Impacts of the Dynamic High-Pressure Pre-Treatment and Post-Treatment of Whey Protein Aggregates on Their Physicochemical Properties and Emulsifying Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Materials
2.2. Preparation of a WPI Solution
2.3. Preparation of WPI Aggregates
2.4. Particle Characterization of WPI Aggregates
2.5. Fluorescence Spectroscopic Analysis of WPI Aggregates
2.6. Characterization of Emulsions Containing WPI Aggregates
2.7. Statistical Analysis
3. Results and Discussion
3.1. Influence of DHP Treatment on WPI Physicochemical Characteristics
3.2. Influence of DHP Pretreatment on the Physicochemical Characteristics of WPI Aggregates
3.3. Influence of DHP Post-Treatment on the Physicochemical Characteristics of the WPI Aggregates
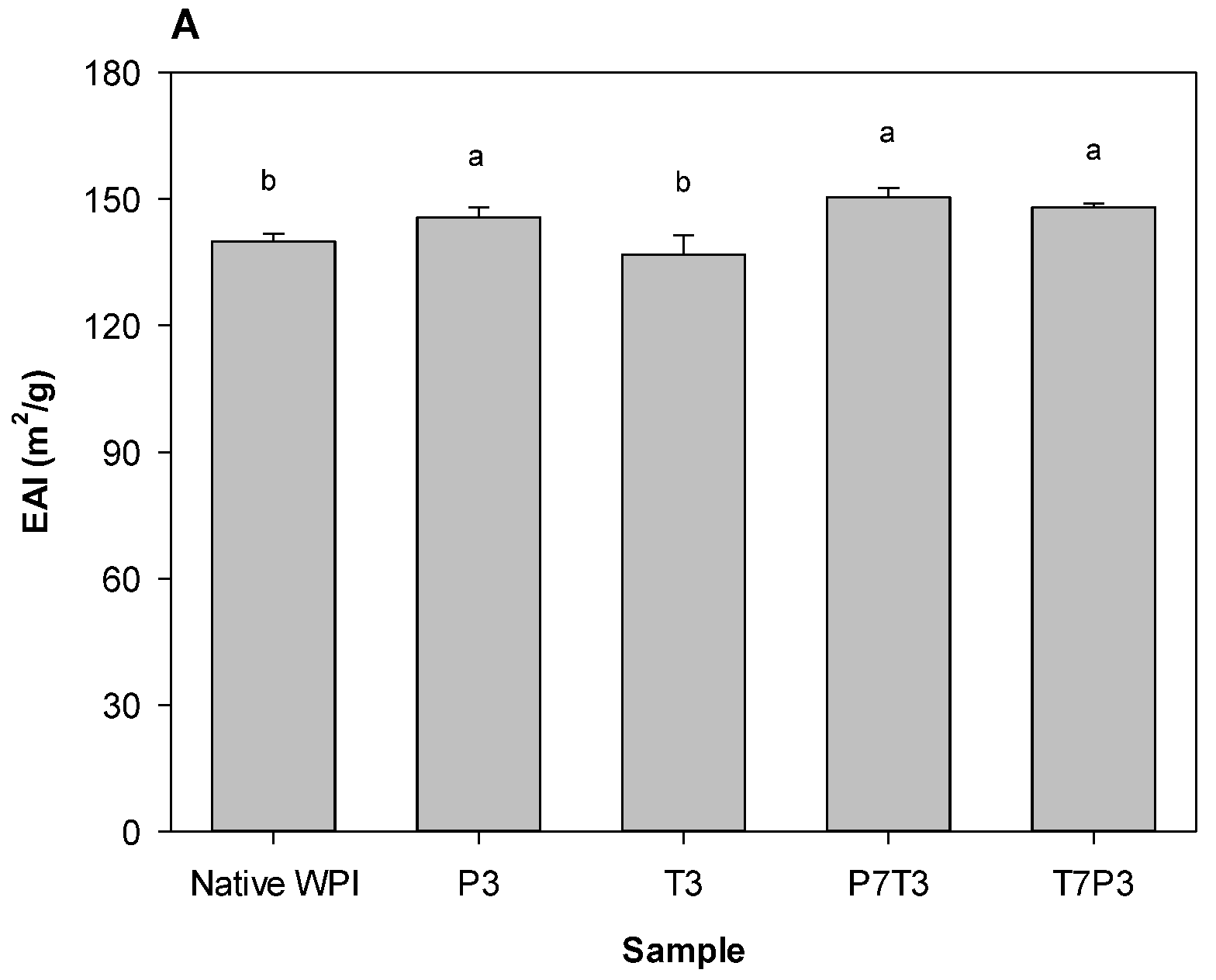
3.4. Emulsifying Properties of the WPI Aggregates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2020, 10, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Ubbink, J.; Krüger, J. Physical approaches for the delivery of active ingredients in foods. Trends Food Sci. Technol. 2006, 17, 244–254. [Google Scholar] [CrossRef]
- Peng, X.; Yao, Y. Carbohydrates as fat replacers. Annu. Rev. Food Sci. Technol. 2017, 8, 331–351. [Google Scholar] [CrossRef]
- Yashini, M.; Sunil, C.K.; Sahana, S.; Hemanth, S.D.; Chidanand, D.V.; Rawson, A. Protein-based fat replacer—A review of recent advances. Food Rev. Int. 2021, 37, 197–223. [Google Scholar]
- Clark, A.H.; Kavanagh, G.M.; Ross-Murphy, S.B. Globular protein gelation-theory and experiment. Food Hydrocoll. 2001, 15, 383–400. [Google Scholar] [CrossRef]
- Durand, D.; Gimel, J.C.; Nicolai, T. Aggregation, gelation and phase separation of heat denatured globular proteins. Phys. A Stat. Mech. Its Appl. 2002, 304, 253–265. [Google Scholar] [CrossRef]
- Gimel, C.J.; Durand, D.; Nicolai, T. Structure and distribution of aggregates formed after heat-induced denaturation of globular proteins. Macromolecules 1994, 27, 583–589. [Google Scholar] [CrossRef]
- Bolder, S.G.; Hendrickx, H.; Sagis, L.M.C.; van der Linden, E. Fibril assemblies in aqueous whey protein mixtures. J. Agric. Food Chem. 2006, 54, 4229–4234. [Google Scholar] [CrossRef]
- Oboroceanu, D.; Wang, L.; Brodkorb, A.; Magner, E.; Auty, M.A.E. Characterization of β-lactoglobulin fibrillar assembly using atomic force microscopy, polyacrylamide gel electrophoresis, and in situ Fourier transform infrared spectroscopy. J. Agric. Food Chem. 2010, 58, 3667–3673. [Google Scholar] [CrossRef]
