailed p value). The limit of statistically significant differences between mean values was set at p-value < 0.05. Calculation of the number of animals randomized for each experimental group was performed by using the “Resource Equation” n = (E + T)/T (10 ≤ E ≤ 20) [35].
3. Results and Discussion
3.1. Phytochemical Analyses
3.1.1. HPLC–DAD–MS Analysis
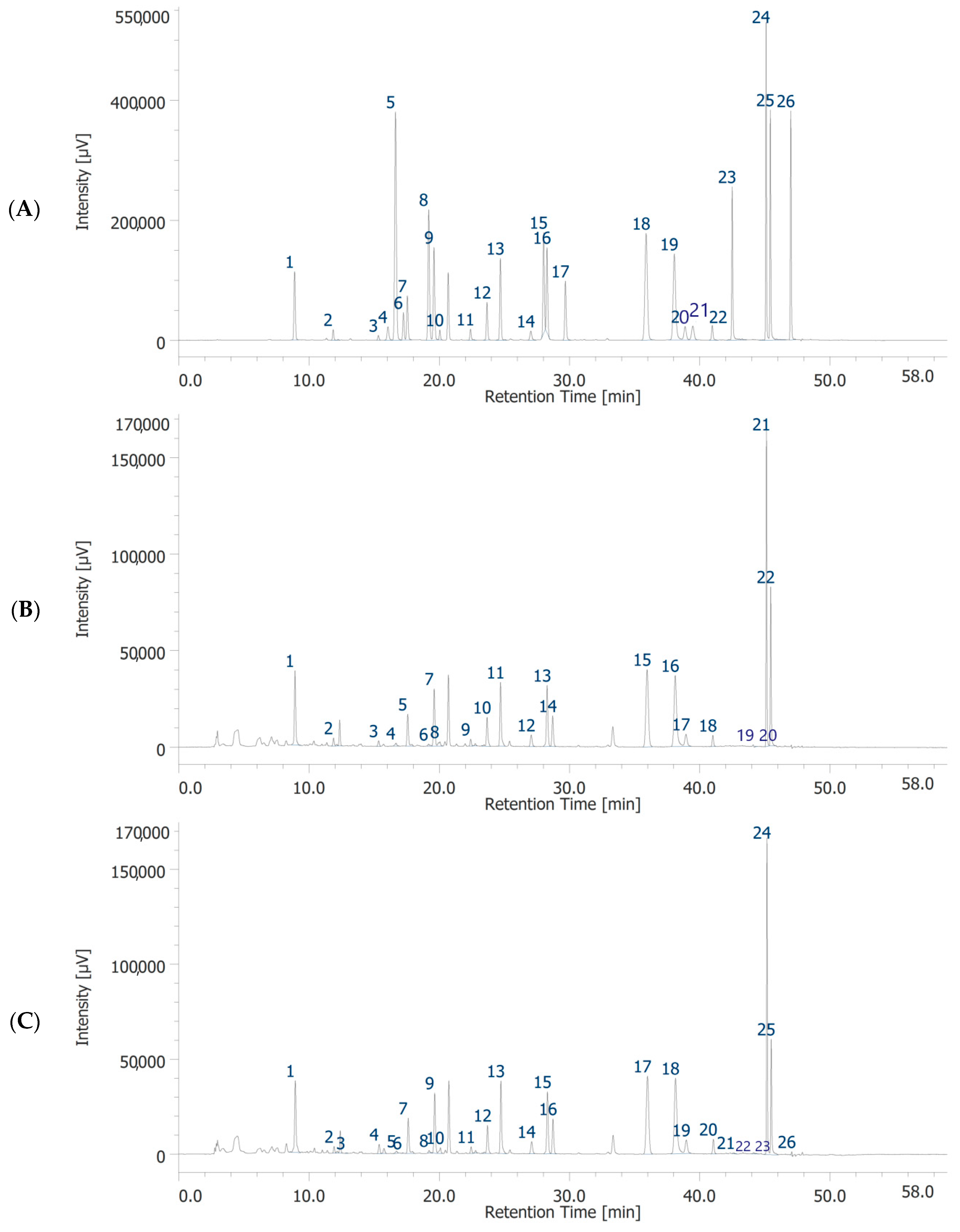
Both garlic extracts were analyzed for the measurement of the levels of selected flavonoids and phenols by quantitative HPLC–DAD–MS analysis. The list of the polyphenolic compounds studied, as well as the retention time, wavelength, and the m/z ratio for their determination are reported in Table S2. Quantification was performed as previously reported [23]. The characterization of the investigated phenolic compounds is reported in Table 1. A variety of factors influence composition of garlic extracts, such as source of garlic strain, conditions of storage, processing type, and aging [36,37]. The biological activity of garlic components has also been reported to be dependent on extraction, temperature, preparation, and storage [38]. In addition, the data are not easily comparable with literature, being chiefly related to the garlic clove and garlic powder rather than to the separated parts [39,40]. The chromatographic analysis of GHE and GWE confirmed the presence of 19 and 18 phytochemicals, respectively (Figure 1 and Table 1). In the present study, the prominent compound found in GHE was catechin, while the prominent compounds found in GWE were catechin and benzoic acid. Gallic acid, chlorogenic acid, p-coumaric acid, t-ferulic acid, benzoic acid, resveratrol, naringenin, hesperetin, and flavone were present at concentrations ranging from 22.66 to 13.33 μg/mL in GHE, and from 12.73 to 21.51 μg/mL in GWE. On the other hand, concentrations of 3-hydroxytyrosol, caffeic acid, epicatechin, syringaldehyde, p-coumaric acid, t-cinnamic acid, quercetin, and 3-hydroxyflavone ranged from 8.94 to 11.83 μg/mL in GHE, and from 7.23 to 11.59 μg/mL in GWE. Caftaric acid was detected only in GHE, at very lower concentration (0.93 μg/mL). Interestingly, GHE was richer than GWE in catechin, epicatechin, t-ferulic acid, benzoic acid, resveratrol, quercetin, naringenin, and hesperetin. On the other hand, GWE showed higher levels of 3-hydroxytyrosol and 3-hyroxyflavone as compared to GHE.

Table 1.
Quantification of the investigated phenolic compounds detected in garlic hydroalcoholic extract (GHE) and garlic water extract (GWE) (μg/mL).
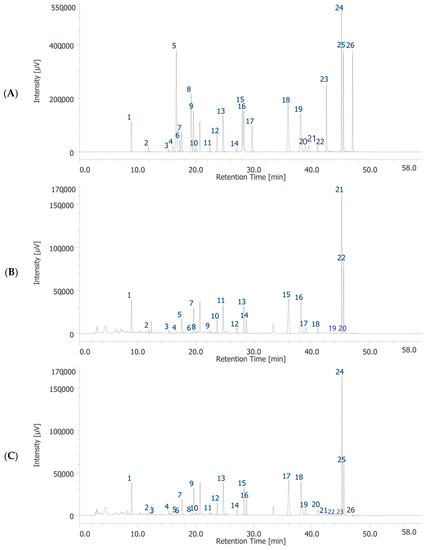
Figure 1.
HPLC-DAD chromatograms of standards solution (A), garlic water extract (GWE) (B) and garlic hydroalcoholic extract (GHE) (C). Chromatographic analysis of GWE (B). The chromatographic analysis showed the presence of 18 phytochemicals: gallic acid (peak #1), 3-hydroxytyrosol (peak #2), catechin (peak #3), chlorogenic acid (peak #5), caffeic acid (peak #7), epicatechin (peak #8), syringaldehyde (peak #9), p-coumaric acid (peak #10), t-ferulic acid (peak #11), benzoic acid (peak #12), rutin (peak #13), resveratrol (peak #14), t-cinnamic acid (peak #15), quercetin (peak #16), naringenin (peak #17), hesperetin (peak #18), flavone (peak #21), 3-hydroxyflavone (peak #22). Chromatographic analysis of GHE (C). The chromatographic analysis showed the presence of 19 phytochemicals: gallic acid (peak #1), 3-hydroxytyrosol (peak #2), caftaric acid (peak #3), catechin (peak #4), chlorogenic acid (peak #7), caffeic acid (peak #9), epicatechin (peak #10), syringaldehyde (peak #11), p-coumaric acid (peak #12), t-ferulic acid (peak #13), benzoic acid (peak #14), rutin (peak #15), resveratrol (peak #16), t-cinnamic acid (peak #17), quercetin (peak #18), naringenin (peak #19), hesperetin (peak #20), flavone (peak #24), 3-hydroxyflavone (peak #25).
3.1.2. HS–SPME–GC–MS and DI–ESI–MS Analysis
The compounds identified through the HS–SPME–GC–MS were clustered in three main classes (Table 2), namely the monoterpenes and their oxygen derivatives (61.2%), the sulfur-containing-compounds (SCC, 22.8%) and finally the aldehydes (3%). Thymol and carvacrol, two monoterpenoids, are the most abundant species (22.4% and 36.1%, respectively), followed by allyl disulfide (9.52%) and the 3-vinyl-1,2-dithiacyclohexene isomers (7.14%) belonging to the SSC class.

Table 2.
HS–SPME–GC–MS analysis of dry residue of garlic water extract (GWE).
