Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Apple Pomace Powder
2.3. Apple Pomace Powder Characterization
2.3.1. Physicochemical Analysis
2.3.2. Pectin Content
2.4. Apple Pomace Extract Characterization
2.4.1. Production of Apple Pomace Extract
2.4.2. Total Polyphenols and Flavonoids by Folin–Ciocalteu
2.4.3. Total Tannins by Folin–Ciocalteu
2.4.4. Total Carotenoids
2.4.5. Antioxidant Activity by Reaction with DPPH Radical
2.5. Production of Yogurt with Apple Pomace (YAP) Powder
2.6. Yogurt with Apple Pomace Powder Characterization
2.6.1. Physicochemical Analysis
2.6.2. Color Analysis
2.6.3. Antioxidant Analysis by Reaction with DPPH Radical
2.6.4. Sensory Analysis
2.6.5. Texture Profile Analysis
2.6.6. Syneresis
2.7. Mathematical Modeling
2.8. Statistical Analysis
3. Results
3.1. Apple Pomace Powder Characterization
3.1.1. Physicochemical Analysis of the Apple Pomace Powder
3.1.2. Antioxidant Compounds and Antioxidant Activity in Apple Pomace Powder
3.2. Physicochemical, Color and Antioxidant Analysis of Yogurt with Apple Pomace Powder
3.3. Evolution of the Yogurt with Apple Pomace Powder Characteristics during Storage
3.3.1. Sensory Analysis
3.3.2. pH Evolution
3.3.3. Texture Profile Analysis
3.3.4. Syneresis
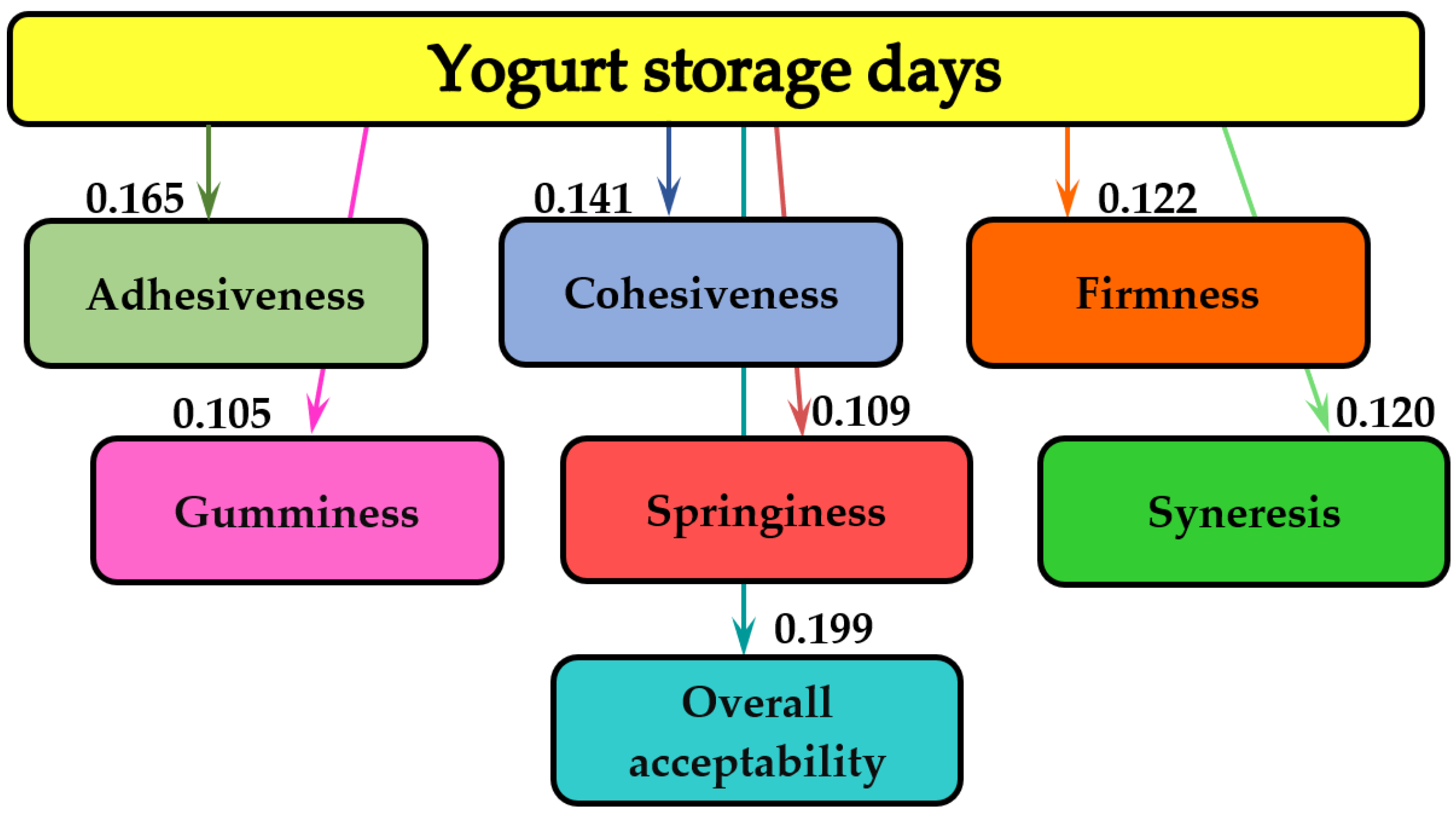
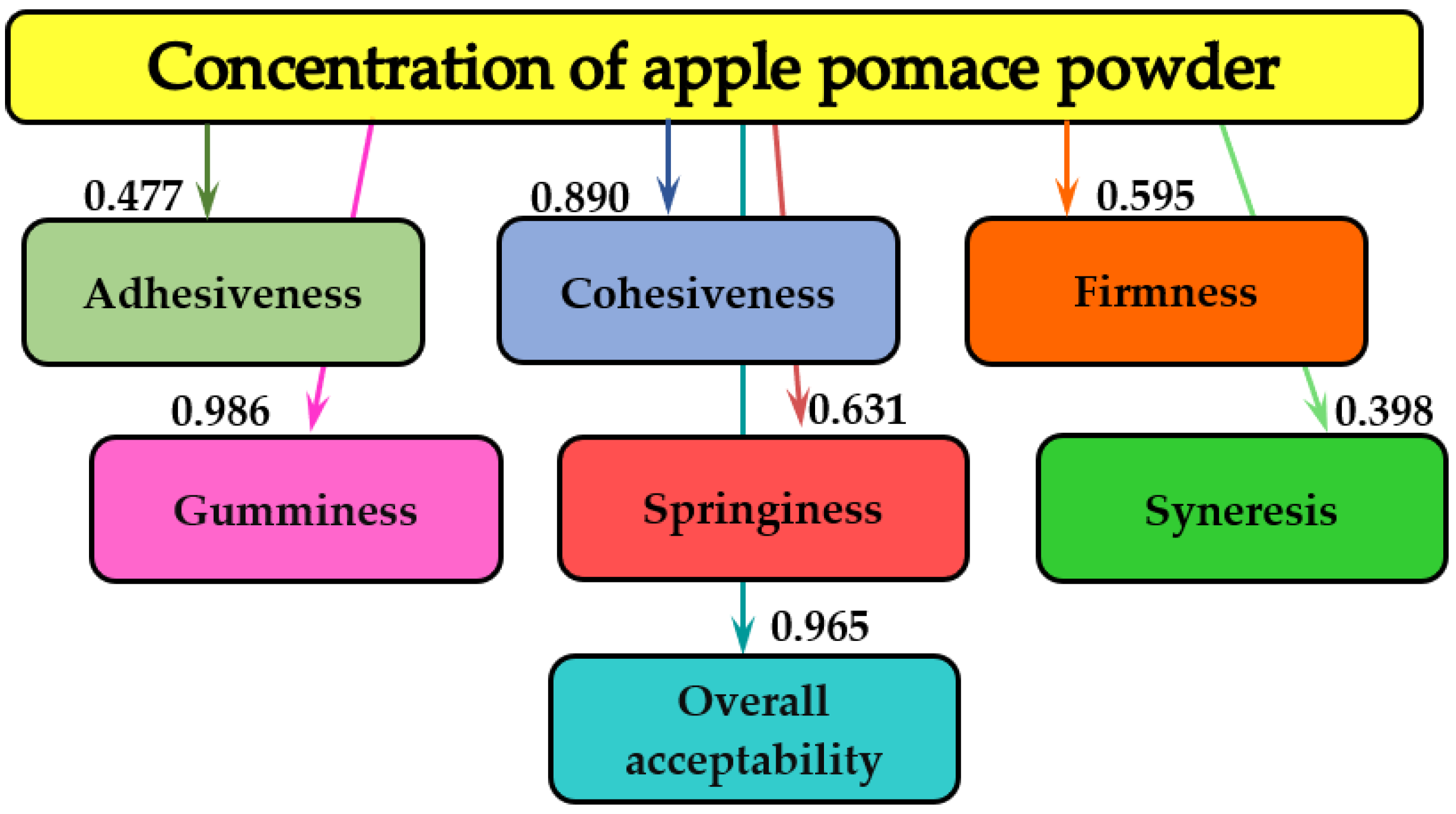
3.4. Mathematical Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dueñas, M.; García-Estévez, I. Agricultural and Food Waste: Analysis, Characterization and Extraction of Bioactive Compounds and Their Possible Utilization. Foods 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of Antioxidants from Apple Pomace by Supercritical Fluid Extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Adil, İ.H.; Çetin, H.İ.; Yener, M.E.; Bayındırlı, A. Subcritical (Carbon Dioxide+ethanol) Extraction of Polyphenols from Apple and Peach Pomaces, and Determination of the Antioxidant Activities of the Extracts. J. Supercrit. Fluids 2007, 43, 55–63. [Google Scholar] [CrossRef]
- Cruz, M.G.; Bastos, R.; Pinto, M.; Ferreira, J.M.; Santos, J.F.; Wessel, D.F.; Coelho, E.; Coimbra, M.A. Waste Mitigation: From an Effluent of Apple Juice Concentrate Industry to a Valuable Ingredient for Food and Feed Applications. J. Clean. Prod. 2018, 193, 652–660. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of Apple Processing Wastes as Low-Cost Substrates for Bioproduction of High Value Products: A Review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Will, F.; Olk, M.; Hopf, I. Characterization of Polyphenol Extracts from Apple Juice. Dtsch. Lebensm.-Rundsch. 2006, 102, 297–302. [Google Scholar]