- Farr, D. High pressure technology in the food industry. Trends Food Sci. Technol. 1990, 1, 14–16. [Google Scholar] [CrossRef]
- Zamora, A.; Guamis, B. Opportunities for ultra-high-pressure homogenisation (UHPH) for the food industry. Food Eng. Rev. 2015, 7, 130–142. [Google Scholar] [CrossRef]
- Guo, X.; Chen, M.; Li, Y.; Dai, T.; Shuai, X.; Chen, J.; Liu, C. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234. [Google Scholar] [CrossRef]
- Chawla, R.; Patil, G.R.; Singh, A.K. High hydrostatic pressure technology in dairy processing: A review. J. Food Sci. Technol. -Mysore 2011, 48, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.M.; He, Y.; Bhandari, B. Nano-emulsion production by sonication and microfluidization—A comparison. Int. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Maa, Y.-F.; Hsu, C.C. Performance of sonication and microfluidization for liquid-liquid emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar] [CrossRef]
- Dissanayake, M.; Vasiljevic, T. Functional properties of why proteins affected by heat treatment and hydrodynamic high-pressure shearing. J. Dairy Res. 2009, 92, 1387–1397. [Google Scholar]
- Shen, L.; Tang, C.-H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Djemaoune, Y.; Cases, E.; Saurel, R. The effect of high-pressure microfluidization treatment on the foaming properties of pea albumin aggregates. J. Food Sci. 2019, 84, 2242–2249. [Google Scholar] [CrossRef]
- Iordache, M.; Jelen, P. High pressure microfluidization treatment of heat denatured why proteins for improved functionality. Innov. Food Sci. Emerg. Technol. 2003, 4, 367–376. [Google Scholar] [CrossRef]
- Moll, P.; Salminen, H.; Schmitt, C.; Weiss, J. Impact of microfluidization on colloidal proeprties of insoluble pea protein fractions. Eur. Food Res. Technol. 2021, 247, 545–554. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S.; Salem, A. Factors affecting the functional properties of whey protein products: A review. Food Rev. Int. 2009, 25, 251–270. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P.; Doucet, D.; McGuffey, M.K. Advances in modifying and understanding whey protein functionality. Trends Food Sci. Technol. 2002, 13, 151–159. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, J.; Xiong, Y.L. Coomassie Brilliant Blue-binding: A simple and effective method for the determination of water-insoluble protein surface hydrophobicity. Anal. Methods 2016, 8, 790–795. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Fox, P.F. Milk: An Overview. In Milk Proteins: From Expression to Food, 2nd ed.; Singh, H., Boland, M., Thompson, A., Eds.; Academic Press: Waltham, MA, USA, 2014; pp. 19–73. [Google Scholar]