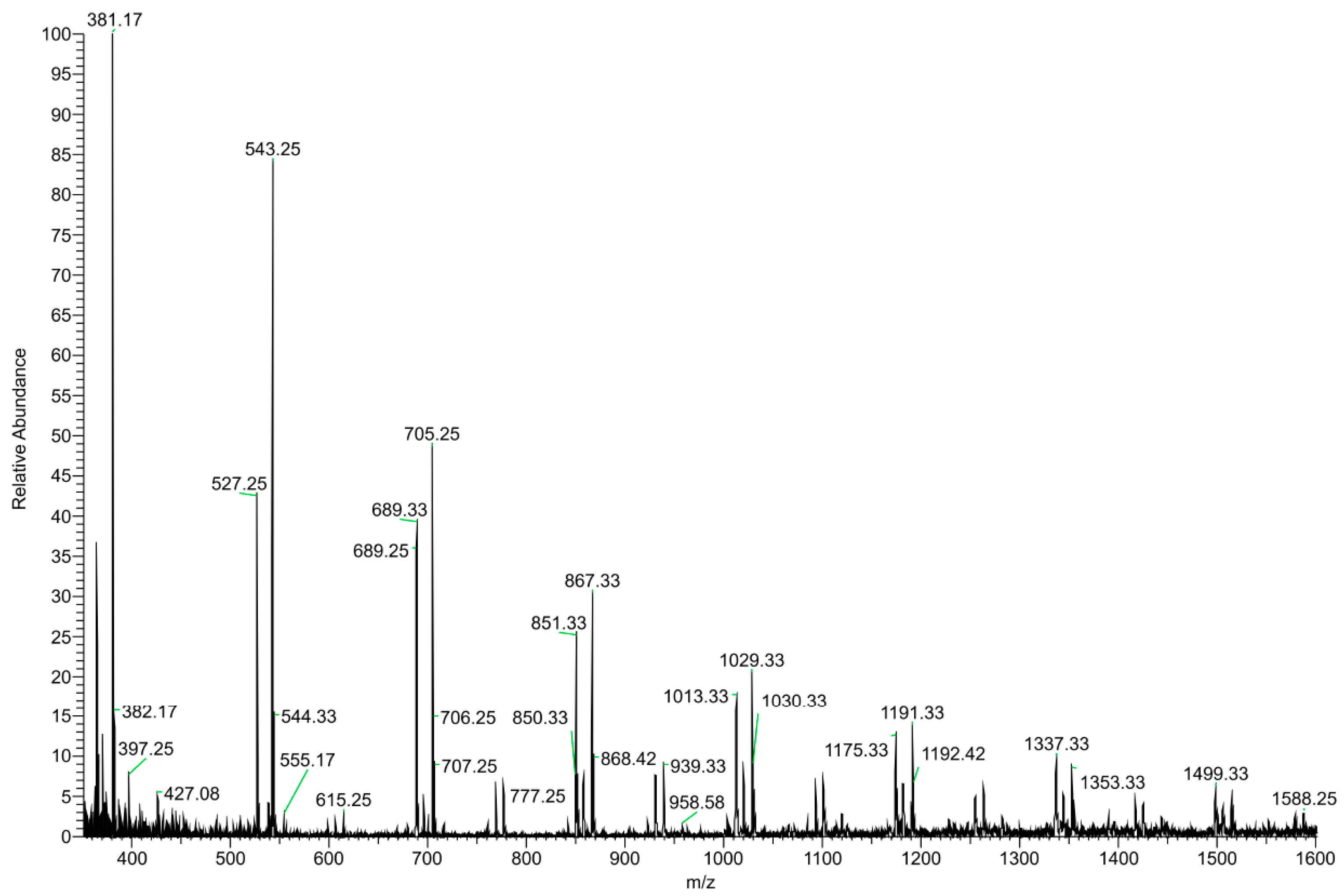
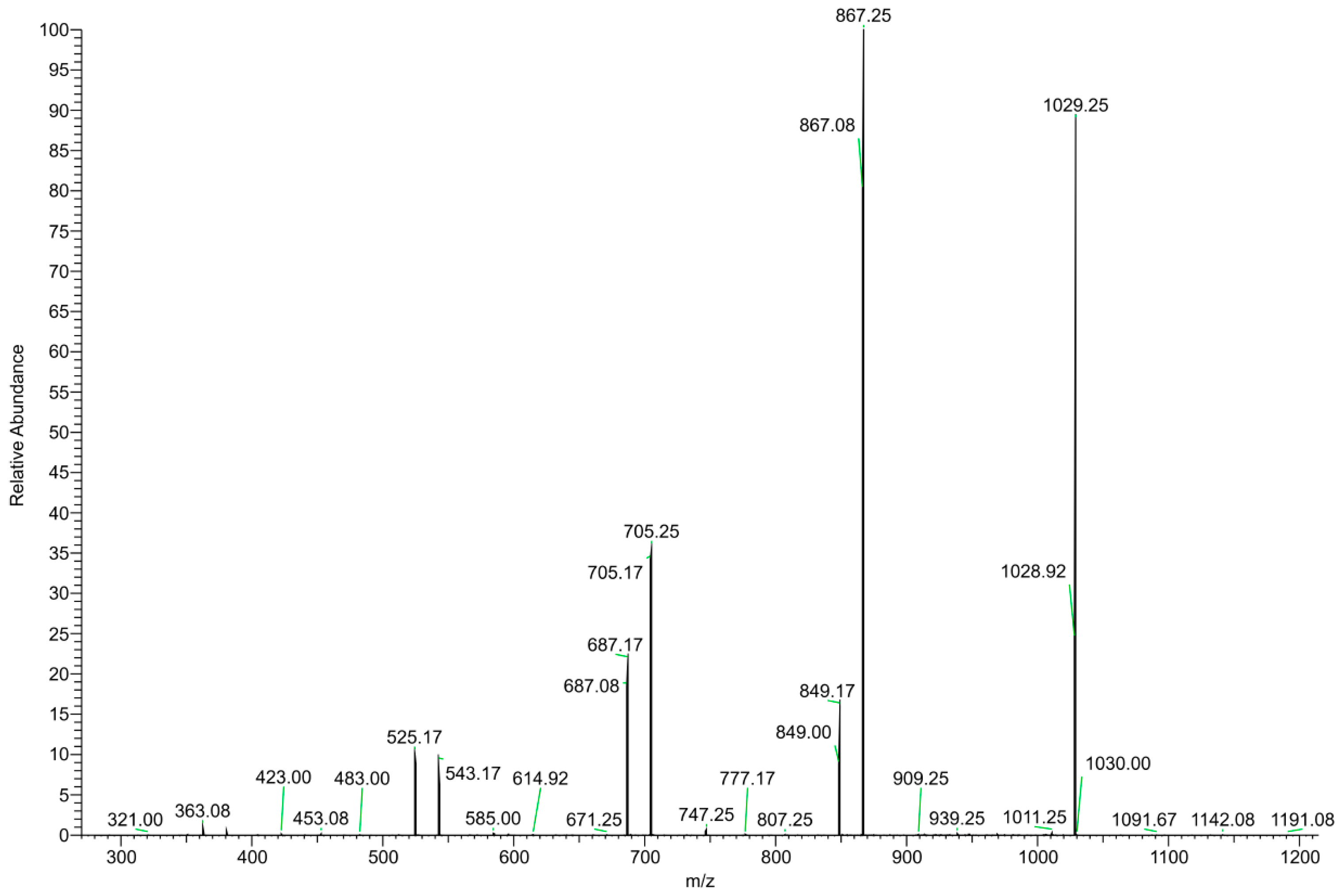
The DI–ESI–MS full spectrum was dominated by the regular repetition of two monocharged species differing by 16 mass units (Figure 2), that suggests the presence of species complexing Na+ and K+ cations. The 162 Da intervals correspond to the difference of one -C6H10O5- unit, typical of a homologous series of polysaccharides. The MS/MS spectrum of the 1029 m/z ion, reported in Figure 3 as representative ion of the series, showed the sequential loss of 162 Da (=C6H10O5) and 18 Da (=H2O) according to the MS2 fragmentation of a polysaccharide. The detected oligosaccharides, most probably fructans [41], are characterized by a wide degree of polymerization (DP), namely in the 2-11 DP range (Table 3). The Na+ and K+ ions distribution emerging from the fructans series indicates a larger content of the latter, despite what one should expect considering the ubiquitous presence of Na+ cation in the ESI–MS experiments. In addition to the fructans, the full scan showed two intense signals at m/z 175 and 214, corresponding to the protonated L-arginine and to N-butylbenzene sulfonamide (a plasticizer contaminant), respectively (Table 3).
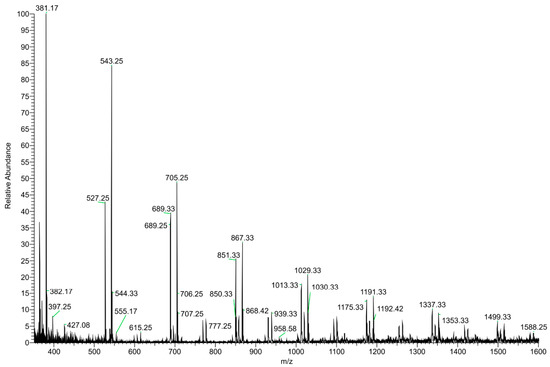
Figure 2.
(+) ESI–MS full scan of the dry residue of garlic water extract (GWE).