- Lu, Y.; Yeap Foo, L. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Schieber, A.; Hilt, P.; Conrad, J.; Beifuss, U.; Carle, R. Elution Order of Quercetin Glycosides from Apple Pomace Extracts on a New HPLC Stationary Phase with Hydrophilic Endcapping. J. Sep. Sci. 2002, 25, 361–364. [Google Scholar] [CrossRef]
- Gómez-Gallego, C.; Gueimonde, M.; Salminen, S. The Role of Yogurt in Food-Based Dietary Guidelines. Nutr. Rev. 2018, 76, 29–39. [Google Scholar] [CrossRef]
- Das, K.; Choudhary, R.; Thompson-Witrick, K.A. Effects of New Technology on the Current Manufacturing Process of Yogurt-to Increase the Overall Marketability of Yogurt. LWT 2019, 108, 69–80. [Google Scholar] [CrossRef]
- Loveday, S.M.; Sarkar, A.; Singh, H. Innovative Yoghurts: Novel Processing Technologies for Improving Acid Milk Gel Texture. Trends Food Sci. Technol. 2013, 33, 5–20. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Witek, M.; Żmudziński, D.; Ptaszek, A. Changes in the Viscosity, Textural Properties, and Water Status in Yogurt Gel upon Supplementation with Green and Pu-Erh Teas. J. Dairy Sci. 2020, 103, 11039–11049. [Google Scholar] [CrossRef] [PubMed]
- Miocinovic, J.; Tomic, N.; Dojnov, B.; Tomasevic, I.; Stojanovic, S.; Djekic, I.; Vujcic, Z. Application of New Insoluble Dietary Fibres from Triticale as Supplement in Yoghurt—Effects on Physico-Chemical, Rheological and Quality Properties: Triticale Fibre as a Supplement in Yoghurt. J. Sci. Food Agric. 2018, 98, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Sendra, E.; Kuri, V.; Fernández-López, J.; Sayas-Barberá, E.; Navarro, C.; Pérez-Alvarez, J.A. Viscoelastic Properties of Orange Fiber Enriched Yogurt as a Function of Fiber Dose, Size and Thermal Treatment. LWT-Food Sci. Technol. 2010, 43, 708–714. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The Structure of the Casein Micelle of Milk and Its Changes during Processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Meng, Y.; Zhang, F.; Shao, Z.; Hu, L. Effect of the Modified High Methoxyl Pectin on the Stability of the Fermented Milk Beverage. Int. J. Food Prop. 2018, 21, 2075–2086. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, S.; Lim, J.; Streletskaya, N.A. Clean Label Trade-Offs: A Case Study of Plain Yogurt. Front. Nutr. 2021, 8, 704473. [Google Scholar] [CrossRef] [PubMed]
- ISO 750:1998; Fruit and Vegetable Products—Determination of Titratable Acidity. International Organization for Standardization: Geneva, Switzerland, 1998.
- Nollet, L.M.L. Handbook of Food Analysis, 2nd ed.; Rev. and Expanded. M. Dekker: New York, NY, USA, 2004; p. 912. ISBN 978-0-8247-5036-7. [Google Scholar]