- Delavari, B.; Saboury, A.A.; Atri, M.S.; Ghasemi, A.; Bigdeli, B.; Khammari, A.; Maghami, P.; Moosavi-Movahedi, A.A.; Haertlé, T.; Goliaei, B. Alpha-lactalbumin: A new carrier for vitamin D3 food enrichment. Food Hydrocoll. 2015, 45, 124–131. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Huang, G.; Yan, L. Predicting protein surface property with its surface hydrophobicity. Protein Pept. Lett. 2021, 28, 938–944. [Google Scholar] [CrossRef]
- Alizadeh-Pasdar, N.; Li-Chan, E.C.Y. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef]
- Möller, M.; Denicola, A. Protein tryptophan accessibility studied by fluorescence quenching. Biochem. Mol. Biol. Educ. 2002, 30, 175–178. [Google Scholar] [CrossRef]
- Albani, J.R.; Vogelar, J.; Bretesche, B.; Kmiecik, D. Tryptophan 19 residue is the origin of bovine β-lactoglobulin fluorescence. J. Pharm. Biomed. Anal. 2014, 91, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhong, J.-Z.; Liu, W.; Tu, Z.-C.; Wan, J.; Cai, X.-F.; Song, X.-Y. Relationship between functional properties and aggregation changes of whey protein induced by high pressure microfluidization. J. Food Sci. 2011, 76, E341–E347. [Google Scholar] [CrossRef]
- Shi, R.; Li, T.; Li, M.; Munkh-Amgalan, G.; Qayum, A.; Bilawal, A.; Jiang, Z. Consequences of dynamic high-pressure homogenization pretreatment on the physicochemical and functional characteristics of citric acid-treated whey protein isolate. LWT-Food Sci. Technol. 2021, 136, 110303. [Google Scholar] [CrossRef]
- Considine, T.; Patel, H.A.; Anema, S.G.; Singh, H.; Creamer, L.K. Interactions of milk proteins during heat and high hydrostatic pressure treatments—A review. Innov. Food Sci. Emerg. Technol. 2007, 8, 1–23. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, P.; Zheng, X.; Hamzah, S.S.; Zeng, H.; Zhang, Y.; Hu, J. Effect of dynamic high pressure microfluidization on the solubility properties and structure profiles of proteins in water-insoluble fraction of edible bird’s nests. LWT-Food Sci. Technol. 2020, 132, 109923. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Liu, C.; Zhong, Y.; Liu, W.; Wan, J. Activation and conformational changes of mushroom polyphenoloxidase by high pressure microfluidization treatment. Innov. Food Sci. Emerg. Technol. 2009, 10, 142–147. [Google Scholar] [CrossRef]
- Majhi, P.R.; Ganta, R.R.; Vanam, R.P.; Seyrek, E.; Giger, K.; Dubin, P.L. Electrostatically driven protein aggregation: Beta-lactoglobin at low ionic strength. Langmuir 2006, 22, 9150–9159. [Google Scholar] [CrossRef]