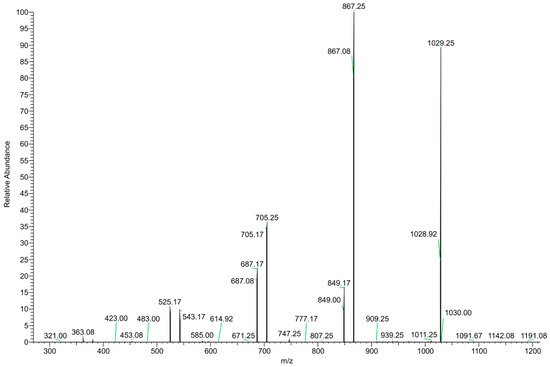
Figure 3.
MS/MS spectrum of the m/z 1029 positively charged ion.

Table 3.
DI–ESI–MS analysis of dry residue of garlic water extract (GWE).
3.1.3. Colorimetric Analysis
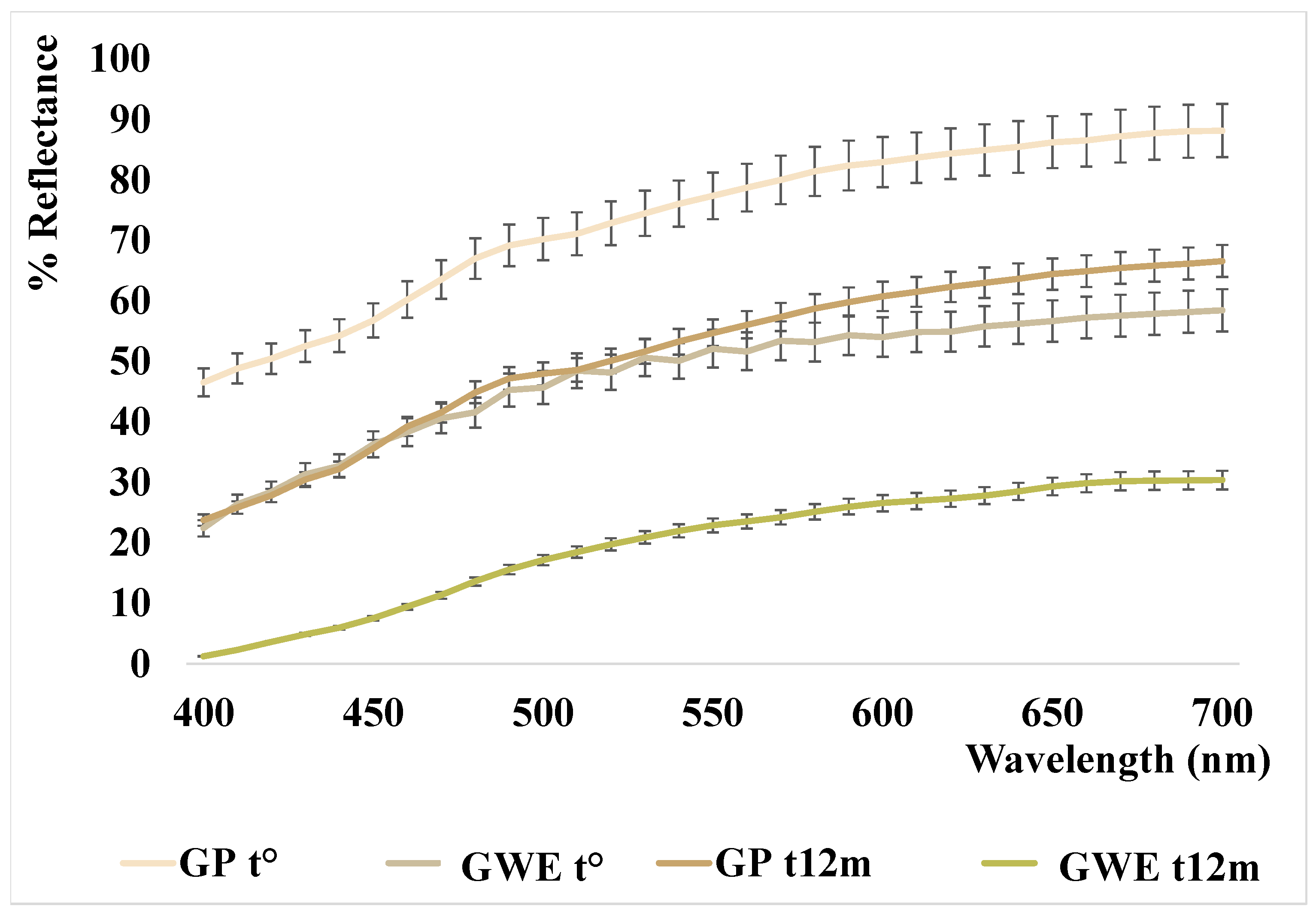
The color parameters and relative reflectance curves of the analyzed samples are shown in Table 4 and Figure 4.

Table 4.
Colorimetric CIEL*a*b* parameters of garlic powder (GP) and garlic water extract (GWE).
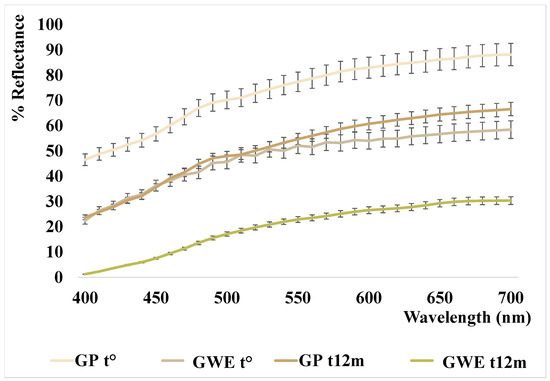
Figure 4.
Reflectance curves of the analyzed garlic samples.
As reported in our previous work [22], the garlic powder analyzed at t° has a very bright color tending to pale yellow (L* = 90.15 and b* = 16.02) that, after eight months of storage, seemed to fade slightly (L* ≈ 94 vs. 90 at t0, b* ≈ 14 vs. 16 at t0, with a ΔE ≈ 2, data previously reported). In this study, the analyses performed at t12m, showed a substantial change in color (ΔE = 12.34), characterized by a strong darkening of the powder (L* ≈ 78; b* ≈ 20), as also shown by the lowering of the reflectance curve reported in Figure 4. The initial light bleaching and subsequent browning were already described in milk powder samples in Cesa and collaborators (2015) [42], and interpreted as carotenoid degradation followed by Maillard reaction. In the case of garlic, bleaching was not previously described. Nevertheless, the presence of carotenoids in Allium spp. phytocomplex was reported in the literature [43] and the carotenoid bleaching seems the simplest hypothesis, despite all our attempts to extract a carotenoid fraction by organic solvents failed. Therefore, the slight bleaching should be attributed to other yellow, hydrosoluble, components. On the other hand, the powder darkening could be mainly due to the different sulfur components (the presence of many different sulfur compounds was confirmed by HS–SPME–GC–MS analysis (see Section 3.1.2)) which, modifying the pH and the activity of polyphenol oxidases, with a not completely known mechanism depending on the state of preservation and on the temperature, cause darkening of the matrix [44,45].
This darkening is also confirmed in the aqueous extract newly prepared and analyzed after 12 months (see Figure 4). In fact, the L* parameter of GWE t° decreases significantly from 76 to 65, as well as the b* parameter increases even more, from 17 to 28, denoting altogether a strong color change characterized by a substantial browning with respect to the starting points (ΔE = 28 respect to GP t° and ΔE = 16 respect to GWE t°).
3.1.4. HPLC–DAD Analysis
The HPLC–DAD analysis was conducted at 254 nm for the identifying benzoic and hydroxycinnamic acids, flavanols and organosulfur compounds. The molecular profile is confirmed by literature even if no chromatograms of aqueous extracts are reported [46,47].
The chromatogram showed the presence of alliin and an its diastereoisomer (361.1 ± 17.3 µg/g dry extract) as reported by Dethier et al., 2012 [48]. Furthermore, chlorogenic acid, caffeic acid, and epicatechin (see Section 3.1.1 for the relevant quantification) were also shown.
These results are only partially comparable to those present in the literature, because the garlic phytocomplex and relative aqueous extracts are very variable according to the different cultivar, geographic area, and storage conditions. There are no references in the literature on quantitative data related to garlic aqueous extracts. Existing data refer to methanolic or hydroalcoholic extracts reporting alliin content in range of 0.5–33 expressed in mg/g dry weight [47,49]. Compared to the hydroalcoholic extract, reported in our previous work [22], alliin values found in GWE are significantly lower (approximately 360 vs. 1200 μg/g dry extract).