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- AOAC 985.29; Total Dietary Fibre in Foods. Enzymatic-Gravimetric Method. Official Methods of Analysis, 14th ed. Association of Official Analytical Chemists: Washington, DC, USA, 1985.
- AOAC 991.42; Insoluble Dietary Fibre in Foods and Food Products. Enzymatic-Gravimetric Method. Official Methods of Analysis, 14th ed. Association of Official Analytical Chemists: Washington, DC, USA, 1985.
- Marcon, M.V.; Vriesmann, L.C.; Wosiacki, G.; Beleski-Carneiro, E.; Petkowicz, C.L. Pectin from Apple Pomace. Polímeros Ciênc. E Tecnol. 2005, 15, 127–129. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Talbaoui, A.; Moussaoui, N.E.; Abrini, J.; Bakri, Y. Phenolic Contents and Antiradical Capacity of Vegetable Oil from Pistacia Lentiscus (L). J. Mater. Environ. Sci. 2018, 9, 1518–1524. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publication: Oxford, UK, 1994; p. 248. [Google Scholar]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Niculaua, M. Rose Hips, a Valuable Source of Antioxidants to Improve Gingerbread Characteristics. Molecules 2020, 25, 5659. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen, S.; Pfander, H.P. Carotenoids, Vol. 1A: Isolation and Analysis; Birkhauser Verlag: Basel, Switzerland, 1995; pp. 104–107. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ISO 1211:2010|IDF 1:2010; Milk—Determination of Fat Content—Gravimetric Method (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 6731:2010|IDF 21:2010; Milk, Cream and Evaporated Milk—Determination of Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2010.
- Yilmaz-Ersan, L.; Topcuoglu, E. Evaluation of Instrumental and Sensory Measurements Using Multivariate Analysis in Probiotic Yogurt Enriched with Almond Milk. J. Food Sci. Technol. 2022, 59, 133–143. [Google Scholar] [CrossRef]
- Abd El-Fat, F.; Hassan Sal, H.; Mosbah El, S.; Samir El-S, H.; Abdel-Hady, H. Utilization of Natural Antimicrobial and Antioxidant of Moringa Oleifera Leaves Extract in Manufacture of Cream Cheese. J. Biol. Sci. 2018, 18, 92–106. [Google Scholar] [CrossRef] [Green Version]
- ISO 22935-3:2009|IDF 99-3:2009; Milk and Milk Products—Sensory Analysis—Part 3: Guidance on a Method for Evaluation of Compliance with Product Specifications for Sensory Properties by Scoring. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Decision of the Government of the Republic of Moldova No. 158 of 07.03.2019 Regarding the Approval of the Quality Requirements for Milk and Dairy Products. 2019. Available online: https://www.legis.md/cautare/getResults?doc_id=113282&lang=ro (accessed on 21 August 2022).
- Varnaitė, L.; Keršienė, M.; Šipailienė, A.; Kazernavičiūtė, R.; Venskutonis, P.R.; Leskauskaitė, D. Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production. Foods 2022, 11, 758. [Google Scholar] [CrossRef]
- Paninski, L. Estimation of Entropy and Mutual Information. Neural Comput. 2003, 15, 1191–1253. [Google Scholar] [CrossRef] [Green Version]
- Erinle, T.J.; Adewole, D.I. Fruit Pomaces—Their Nutrient and Bioactive Components, Effects on Growth and Health of Poultry Species, and Possible Optimization Techniques. Anim. Nutr. 2022, 9, 357–377. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef] [Green Version]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple Pomace as a Source of Dietary Fiber and Polyphenols and Its Effect on the Rheological Characteristics and Cake Making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Sato, M.D.F.; Rigoni, D.C.; Canteri, M.H.G.; Petkowicz, C.L.D.O.; Nogueira, A.; Wosiacki, G. Chemical and Instrumental Characterization of Pectin from Dried Pomace of Eleven Apple Cultivars. Acta Sci. Agron. 2011, 33, 383–389. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple Pomace as Food Fortification Ingredient: A Systematic Review and Meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional Properties, Phenolic Constituents and Antioxidant Potential of Industrial Apple Pomace for Utilization as Active Food Ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Hassan, Y.I.; Das, Q.; Lepp, D.; Hernandez, M.; Godfrey, D.V.; Orban, S.; Ross, K.; Delaquis, P.; Diarra, M.S. Dietary Organic Cranberry Pomace Influences Multiple Blood Biochemical Parameters and Cecal Microbiota in Pasture-Raised Broiler Chickens. J. Funct. Foods 2020, 72, 104053. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, J.; Xu, Y. Co-Preparation of Pectin and Cellulose from Apple Pomace by a Sequential Process. J. Food Sci. Technol. 2019, 56, 4091–4100. [Google Scholar] [CrossRef]
- Diñeiro García, Y.; Valles, B.S.; Picinelli Lobo, A. Phenolic and Antioxidant Composition of By-Products from the Cider Industry: Apple Pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Krasnova, I.; Segliņa, D. Content of Phenolic Compounds and Antioxidant Activity in Fresh Apple, Pomace and Pomace Water Extract—Effect of Cultivar. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2019, 73, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of Wild Apple (Malus Spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants 2018, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Sabater, C.; Abad-García, C.; Delgado-Fernández, P.; Corzo, N.; Montilla, A. Carbohydrate Fraction Characterisation of Functional Yogurts Containing Pectin and Pectic Oligosaccharides through Convolutional Networks. J. Food Compos. Anal. 2020, 90, 103484. [Google Scholar] [CrossRef]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. Optimisation of the Phenolic Content and Antioxidant Activity of Apple Pomace Aqueous Extracts. CyTA-J. Food 2015, 13, 293–299. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Wusigale; Liang, L.; Luo, Y. Casein and Pectin: Structures, Interactions, and Applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Yang, H.; Zhu, F.; Tang, D.; Cheng, J.; Liu, X. Changes of Phenolic Profile and Antioxidant Activity during Cold Storage of Functional Flavored Yogurt Supplemented with Mulberry Pomace. Food Control 2022, 132, 108554. [Google Scholar] [CrossRef]
- Ivanova, I.; Dimitrova, M.; Ivanov, G. Antioxidant Capacity of Yoghurt Fortified with Polyphenol Extract from Strawberry Pomace. J. Hyg. Eng. Des. 2021, 33, 101–107. [Google Scholar]