3.2. Toxicological and Pharmacological Studies
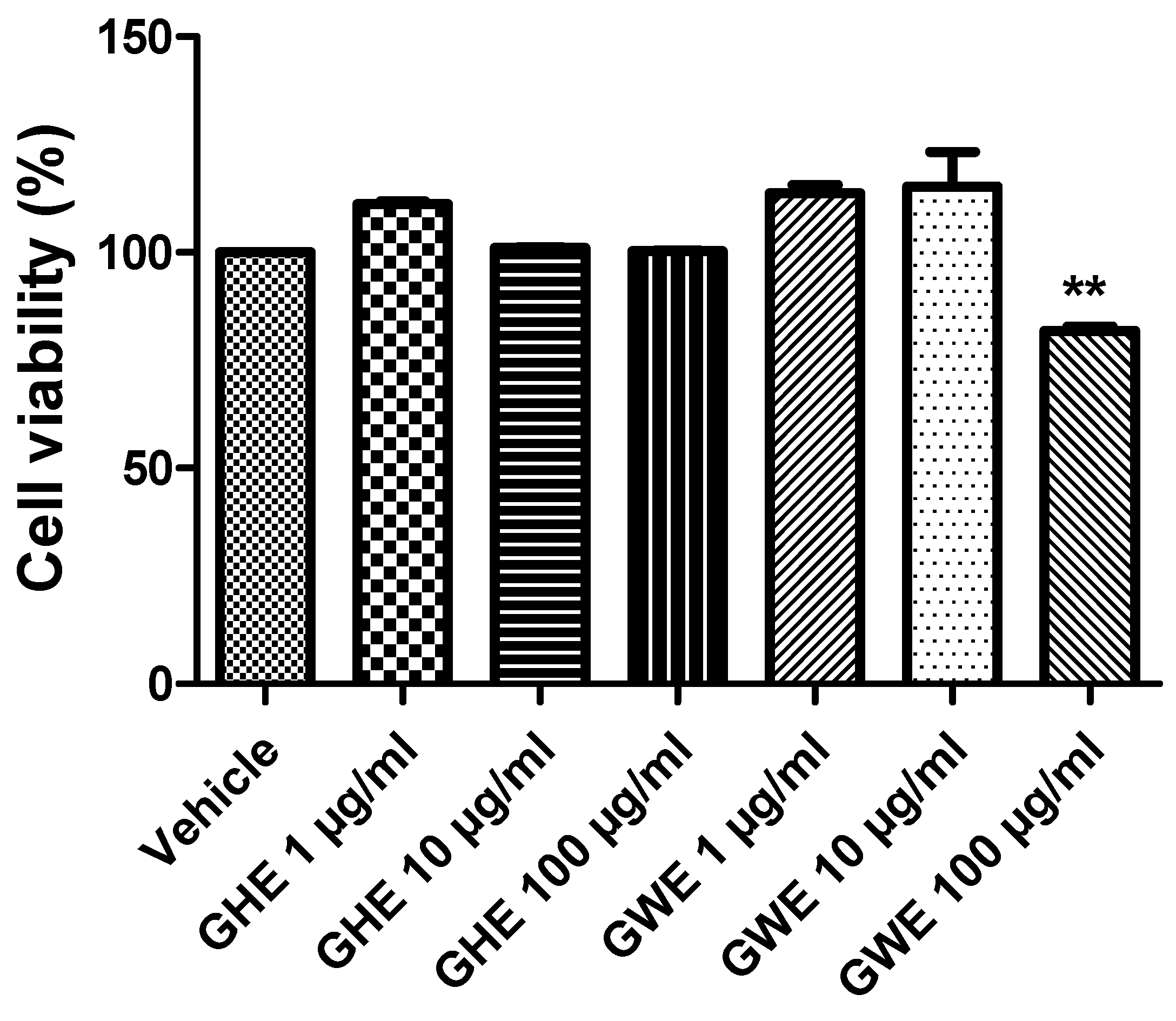
In a previous study of ours, GHE (1–100 μg/mL) was not able to modify cell viability of H9c2 cells (rat cardiomyoblasts), in basal conditions, confirming its good biocompatibility [22]. Moreover, the same extract (10–100 μg/mL) was effective in protecting cells from cytotoxicity induced by H2O2 (200 μM) [22]. In the present study, we investigated the effects of GHE and GWE, in the dose range 1–100 μg/mL, on colon cancer SW480 cell line viability, in basal conditions. Compared to the control group, GHE was not able to affect SW480 cell viability (Figure 5). However, GWE (100 μg/mL) significantly suppressed SW480 cell viability, even if the cell viability was not under the biocompatibility limits (70% viability compared to control, respectively) (Figure 5).
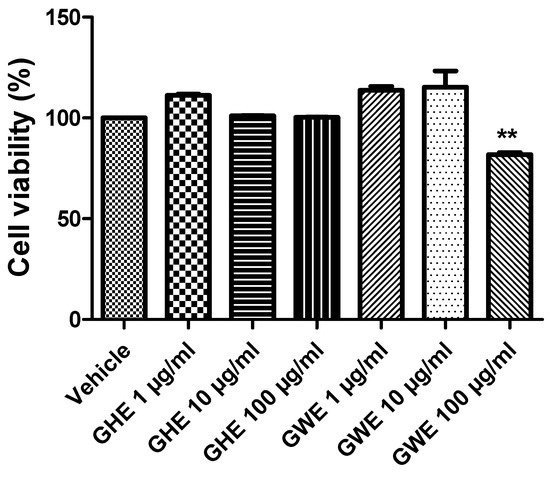
Figure 5.
MTT assay of SW480 cell line treated with garlic hydroalcoholic extract (GHE) (1, 10, and 100 μg/mL), garlic water extract (GWE) (1, 10, and 100 μg/mL), and vehicle (RPMI) for 48 h, in basal conditions. Data are displayed as the means ± SEM. ANOVA, p < 0.001; ** p < 0.01 vs. vehicle.
Accordingly, both GHE and GWE were not able to modify apoptosis of SW480 cell lines following 48 h of treatment in basal conditions (Figure 6).
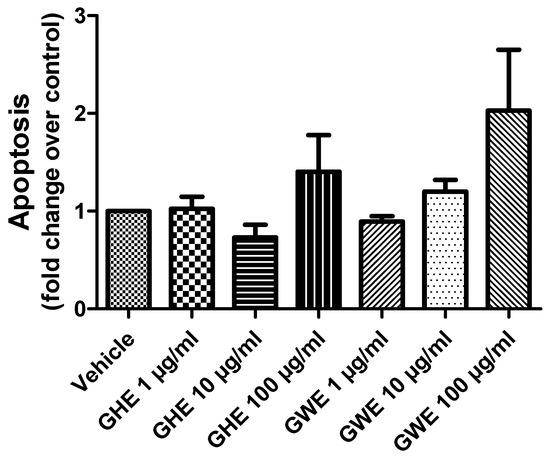
Figure 6.
Apoptosis assay in SW480 cell line treated with garlic hydroalcoholic extract (GHE) (1, 10, and 100 μg/mL), garlic water extract (GWE) (1, 10, and 100 μg/mL), and vehicle (RPMI) for 48 h, in basal conditions.
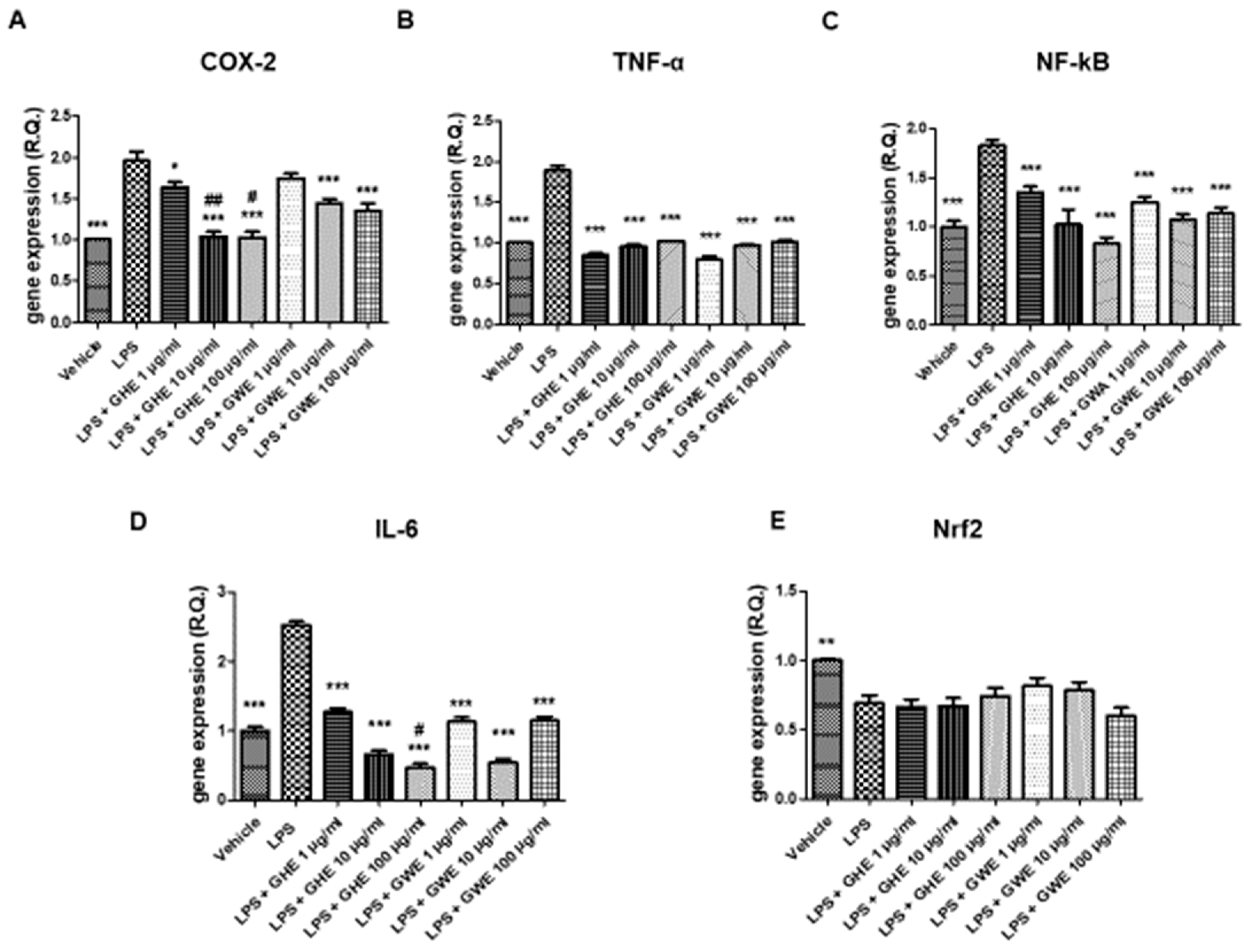
GHE and GWE, in the dose range 1–100 μg/mL, were then tested to evaluate their potential protective activities on oxidative and inflammation pathways in mouse colon specimens treated with LPS. In particular, GHE, in the tested dose range, was effective in suppressing LPS-induced gene expression of pro-inflammatory markers strongly involved in colon inflammation, including COX-2, TNF-α, NF-κB, and IL-6 (Figure 7A–E) [50,51,52,53]. In the same experimental paradigm, GWE (1–100 μg/mL) inhibited LPS-induced TNF-α, NF-κB, and IL-6 (Figure 7A–E) gene expression. Moreover, the higher concentrations of GWE suppressed COX-2 (Figure 7A) gene expression induced by LPS treatment. On the other hand, GHE and GWE did not modify LPS-induced Nrf2 gene expression (Figure 7E), ruling out a possible role of this mediator in mediating the protective effects exerted by GWE and GHE in mouse colon.
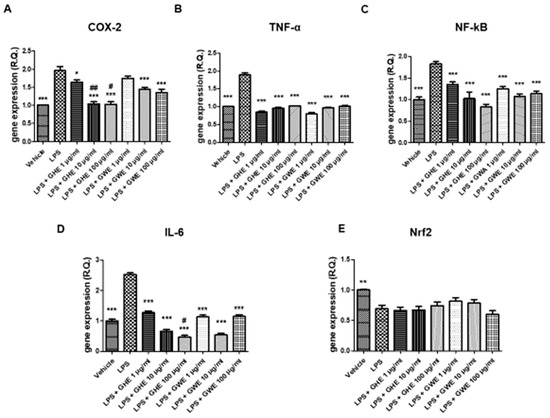
Figure 7.
Effects of garlic hydroalcoholic extract (GHE) (1, 10, and 100 μg/mL), garlic water extract (GWE) (1, 10, and 100 μg/mL) and vehicle (RPMI) on LPS-induced cyclooxygenase (COX)-2 (A), tumor necrosis factor (TNF)-α (B) nuclear factor-kB (NF-kB (C)), interleukin (IL)-6 (D), nuclear factor erythroid 2-related factor 2 (Nrf2) (E) gene expression (RQ, relative quantification) in mouse colon specimens. Data are displayed as the means ± SEM. ANOVA, p < 0.01; * p < 0.05, ** p < 0.01 vs. LPS, and *** p < 0.001 vs. LPS; # p < 0.05 vs. LPS+GWE 100 μg/mL; ## p < 0.01 vs. GWE 10 μg/mL.
Accordingly, Hodge and collaborators (2002) [54] showed that, in peripheral blood monocytes, production of various leukocyte pro-inflammatory cytokines, such as IL-6, IL-8 and TNF-α, was reduced by a garlic extract (≥10.0 µg/mL). On the other hand, it also stimulated IL-10 synthesis in the same experimental model. In this context, the same authors suggested a possible therapeutic use of garlic in the management of inflammatory conditions, including inflammatory bowel disease [54]. Our present findings also agree with those by Shin and collaborators (2013) [55], who observed inhibitory effects induced by fresh and heated raw garlic extracts (FRGE and HRGE) on LPS-induced release of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, in RAW 264.7 macrophages. Moreover, we investigated the effects of the extracts on LPS-induced levels of PGE2, a pro-inflammatory mediator generated by COX-2, which is strongly involved in the pathophysiology of inflammatory bowel disease [56]. Our present results showed that both GHE (1–100 μg/mL) and GWE (10–100 μg/mL) suppressed LPS-induced PGE2 levels in isolated colon specimens (Figure 8), further supporting the anti-inflammatory effects exerted by these extracts.
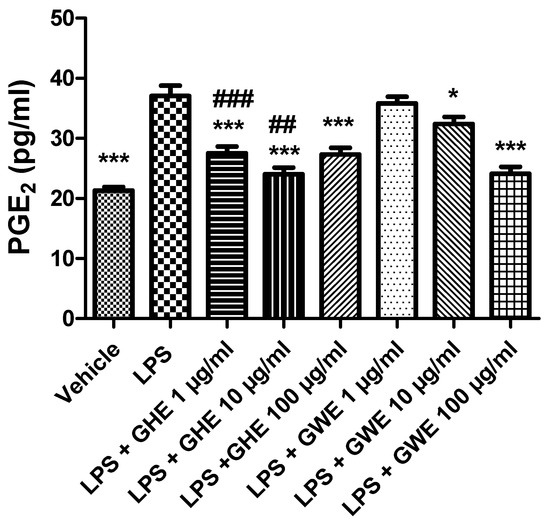
Figure 8.
Effects of garlic hydroalcoholic extract (GHE) (1, 10, and 100 μg/mL), garlic water extract (GWE) (1, 10, and 100 μg/mL) and vehicle (RPMI) on LPS-induced prostaglandin E2 (PGE2) levels in mouse colon specimens. Data are shown as the means ± SEM. ANOVA, p < 0.0001; * p < 0.05, and *** p < 0.001 vs. LPS; ## p < 0.01, ### p < 0.001 vs. co-respective treatment with GWE.
Accordingly, we previously reported that GWE exerted cardioprotective activities, by suppressing LPS-induced COX-2, IL-6, and NF-kB gene expression, as well as PGE2 levels, in heart specimens. To this regard, we hypothesized that the suppression of NF-kB gene expression could be involved in modulating the inhibitory effects induced by GWE on COX-2 gene expression and PGE2 production [22]. Accordingly, ethyl linoleate from garlic was reported to suppress LPS-induced COX-2 mRNA and protein expression as well as PGE2 production in RAW264.7 cells, by suppressing NF-kB activation [57]. Actually, the anti-inflammatory effects exerted by GHE and GWE could be related, at least in part, to their phenol and flavonoid content [58,59,60,61,62,63,64,65,66,67], with particular regards to catechin [58]. To this regard, catechin was suggested to act as a potential therapeutic agent in the prevention of inflammation. In particular, catechin exhibited anti-inflammatory properties by suppressing inducible nitric oxide synthase (iNOS), and COX-2 protein expression, as well as IL-6 and TNF-α mRNA levels in LPS-treated RAW264.7 cells [68]. Interestingly, GHE was shown more effective than GWE in decreasing gene expression of COX-2, and IL-6 (Figure 7A,D), as well as PGE2 levels (Figure 9) in isolated mouse colon specimens following LPS challenge. This finding could be related to its major content in catechin [69], resveratrol [70], naringenin [71], and hesperetin [72]. Accumulating evidence showed that 5-HT is a pro-inflammatory mediator critically involved in the pathogenesis of intestinal disorders, such as IBD [73,74,75], irritable bowel syndrome [73,76,77], as well as DSS-induced experimental colitis [78]. In the present study, we also investigated the effects of the garlic extracts on the LPS-induced 5HIIA/5-HT ratio, which is known as a useful index of 5-HT turnover, in vivo [79,80], deeply related to the activity of monoamine oxidase (MAO)-A [81].
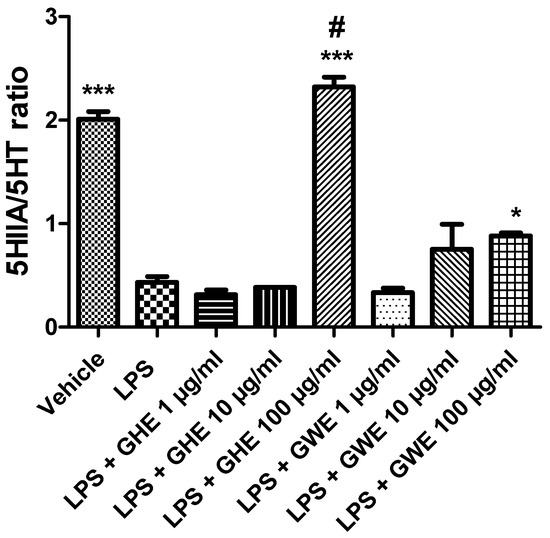
Figure 9.