- Szołtysik, M.; Kucharska, A.Z.; Dąbrowska, A.; Zięba, T.; Bobak, Ł.; Chrzanowska, J. Effect of Two Combined Functional Additives on Yoghurt Properties. Foods 2021, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodrigues, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and Phytochemical Properties of Cold and Hot Water Extraction from Hibiscus Sabdariffa. J. Food Sci. 2011, 76, C428–C435. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt Enrichment with Grape Pomace: Effect of Grape Cultivar on Physicochemical, Microbiological and Sensory Properties: Grape Skin Flour and Yogurt Quality. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, M.; Zlatanović, S.; Micić, D.; Bacić, D.; Mitić-Ćulafić, D.; Đuriš, M.; Gorjanović, S. Functionality and Palatability of Yogurt Produced Using Beetroot Pomace Flour Granulated with Lactic Acid Bacteria. Foods 2021, 10, 1696. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology Food Science and Technology, 2nd ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; p. 291. ISBN 978-0-8247-2763-5. [Google Scholar]
- Wang, X.; Kristo, E.; LaPointe, G. The Effect of Apple Pomace on the Texture, Rheology and Microstructure of Set Type Yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-Based Prebiotics and Synbiotics: Potential for Cancer Chemoprevention. Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Delikanli, B.; Ozcan, T. Improving the Textural Properties of Yogurt Fortified with Milk Proteins: Textural Properties of Yogurt. J. Food Process. Preserv. 2017, 41, e13101. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Texture Profile Analysis of Yogurt as Influenced by Partially Hydrolyzed Guar Gum and Process Variables. J. Food Sci. Technol. 2017, 54, 3810–3817. [Google Scholar] [CrossRef]
- do Espírito Santo, A.P.; Perego, P.; Converti, A.; Oliveira, M.N. Influence of Milk Type and Addition of Passion Fruit Peel Powder on Fermentation Kinetics, Texture Profile and Bacterial Viability in Probiotic Yoghurts. LWT 2012, 47, 393–399. [Google Scholar] [CrossRef]
- Puvanenthiran, A.; Stevovitch-Rykner, C.; McCann, T.H.; Day, L. Synergistic Effect of Milk Solids and Carrot Cell Wall Particles on the Rheology and Texture of Yoghurt Gels. Food Res. Int. 2014, 62, 701–708. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine Grape Pomace as Antioxidant Dietary Fibre for Enhancing Nutritional Value and Improving Storability of Yogurt and Salad Dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Mahomud, M.S.; Haque, M.E. Heat-Induced Interaction of Milk Proteins: Impact on Yoghurt Structure. Int. J. Food Sci. 2021, 2021, 5569917. [Google Scholar] [CrossRef]
- Demirkol, M.; Tarakci, Z. Effect of Grape (Vitis Labrusca L.) Pomace Dried by Different Methods on Physicochemical, Microbiological and Bioactive Properties of Yoghurt. LWT 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Khubber, S.; Chaturvedi, K.; Thakur, N.; Sharma, N.; Yadav, S.K. Low-Methoxyl Pectin Stabilizes Low-Fat Set Yoghurt and Improves Their Physicochemical Properties, Rheology, Microstructure and Sensory Liking. Food Hydrocoll. 2021, 111, 106240. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Lopes, C.; Almeida, D.P.F.; Pintado, M. Incorporation of Strawberries Preparation in Yoghurt: Impact on Phytochemicals and Milk Proteins. Food Chem. 2015, 171, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Bulgaru, V.; Popescu, L.; Netreba, N.; Ghendov-Mosanu, A.; Sturza, R. Assessment of Quality Indices and Their Influence on the Texture Profile in the Dry-Aging Process of Beef. Foods 2022, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Padureanu, S.; Deseatnicova, O.; Turculet, N.; Boestean, O.; Niculaua, M. Potential Application of Hippophae Rhamnoides in Wheat Bread Production. Molecules 2020, 25, 1272. [Google Scholar] [CrossRef] [PubMed]