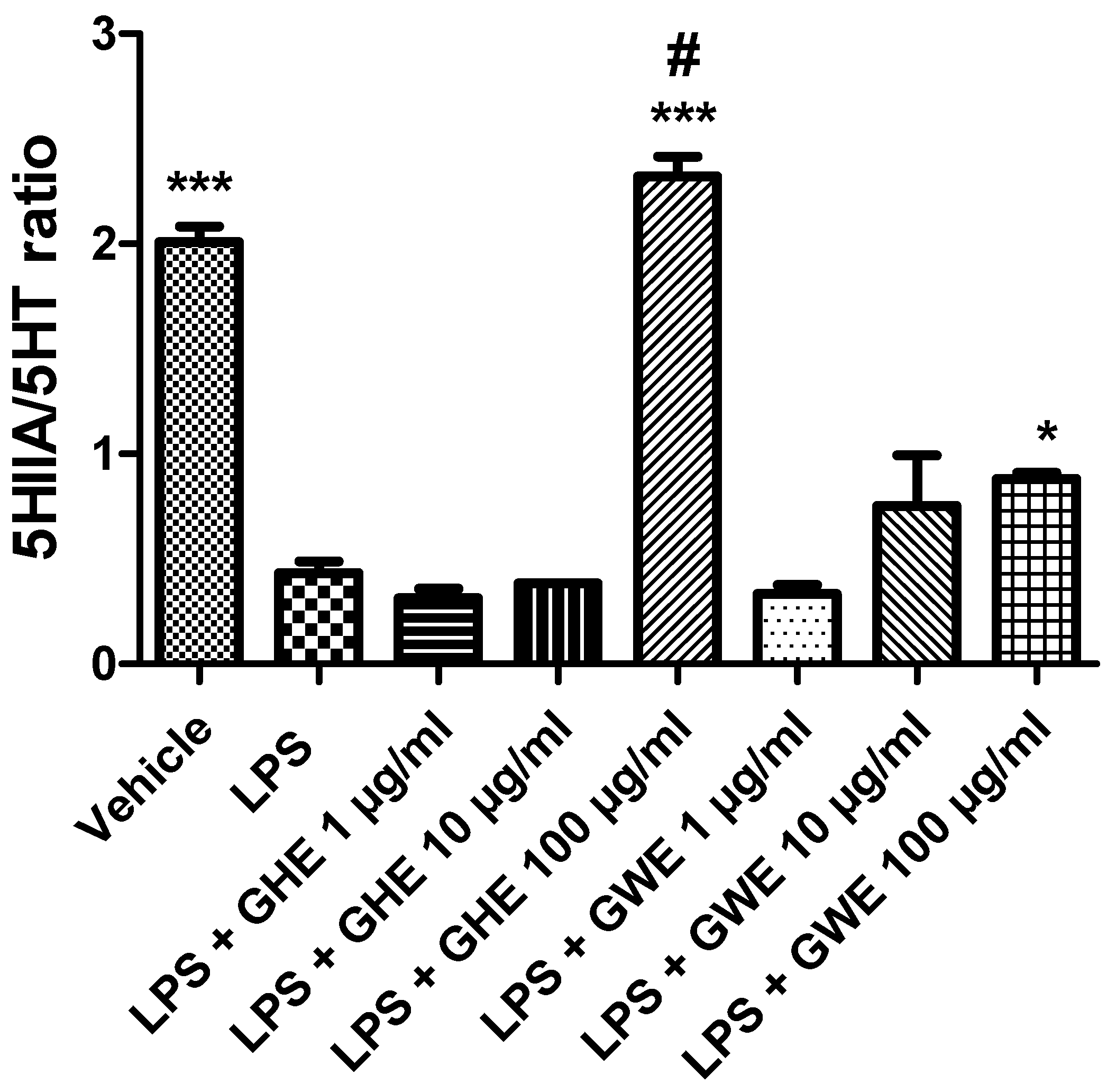
Effects of garlic hydroalcoholic extract (GHE) (1, 10, and 100 μg/mL), garlic water extract (GWE) (1, 10, and 100 μg/mL) and vehicle (RPMI) on the 5-HIIA/5-HT ratio in mouse colon specimens treated with vehicle. Data are displayed as the means ± SEM. ANOVA, p < 0.0001; * p < 0.05, and *** p < 0.001 vs. LPS; # p < 0.001 vs. LPS + GWE 100 μg/mL.
Consistently with the literature data [33], we found that LPS reduced the 5HIIA/5-HT ratio (Figure 9) in isolated mouse colon specimens.
However, GHE and GWE (100 μg/mL) prevented LPS-induced reduction in 5-HT turnover. The decreased levels of 5-HT, measured as the 5HIIA/5-HT ratio, could further account for the anti-inflammatory effects exerted by garlic extracts. To this regard, we have previously found that anti-inflammatory herbal extracts suppressed 5-HT levels in isolated rat colon treated with LPS [82,83]. In particular, GHE was found more effective than GWE in counteracting the LPS-induced decrease in the 5HIIA/5-HT ratio (Figure 9). Actually, the higher activity of GHE compared to GWE could be related to its higher content in benzoic acid and flavonoids, such as quercetin [84,85].
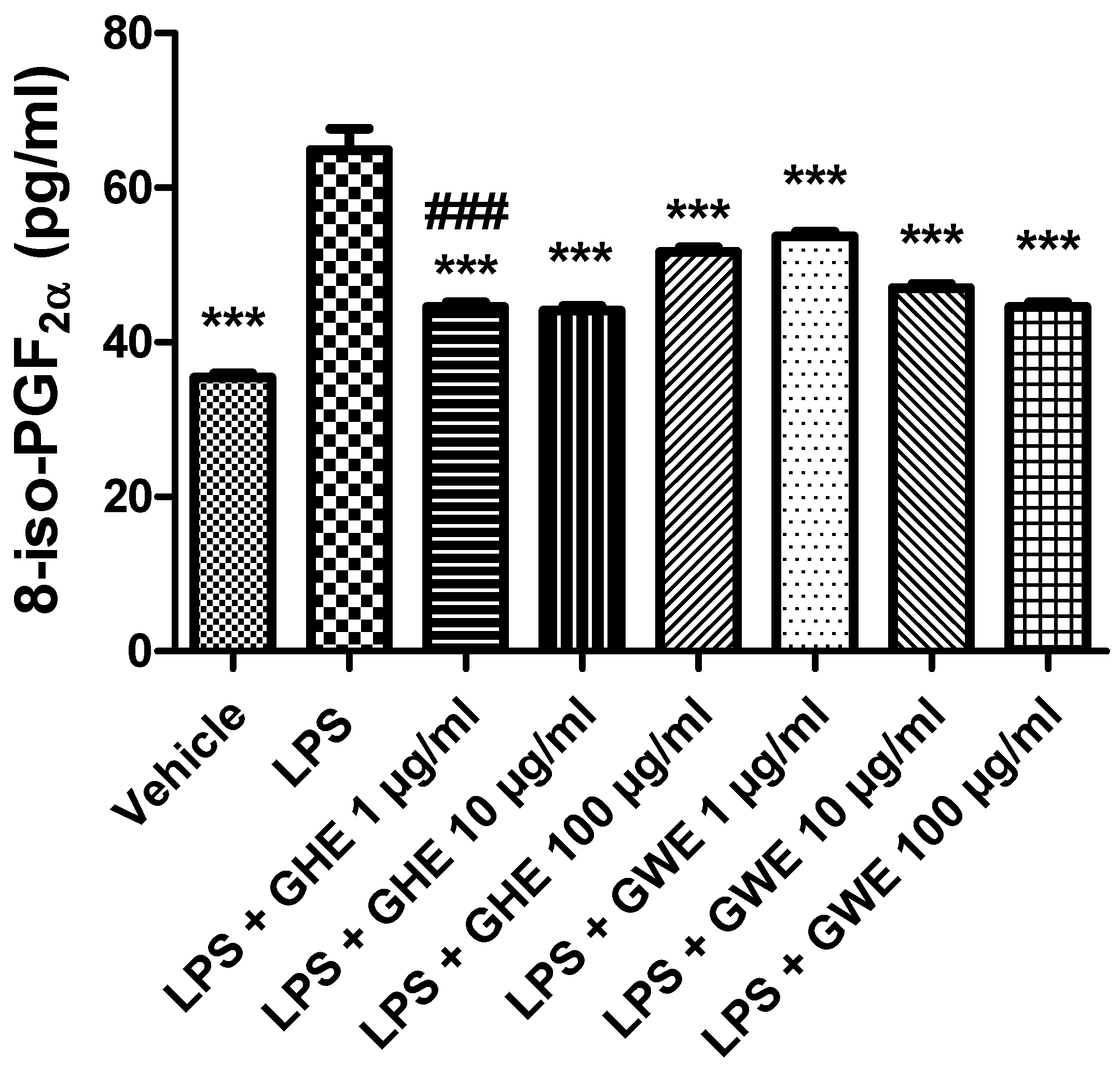
A wide body of evidence showed that imbalance between the oxidative reactions and antioxidant defense mechanisms played a key role in the initiation and progression of IBD. This imbalance generates oxidative stress resulting from either reactive oxygen species (ROS) overproduction or a reduction in antioxidant activity [86,87]. In particular, ROS overproduction was suggested to be involved in functional disruption of the enteric mucosa [52]. Increased production of ROS is known to damage cellular lipids, proteins, as well as nucleic acids, and finally disrupt gastrointestinal barrier integrity [88]. 8-iso-PGF2α is an isomer of prostaglandins produced from membrane arachidonic acid by free radical-catalyzed peroxidation, which is regarded as a stable marker of lipid peroxidation and oxidative stress [89]. As shown in Figure 10, both GHE and GWE (1–100 μg/mL) were able to counteract 8-iso-PGF2α levels induced by LPS treatment in isolated mouse colon specimens. In particular, GHE (1 μg/mL) showed higher efficacy in inhibiting 8-iso-PGF2α levels (Figure 10).