| Sensory Characteristics | Description |
|---|---|
| Appearance and consistency | The curd is fine, homogeneous, has a fluid consistency, without gas bubbles. A slight removal of whey, to a maximum of 2%, is allowed. |
| Color | White, yellowish-white or specific to the ingredients. |
| Odor | Specific to the type, with lactic fermentation characteristics, pleasant. With the odor of ingredients used in the case of those with added fruit. No foreign odor was allowed. |
| Taste | Specific to the type, with lactic fermentation characters, pleasant and sour. With the taste of ingredients used in the case of those with added fruit. No foreign taste was allowed. |
| Parameters | Value |
|---|---|
| Moisture, % Titrabile acidity, % expressed in malic acid Soluble solids content, oBrix Fat content, % Protein content, % Total dietary fibers content, % Pectin content, % Insoluble dietary fibers content, % Ash content, % | 7.84 ± 0.05 0.22 ± 0.01 15.82 ± 0.01 3.03 ± 0.18 5.27 ± 0.09 62.73 ± 1.46 23.12 ± 1.70 14.05 ± 0.44 1.67 ± 0.02 |
| Compounds | Value |
|---|---|
| Total polyphenol content, mg GAE/100 g DW Total flavonoid content, mg QE/100 g DW Total tannins, mg TAE/100 g DW Total carotenoids, mg/100 g DW Antioxidant activity (DPPH), µmol TE/100 g DW | 728.8 ± 25.5 246.5 ± 31.2 63.54 ± 5.71 4.93 ± 0.27 2433 ± 44.3 |
| Values | Samples | |||||
|---|---|---|---|---|---|---|
| Y | 0.2%YAP | 0.4%YAP | 0.6%YAP | 0.8%YAP | 1.0%YAP | |
| Total solids content, % | 14.40 ± 0.01 a | 14.55 ± 0.02 b | 14.69 ± 0.01 c | 14.82 ± 0.02 d | 14.89 ± 0.01 d | 15.04 ± 0.02 e |
| Fat content, % | 0.15 ± 0.002 a | 0.15 ± 0.001 a | 0.16 ± 0.002 b | 0.17 ± 0.001 c | 0.17 ± 0.001 c | 0.18 ± 0.002 d |
| Total dietary fibers content, % | n.d. | 0.13 ± 0.01 a | 0.25 ± 0.02 b | 0.38 ± 0.02 c | 0.50 ± 0.0 d | 0.63 ± 0.03 e |
| Insoluble dietary fiber content, % | n.d. | 0.03 ± 0.01 a | 0.06 ± 0.01 b,c | 0.08 ± 0.01 c | 0.11 ± 0.02 d,e | 0.14 ± 0.01 e |
| Luminance (L*) | 77.27 ± 0.10 d | 76.79 ± 0.04 d | 74.87 ± 0.02 c | 74.37 ± 0.08 c | 73.9 ± 0.02 b | 73.36 ± 0.05 a |
| Red/green component (a*) | −2.56 ± 0.04 a | −1.96 ± 0.02 b | −1.57 ± 0.02 c | −1.5 ± 0.03 c,d | −1.3 ± 0.06 d,e | −1.25 ± 0.03 e |
| Yellow/blue component (b*) | 6.27 ± 0.03 a | 6.35 ± 0.09 a,b | 6.59 ± 0.05 b | 7.56 ± 0.04 c | 7.5 ± 0.01 c | 8.06 ± 0.04 d |
| Total color differences (ΔE*) | - | 0.77 ± 0.05 a | 2.62 ± 0.12 b | 3.35 ± 0.07 c | 3.80 ± 0.08 d | 4.50 ± 0.04 e |
| Antioxidat Activitity (DPPH), μmol TE/100 g | 0.52 ± 0.03 a | 0.67 ± 0.07 a | 1.75 ± 0.14 a | 7.15 ± 0.83 b | 11.01 ± 1.96 c | 29.80 ± 0.79 d |
| Sensory Properties | Storage Period | Samples | |||||
|---|---|---|---|---|---|---|---|
| Y | 0.2%YAP | 0.4%YAP | 0.6%YAP | 0.8%YAP | 1.0%YAP | ||
| Appearance and consistency | 1 7 14 17 20 | 4.05 ± 0.04 f 4.05 ± 0.03 f 4.00 ± 0.05 e,f 3.96 ± 0.04 e 3.82 ± 0.01 d | 4.23 ± 0.06 h,i 4.23 ± 0.05 h,i 4.20 ± 0.07 g,h 4.20 ± 0.05 g,h 4.12 ± 0.04 f,g | 4.41 ± 0.02 j 4.41 ± 0.05 j 4.52 ± 0.04 k,l 4.54 ± 0.03 k,l 4.60 ± 0.03 l | 4.41 ± 0.03 j 4.41 ± 0.02 j 4.52 ± 0.04 k,l 4.54 ± 0.02 k,l 4.60 ± 0.03 l | 4.83 ± 0.05 n,o 4.83 ± 0.03 n,o 4.89 ± 0.04 o,p 4.92 ± 0.02 o,p 4.90 ± 0.01 o | 3.48 ± 0.04 b,c 3.48 ± 0.05 b,c 3.40 ± 0.06 b 3.38 ± 0.01 b 3.26 ± 0.02 a |
| Color | 1 7 14 17 20 | 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p | 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p | 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p | 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p | 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p 5.00 ± 0.00 p | 4.22 ± 0.03 g,h 4.21 ± 0.02 g,h 4.21 ± 0.05 g,h 4.20 ± 0.04 g,h 4.20 ± 0.04 g,h |
| Odor | 1 7 14 17 20 | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.62 ± 0.02 l 4.60 ± 0.03 l 4.56 ± 0.04 k,l | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.88 ± 0.03 o 4.84 ± 0.01 o 4.84 ± 0.01 o | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.92 ± 0.04 o,p 4.88 ± 0.02 o 4.86 ± 0.03 o | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.80 ± 0.02 n 4.80 ± 0.01 n 4.78 ± 0.03 n | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.80 ± 0.01 n 4.78 ± 0.02 n 4.72 ± 0.01 m | 4.64 ± 0.02 l,m 4.58 ± 0.02 l 4.48 ± 0.03 j,k 4.48 ± 0.04 j,k 4.24 ± 0.03 h |
| Taste | 1 7 14 17 20 | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.80 ± 0.02 n 4.60 ± 0.04 l 4.56 ± 0.03 k,l | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.86 ± 0.03 o 4.84 ± 0.02 n,o 4.76 ± 0.04 m,n | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.92 ± 0.04 o,p 4.88 ± 0.03 o 4.84 ± 0.04 n,o | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.90 ± 0.03 o,p 4.80 ± 0.02 n 4.64 ± 0.02 l,m | 5.00 ± 0.00 p 5.00 ± 0.00 p 4.88 ± 0.03 o 4.78 ± 0.04 n 4.68 ± 0.03 m | 4.57 ± 0.04 k,l 4.42 ± 0.02 j 4.36 ± 0.03 i,j 4.20 ± 0.04 g,h 4.00 ± 0.02 e,f |
| Overall acceptability | 1 7 14 17 20 | 4.76 ± 0.04 m,n 4.76 ± 0.03 m,n 4.61 ± 0.02 l 4.54 ± 0.02 k,l 4.49 ± 0.03 j,k | 4.81 ± 0.02 n,o 4.81 ± 0.01 n,o 4.74 ± 0.02 m,n 4.72 ± 0.01 m 4.68 ± 0.02 m | 4.85 ± 0.02 o 4.85 ± 0.01 o 4.84 ± 0.02 n,o 4.83 ± 0.02 n,o 4.83 ± 0.01 n,o | 4.96 ± 0.01 p 4.93 ± 0.02 o,p 4.90 ± 0.02 o,p 4.88 ± 0.01 o 4.82 ± 0.02 n,o | 4.96 ± 0.01 p 4.96 ± 0.01 p 4.89 ± 0.02 o 4.87 ± 0.02 o 4.83 ± 0.01 n,o | 4.23 ± 0.02 h 4.18 ± 0.01 g 4.11 ± 0.01 g 4.07 ± 0.02 e 3.93 ± 0.01 e |
| Parameter | Times | Samples | |||||
|---|---|---|---|---|---|---|---|
| Y | 0.2%YAP | 0.4%YAP | 0.6%YAP | 0.8%YAP | 1.0%YAP | ||
| Fermentation | |||||||
| pH | 0 h 2 h 4 h 6 h 7 h 7.5 h 8 h | 6.58 ± 0.02 n 6.42 ± 0.01 m 6.04 ± 0.02 k 5.16 ± 0.02 g 4.95 ± 0.03 f 4.85 ± 0.01 e 4.62 ± 0.02 d | 6.58 ± 0.01 n 6.42 ± 0.02 m 5.92 ± 0.01 j 5.02 ± 0.01 f 4.91 ± 0.03 f 4.82 ± 0.02 e 4.60 ± 0.04 c,d | 6.58 ± 0.02 n 6.38 ± 0.01 m 5.85 ± 0.02 i,j 4.92 ± 0.04 e,f 4.76 ± 0.01 e 4.62 ± 0.03 d n.d. | 6.58 ± 0.03 n 6.38 ± 0.02 m 5.85 ± 0.03 i,j 4.92 ± 0.01 f 4.76 ± 0.03 d,e 4.61 ± 0.01 d n.d. | 6.58 ± 0.01 n 6.38 ± 0.04 m 5.75 ± 0.01 i 4.86 ± 0.02 e 4.62 ± 0.02 d n.d. n.d. | 6.58 ± 0.02 n 6.20 ± 0.03 l 5.55 ± 0.01 h 4.85 ± 0.03 e 4.60 ± 0.04 c,d n.d. n.d. |
| Storage | |||||||
| pH | 1 day 7 day 14 day 17 day 20 day | 4.60 ± 0.02 d 4.58 ± 0.01 c,d 4.57 ± 0.03 c,d 4.50 ± 0.02 c 4.47 ± 0.03 c | 4.59 ± 0.05 d 4.57 ± 0.03 c,d 4.55 ± 0.02 c 4.50 ± 0.04 c 4.45 ± 0.01 c | 4.60 ± 0.02 d 4.56 ± 0.01 c 4.50 ± 0.03 c 4.47 ± 0.01 c 4.41 ± 0.02 b,c | 4.59 ± 0.01 d 4.52 ± 0.03 c 4.48 ± 0.02 c 4.42 ± 0.05 b,c 4.39 ± 0.01 b | 4.59 ± 0.03 c,d 4.46 ± 0.02 c 4.38 ± 0.03 b 4.35 ± 0.01 b 4.26 ± 0.04 a,b | 4.58 ± 0.01 c,d 4.42 ± 0.02 b,c 4.35 ± 0.02 b 4.30 ± 0.04 b 4.24 ± 0.03 a,b |
| Texture Parameters | Storage Period, Days | Samples | |||||
|---|---|---|---|---|---|---|---|
| Y | 0.2%YAP | 0.4%YAP | 0.6%YAP | 0.8%YAP | 1.0%YAP | ||
| Firmness, g | 1 7 14 17 20 | 1235.0 ± 29.2 a 1271.8 ± 42.4 a,b 1316.5 ± 25.5 a,b 1377.2 ± 31.8 b 1390.3 ± 41.7 b,c | 1297.3 ± 26.8 a,b 1307.6 ± 41.9 a,b 1321.1 ± 36.5 a,b 1329.3 ± 28.6 b 1339.0 ± 36.8 b | 1343.6 ± 31.5 b 1351.9 ± 42.9 b 1457.9 ± 53.9 c 1562.9 ± 48.4 d 1574.1 ± 38.1 d | 1442.2 ± 29.6 c 1483.4 ± 35.3 c,d 1646.4 ± 42.8 e 1736.5 ± 51.8 e,f 1804.9 ± 58.5 f,g | 1661.8 ± 46.7 e,f 1751.4 ± 49.1 e,f 2064.7 ± 41.3 i,j 2105.7 ± 49.6 i,j 2185.5 ± 43.7 j,k | 1944.5 ± 36.8 h 1992.4 ± 41.9 h,i 2191.2 ± 39.7 j,k 2269.9 ± 29.8 k 2244.5 ± 32.5 k |