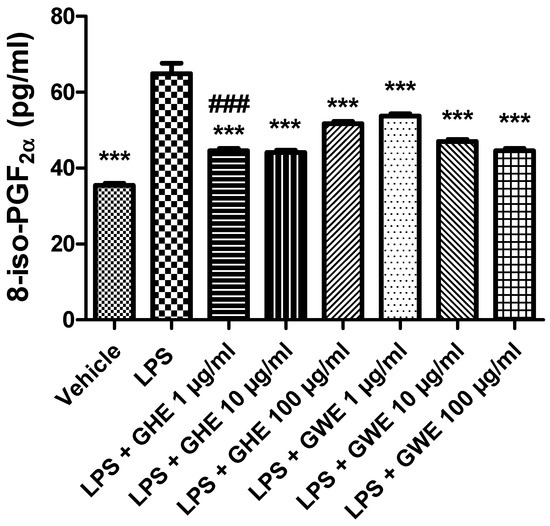
Figure 10.
Effects of garlic hydroalcoholic extract (GHE) (1, 10, and 100 μg/mL), garlic water extract (GWE) (1, 10, and 100 μg/mL) and vehicle (RPMI) on LPS-induced 8-iso-prostaglandin (PG)F2α levels in mouse colon specimens. Data are shown as the means ± SEM. ANOVA, p < 0.0001; *** p < 0.001 vs. LPS; ### p < 0.001 vs. co-respective treatment with GWE.
These results agreed with the antioxidant effects induced by GHE, tested in the same concentration range, in isolated mouse heart [22]. Accordingly, aqueous garlic extract was also shown to possess antioxidant properties by scavenging ROS and increasing cellular antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase [36]. The antioxidant effects induced by GHE and GWE are consistent with their polyphenol content [90], with particular regards to catechin [58]. In this context, catechins were found able to reduce the colonic oxidative damages to the colon, by suppressing oxidative stress, via exerting direct or indirect antioxidant effects, and by enhancing the activity of various antioxidant enzymes including glutathione peroxidases [58].
4. Conclusions
Concluding, GHE and GWE, particularly GHE, showed protective effects, as confirmed by the inhibitory effects on selected pro-inflammatory and pro-oxidant markers, in LPS-stimulated colon, suggesting a potential role in the prevention and management of ulcerative colitis. The phytochemical analyses suggested these effects could be related, albeit partially, to their phenol and flavonoid content, with particular regards to catechin. Moreover, other components of nutraceutical and pharmaceutical interest were detected in these extracts. On the other hand, further studies are necessary to accurately evaluate the in vivo activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11223559/s1. Table S1: MS analysis conditions. Table S2: Retention times, wavelengths of quantification, mass to charge (m/z) ratios, and molecular weight of the investigated phenolic compounds. Table S3: Gradient elution conditions of the HPLC-DAD analyses for investigated polyphenolic compounds in garlic hydroalcoholic and water extracts (GHE and GWE).
Author Contributions
Conceptualization, L.R., L.B. and S.L.; methodology, E.G., A.C. (Annalisa Chiavaroli), C.F. (Caterina Fraschetti), A.F., S.C., F.C. and A.M.; software, G.O., C.F. (Claudio Ferrante) and L.M.; validation, G.O., C.F. (Claudio Ferrante) and L.M.; formal analysis, E.G., A.C. (Annalisa Chiavaroli), C.F. (Caterina Fraschetti), A.F., S.C., F.C., A.M., S.V., P.L., A.C. (Alessandro Cama), C.F. (Claudio Ferrante), S.C.D.S., A.A., M.L.L. and N.; investigation, E.G., A.C. (Annalisa Chiavaroli), C.F. (Caterina Fraschetti), A.F., F.C., A.M., V.C., S.V., P.L., A.C. (Alessandro Cama), G.O., C.F. (Claudio Ferrante), S.C.D.S., A.A., M.L.L. and N.; resources, L.R., E.G., L.B. and S.L.; data curation, L.R., E.G., A.C. (Annalisa Chiavaroli), S.C., A.M. and S.L.; writing—original draft preparation, L.R., E.G., S.C. and S.L.; writing—review and editing, L.R., E.G., L.B. and S.L.; visualization, C.F. (Caterina Fraschetti), A.F., S.C. and F.C.; supervision, L.B.; project administration, L.R., A.C. (Annalisa Chiavaroli), L.B. and S.L.; funding acquisition, L.R., L.B. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by il Grappolo S.r.l. (Soliera, Modena, Italy), [Grants il Grappolo 2019 (Principal Investigators: Luigi Brunetti, and Sheila Leone; Coordinator: Lucia Recinella), grant number [2019] and il Grappolo 2021 (Principal Investigators: Luigi Brunetti, Sheila Leone, and Lucia Recinella; Coordinator: Annalisa Chiavaroli), grant number [2021].
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gómez-Gómez, L.; Ahrazem, O. Tissue-specific accumulation of sulfur compounds and saponins in different parts of garlic cloves from purple and white ecotypes. Molecules 2017, 22, 1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, M.; Zhao, X.; Li, X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 2018, 56, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Waterer, D.; Schmitz, D. Influence of variety and cultural practices on garlic yields in Saskatchewan. Can. J. Plant Sci. 1994, 74, 611–614. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Nazareno, M.A.; Fusari, C.; Camargo, A. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017, 220, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; El-Hack, M.E.A.; Taha, A.E.; Abd-Elhakim, Y.M.; Devkota, H.P. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Malliaraki, N.; Dimoulios, P.D.; Karmiris, K.; Castanas, E.; Kouroumalis, E.A. Decreased Total and Corrected Antioxidant Capacity in Patients with Inflammatory Bowel Disease. Am. J. Dig. Dis. 2004, 49, 1433–1437. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Achitei, D.; Ciobica, A.; Balan, G.; Gologan, E.; Stanciu, C.; Stefanescu, G. Different Profile of Peripheral Antioxidant Enzymes and Lipid Peroxidation in Active and Non-active Inflammatory Bowel Disease Patients. Am. J. Dig. Dis. 2013, 58, 1244–1249. [Google Scholar] [CrossRef]
- Chung, H.-L.; Yue, G.G.-L.; To, K.-F.; Su, Y.-L.; Huang, Y.; Ko, W.-H. Effect of Scutellariae Radix extract on experimental dextran-sulfate sodium-induced colitis in rats. World J. Gastroenterol. 2007, 13, 5605. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, L.; Joubert-Zakeyh, J.; Texier, O.; Lamaison, J.-L.; Vasson, M.-P.; Felgines, C. Aloysia triphylla infusion protects rats against dextran sulfate sodium-induced colonic damage. J. Sci. Food Agric. 2011, 92, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.E.; Abo-Salem, O.M.; El-Sayed, E.-S.M.; Taha, E.I.; El-Halawany, N. L-arginine augments the antioxidant effect of garlic against acetic acid-induced ulcerative colitis in rats. Pak. J. Pharm. Sci. 2009, 22, 373–380. [Google Scholar] [PubMed]
- Kuo, C.H.; Lee, S.H.; Chen, K.M.; Lii, C.K.; Liu, C.T. Effect of garlic oil on neutrophil infiltration in the small intestine of endotoxin-injected rats and its association with levels of soluble and cellular adhesion molecules. J. Agric. Food Chem. 2011, 59, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Balaha, M.; Kandeel, S.; Elwan, W. Garlic oil inhibits dextran sodium sulfate-induced ulcerative colitis in rats. Life Sci. 2016, 146, 40–51. [Google Scholar] [CrossRef]
- Tanrıkulu, Y.; Şen Tanrıkulu, C.; Kılınç, F.; Can, M.; Köktürk, F. Effects of garlic oil (allium sativum) on acetic acid-induced colitis in rats: Garlic oil and experimental colitis. Turk. J. Trauma Emerg. Surg. 2020, 26, 503–508. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Ronci, M.; Menghini, L.; Brunetti, L.; Leone, S.; Tirillini, B.; Angelini, P.; Covino, S.; Venanzoni, R.; et al. Multidirectional Pharma-Toxicological Study on Harpagophytum procumbens DC. ex Meisn.: An IBD-Focused Investigation. Antioxidants 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, R.; Mottese, A.F.; Bua, G.D.; Salvo, A.; Mallamace, D.; Corsaro, C.; Vasi, S.; Giofrè, S.V.; Alfa, M.; Cicero, N.; et al. Statistical Analysis of Mineral Concentration for the Geographic Identification of Garlic Samples from Sicily (Italy), Tunisia and Spain. Foods 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of allicin determined by chemical and biological assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and chemical stability of garlic-derived allicin. J. Agric. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef] [PubMed]
- Florio, R.; De Lellis, L.; Veschi, S.; Verginelli, F.; di Giacomo, V.; Gallorini, M.; Perconti, S.; Sanna, M.; Mariani-Costantini, R.; Natale, A.; et al. Effects of dichloroacetate as single agent or in combination with GW6471 and metformin in paraganglioma cells. Sci. Rep. 2018, 8, 13610. [Google Scholar] [CrossRef] [PubMed]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Menghini, L.; Ferrante, C.; Di Cesare Mannelli, L.; Ghelardini, C.; Brunetti, L.; Leone, S. Protective Effects Induced by Two Polyphenolic Liquid Complexes from Olive (Olea europaea, mainly Cultivar Coratina) Pressing Juice in Rat Isolated Tissues Challenged with LPS. Molecules 2019, 24, 3002. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Micheli, L.; Chiavaroli, A.; Libero, M.L.; Orlando, G.; Menghini, L.; Acquaviva, A.; Di Simone, S.; Ferrante, C.; Ghelardini, C.; et al. Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea europaea, Mainly Cultivar coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis. Nutrients 2022, 14, 1487. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Marconi, G.D.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; Schally, A.V.; et al. Antinflammatory, antioxidant, and behavioral effects induced by administration of growth hormone-releasing hormone analogs in mice. Sci. Rep. 2020, 10, 4850. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Di Valerio, V.; Veschi, S.; Orlando, G.; Ferrante, C.