| Springiness, % | 1 7 14 17 20 | 1.001 ± 0.001 a 1.004 ± 0.003 a 1.075 ± 0.005 a 1.093 ± 0.006 a 1.098 ± 0.002 a | 1.290 ± 0.005 b 1.319 ± 0.006 b 1.339 ± 0.008 b 1.391 ± 0.007 c 1.403 ± 0.011 c,d | 1.303 ± 0.008 b,c 1.300 ± 0.006 b,c 1.410 ± 0.012 c,d 1.431 ± 0.017 c 1.447 ± 0.021 c | 1.329 ± 0.012 b,c 1.391 ± 0.008 c 1.620 ± 0.019 e 1.920 ± 0.020 f 2.080 ± 0.027 g,h | 1.375 ± 0.009 c 1.495 ± 0.005 d 1.983 ± 0.018 f,g 2.148 ± 0.021 h 2.218 ± 0.027 h,i | 1.401 ± 0.011 c,d 1.607 ± 0.007 e 2.064 ± 0.016 g 2.182 ± 0.027 h 2.269 ± 0.030 i |
| Cohesiveness, % | 1 7 14 17 20 | 0.278 ± 0.001 a 0.280 ± 0.002 a 0.308 ± 0.003 a 0.320 ± 0.001 a 0.406 ± 0.004 b | 0.433 ± 0.009 b 0.443 ± 0.012 b 0.456 ± 0.014 b 0.463 ± 0.011 b 0.470 ± 0.018 b,c | 0.448 ± 0.014 b 0.534 ± 0.021 c 0.704 ± 0.028 d,e 0.760 ± 0.021 e 0.779 ± 0.024 e,f | 0.643 ± 0.003 d 0.745 ± 0.011 e 0.851 ± 0.019 f 0.859 ± 0.022 f 0.897 ± 0.025 f,g | 0.682 ± 0.009 d 0.868 ± 0.011 f 0.912 ± 0.021 g 0.983 ± 0.011 h 0.993 ± 0.017 h | 0.703 ± 0.011 d 0.959 ± 0.020 g,h 0.967 ± 0.018 g,h 0.986 ± 0.011 h 0.998 ± 0.006 h |
| Adhesiveness, g·s | 1 7 14 17 20 | 1477.9 ± 5.9 h 1368.3 ± 1.6 g 1291.5 ± 6.2 e 1227.0 ± 4.8 d 1212.8 ± 2.6 d | 1306.9 ± 5.7 f 1304.1 ± 3.6 f 1213.9 ± 2.9 d 1197.5 ± 2.3 c 1187.7 ± 4.8 c | 1299.3 ± 3.8 e,f 1293.3 ± 2.7 e 1200.6 ± 5.2 c,d 1186.7 ± 3.8 c 1175.3 ± 4.2 c | 1276.2 ± 4.6 e 1270.8 ± 3.1 e 1169.5 ± 2.8 b,c 1133.9 ± 2.5 a,b 1129.5 ± 3.3 a | 1232.1 ± 2.9 d 1223.1 ± 3.1 d 1134.7 ± 2.5 a,b 1129.5 ± 2.6 a 1114.0 ± 1.9 a | 1219.1 ± 6.2 d 1181.2 ± 5.4 c 1121.5 ± 4.7 a 1111.7 ± 6.1 a 1110.5 ± 2.9 a |
| Gumminess, % | 1 7 14 17 20 | 0.999 ± 0.002 j 0.965 ± 0.001 j 0.816 ± 0.006 h 0.801 ± 0.005 g,h 0.782 ± 0.004 g | 0.874 ± 0.004 i 0.845 ± 0.008 h 0.763 ± 0.010 g 0.771 ± 0.009 g 0.761 ± 0.006 g | 0.689 ± 0.005 f 0.669 ± 0.009 f 0.672 ± 0.011 f 0.642 ± 0.008 e,f 0.622 ± 0.013 e | 0.444 ± 0.011 d 0.438 ± 0.007 d 0.434 ± 0.009 c,d 0.424 ± 0.010 c,d 0.411 ± 0.007 c | 0.409 ± 0.006 c 0.405 ± 0.012 c 0.403 ± 0.009 c 0.377 ± 0.008 b,c 0.361 ± 0.011 b | 0.382 ± 0.012 b 0.372 ± 0.009 b,c 0.327 ± 0.005 a,b 0.319 ± 0.006 a,b 0.308 ± 0.011 a |
| Parameter | Storage Period, Days | Samples | |||||
|---|---|---|---|---|---|---|---|
| Y | 0.2%YAP | 0.4%YAP | 0.6%YAP | 0.8%YAP | 1.0%YAP | ||
| Syneresis, % | 1 7 14 17 20 | 26.65 ± 0.12 h 27.05 ± 0.32 i 27.39 ± 0.10 i 27.97 ± 0.04 j 28.13 ± 0.31 j | 25.72 ± 0.08 g 25.58 ± 0.11 g 24.89 ± 0.22 f 24.62 ± 0.09 e 24.46 ± 0.16 e | 24.94 ± 0.14 f 24.87 ± 0.12 f 24.33 ± 0.10 e 23.89 ± 0.09 d 23.63 ± 0.15 c,d | 24.55 ± 0.08 e 24.29 ± 0.05 e 23.96 ± 0.11 d 23.72 ± 0.07 c,d 23.16 ± 0.09 b,c | 24.48 ± 0.11 e 24.21 ± 0.09 d,e 23.61 ± 0.07 c,d 23.22 ± 0.05 c 22.53 ± 0.06 a | 24.38 ± 0.09 e 24.03 ± 0.07 d 23.39 ± 0.08 c 22.86 ± 0.11 b 22.18 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. https://doi.org/10.3390/foods11223565
Popescu L, Ceșco T, Gurev A, Ghendov-Mosanu A, Sturza R, Tarna R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods. 2022; 11(22):3565. https://doi.org/10.3390/foods11223565
Chicago/Turabian StylePopescu, Liliana, Tatiana Ceșco, Angela Gurev, Aliona Ghendov-Mosanu, Rodica Sturza, and Ruslan Tarna. 2022. "Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt" Foods 11, no. 22: 3565. https://doi.org/10.3390/foods11223565
APA StylePopescu, L., Ceșco, T., Gurev, A., Ghendov-Mosanu, A., Sturza, R., & Tarna, R. (2022). Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods, 11(22), 3565. https://doi.org/10.3390/foods11223565