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; et al. Protective effects of growth hormone-releasing hormone analogs in DSS-induced colitis in mice. Sci. Rep. 2021, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Veschi, S.; Cama, A.; Marconi, G.D.; Diomede, F.; Gesmundo, I.; Granata, R.; et al. Effects of growth hormone-releasing hormone receptor antagonist MIA-602 in mice with emotional disorders: A potential treatment for PTSD. Mol. Psychiatry 2021, 26, 7465–7474. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Veschi, S.; Di Valerio, V.; Lattanzio, R.; Orlando, G.; Ferrante, C.; Gesmundo, I.; Granata, R.; Cai, R.; et al. Antagonist of growth hormone-releasing hormone MIA-690 attenuates the progression and inhibits growth of colorectal cancer in mice. Biomed. Pharmacother. 2022, 146, 112554. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Ferrante, C.; Menghini, L.; Orlando, G.; Brunetti, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Leporini, L.; et al. New pharmacological targets of three Asphodeline species using in vitro and ex vivo models of inflammation and oxidative stress. Int. J. Environ. Health Res. 2019, 29, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Sivam, G.P. Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr. 2001, 131, 1106S–1108S. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Altamirano, J.C.; Camargo, A.B. Multi-phytochemical determination of polar and non-polar garlic bioactive compounds in different food and nutraceutical preparations. Food Chem. 2021, 337, 127648. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Zhao, R.; Zhao, M.; Cui, X.; Xu, Y.; Qiao, X. In vitro Prebiotic Properties of Garlic Polysaccharides and Its Oligosaccharide Mixtures Obtained by Acid Hydrolysis. Front. Nutr. 2021, 8, 798450. [Google Scholar] [CrossRef]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kim, J.K.; Kim, H.H.; Lee, S.Y.; Park, N.I.; Park, S.U. Carotenoid accumulation and characterization of cDNAs encoding phytoene synthase and phytoene desaturase in garlic (Allium sativum). J. Agric. Food Chem. 2011, 59, 5412–5417. [Google Scholar] [CrossRef]
- Hong, S.I.; Kim, D.M. Storage quality of chopped garlic as influenced by organic acids and high-pressure treatment. J. Sci. Food Agric. 2001, 81, 397–403. [Google Scholar] [CrossRef]
- Liu, P.; Lu, X.; Li, N.; Zheng, Z.; Zhao, R.; Tang, X.; Qiao, X. Effects and mechanism of free amino acids on browning in the processing of black garlic. J. Sci. Food Agric. 2019, 99, 4670–4676. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ryu, K.; Yoshida, J.; Ide, N.; Kodera, Y.; Sasaoka, T.; Rosen, R.T. Identification of six phenylpropanoids from garlic skin as major antioxidants. J. Agric. Food Chem. 2003, 51, 7313–7317. [Google Scholar] [CrossRef]
- Eghdami, A.; Sohi, S.M.H.; Asli, D.E.; Houshmandfar, A. Antioxidant activity of methanolic and hydroalcohlic extracts of garlic plant. Adv. Environ. Biol. 2011, 5, 1575–1578. [Google Scholar]
- Dethier, B.; Laloux, M.; Hanon, E.; Nott, K.; Heuskin, S.; Wathelet, J.P. Analysis of the diastereoisomers of alliin by HPLC. Talanta 2012, 101, 447–452. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of nitrogen and sulfur fertilization on the alliin content of onions and garlic. J. Plant Nutr. 2005, 27, 1827–1839. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Bouguen, G.; Chevaux, J.B.; Peyrin-Biroulet, L. Recent advances in cytokines: Therapeutic implications for inflammatory bowel diseases. World J. Gastroenterol. 2011, 17, 547–556. [Google Scholar] [CrossRef]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-kB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hodge, G.; Hodge, S.; Han, P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry 2002, 48, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Ryu, J.H.; Kang, M.J.; Hwang, C.R.; Han, J.; Kang, D. Short-term heating reduces the anti-inflammatory effects of fresh raw garlic extracts on the LPS-induced production of NO and pro-inflammatory cytokines by downregulating allicin activity in RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 545–551. [Google Scholar] [CrossRef]
- Dey, I.; Lejeune, M.; Chadee, K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br. J. Pharmacol. 2006, 149, 611–623. [Google Scholar] [CrossRef]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, D.Y.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, J.; Jiang, B.; Miao, M. Resveratrol and inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1403, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A review of the protective effects of chlorogenic acid against different chemicals. J. Food Biochem. 2022, 46, e14254. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef]
- Duan, L.; Cheng, S.; Li, L.; Liu, Y.; Wang, D.; Liu, G. Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 684486. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, A.; Zhang, Z.; Yu, Z.; Liu, Y.; Peng, S.; Wu, L.; Qin, H.; Wang, W. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-κB pathway. Pharmacol. Rep. 2016, 68, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Wang, J.; Le, T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-kappaB, attenuates bacterial peptidoglycan polysaccharide induced colitis in rats. J. Pharmacol. Exp. Ther. 2001, 299, 915–920. [Google Scholar]
- Liu, S.H.; Lu, T.H.; Su, C.C.; Lay, I.S.; Lin, H.Y.; Fang, K.M.; Ho, T.J.; Chen, K.L.; Su, Y.C.; Chiang, W.C.; et al. Lotus leaf (Nelumbo nucifera) and its active constituents prevent inflammatory responses in macrophages via JNK/NF-κB signaling pathway. Am. J. Chin. Med. 2014, 42, 869–889. [Google Scholar] [CrossRef]
- Sunil, M.A.; Sunitha, V.S.; Santhakumaran, P.; Mohan, M.C.; Jose, M.S.; Radhakrishnan, E.K.; Mathew, J. Protective effect of (+)-catechin against lipopolysaccharide-induced inflammatory response in RAW 264.7 cells through downregulation of NF-κB and p38 MAPK. Inflammopharmacology 2021, 29, 1139–1155. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. (Lond.) 2008, 5, 17. [Google Scholar] [CrossRef]
- Lim, R.; Barker, G.; Wall, C.A.; Lappas, M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol. Hum. Reprod. 2013, 19, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, S.C.; Senthil Kumar, K.J.; Yu, K.N.; Lee Chao, P.D.; Tsai, S.Y.; Hou, Y.C.; Hseu, Y.C. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J. Agric. Food Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.D.; Mahoney, C.R.; Linden, D.R.; Sampson, J.E.; Chen, J.; Blaszyk, H.; Crowell, M.D.; Sharkey, K.A.; Gershon, M.D.; Mawe, G.M.; et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004, 126, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Minderhoud, I.M.; Oldenburg, B.; Schipper, M.E.I.; ter Linde, J.J.M.; Samsom, M. Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin. Gastroenterol. Hepatol. 2007, 5, 714–720. [Google Scholar] [CrossRef]
- Stoyanova, I.I.; Gulubova, M.V. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002, 104, 185–192. [Google Scholar] [CrossRef]
- Bearcroft, C.P.; Perrett, D.; Farthing, M.J.G. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: A pilot study. Gut 1998, 42, 42–46. [Google Scholar] [CrossRef]
- Spiller, R.C.; Jenkins, D.; Thornley, J.P.; Hebden, J.M.; Wright, T.; Skinner, M.; Neal, K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef]
- Chen, M.; Gao, L.; Chen, P.; Feng, D.; Jiang, Y.; Chang, Y.; Jin, J.; Chu, F.F.; Gao, Q. Serotonin-Exacerbated DSS-Induced Colitis Is Associated with Increase in MMP-3 and MMP-9 Expression in the Mouse Colon. Mediat. Inflamm. 2016, 2016, 5359768. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Orexigenic effects of omentin-1 related to decreased CART and CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides 2013, 44, 66–74. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides 2014, 51, 115–121. [Google Scholar] [CrossRef]
- Lee, J.; Chang, C.; Liu, I.; Chi, T.; Yu, H.; Cheng, J. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Ronci, M.; Orlando, G.; Di Simone, S.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Politi, M.; Tirillini, B.; et al. Protective effects induced by alcoholic Phlomis fruticosa and Phlomis herba-venti extracts in isolated rat colon: Focus on antioxidant, anti-inflammatory, and antimicrobial activities in vitro. Phytother. Res. 2019, 33, 2387–2400. [Google Scholar] [CrossRef] [PubMed]
- Batshaw, M.L.; Hyman, S.L.; Coyle, J.T.; Robinson, M.B.; Qureshi, I.A.; Mellits, E.D.; Quaskey, S. Effect of sodium benzoate and sodium phenylacetate on brain serotonin turnover in the ornithine transcarbamylase-deficient sparse-fur mouse. Pediatr. Res. 1988, 23, 368–374. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef]
- Praticò, D.; Lee, V.M.Y.; Trojanoswki, J.Q.; Rokach, J.; FitzGerald, G.A. Increased F2-isoprostanes in Alzheimer’s disease: Evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998, 12, 1777–1783. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).