- Verheul, m.; Roefs, S.P.F.M. Structure of particulate whey protein gels: Effect of NaCl concentration, pH, heating temperature, and protein composition. J. Agric. Food Chem. 1998, 46, 4909–4916. [Google Scholar] [CrossRef]
- Lam, C.W.Y.; Ikeda, S. Physical properties of heat-induced whey protein aggregates formed at pH 5.5 and 7.0. Food Sci. Technol. Res. 2017, 23, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Fu, W.; Luo, Y.; Cui, C.; Suppavorasatit, I.; Liang, L. Protein deamidation to produce processable ingredients and engineered colloids for emerging food applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3788–3817. [Google Scholar] [CrossRef]
- Bouaouina, H.; Desrumaux, A.; Loisel, C.; Legrand, J. Functional properties of whey proteins as affected by dynamic high-pressure treatment. Int. Dairy J. 2006, 16, 275–284. [Google Scholar] [CrossRef]
- Harshe, Y.M.; Lattuada, M. Breakage rate of colloidal aggregates in shear flow through Stokesian dynamics. Langmuir 2012, 28, 283–292. [Google Scholar] [CrossRef]
- Norde, W. Colloids and Interfaces in Life Sciences and Bionanotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- McClements, D.J. Emulsion Ingredients. In Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; McClements, D.J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 99–183. [Google Scholar]
- Tcholakova, S.; Denkov, N.D.; Ivanov, I.B.; Campbell, B. Coalescence stability of emulsions containing globular milk proteins. Adv. Colloid Interface Sci. 2006, 123–126, 259–293. [Google Scholar] [CrossRef]
- Ma, W.; Xie, F.; Zhang, S.; Wang, H.; Hu, M.; Sun, Y.; Zhong, M.; Zhu, J.; Qi, B.; Li, Y. Characterizing the structural and functional properties of soybean protein extracted from full-fat soybean flakes after low-temperature dry extrusion. Molecules 2018, 23, 3265. [Google Scholar] [CrossRef]

| pH of Sample | |||
|---|---|---|---|
| 3 | 5 | 7 | |
| Mean particle diameter (nm) | 183.05 ± 1.72 b | 3626.15 ± 362.06 a | 165.3 ± 3.7 b |
| ζ-potential (mV) | 11.17 ± 0.62 a | −5.03 ± 0.77 b | −20.27 ± 1.00 c |
| H0 (×106) | 92.38 ± 0.39 a | 21.63 ± 0.75 b | 15.92 ± 0.32 c |
| Imax (×106) | 62.74 ± 1.26 a | 54.15 ± 0.78 b | 49.16 ± 1.73 c |
| λmax (nm) | 338.7 ± 1.4 a | 338.3 ± 0.0 a | 337.5 ± 0.2 a |
| Sample | |||
|---|---|---|---|
| P3 | P5 | P7 | |
| Mean particle diameter (nm) | 176.5 ± 1.3 b | 4392.8 ± 281.1 a | 184.6 ± 1.1 b |
| ζ-potential (mV) | 12.42 ± 0.28 a | −4.03 ± 0.30 b | −21.36 ± 0.66 c |
| H0 (×106) | 94.11 ± 1.22 a | 21.53 ± 1.07 b | 15.92 ± 0.32 c |
| Imax (×106) | 63.80 ± 0.56 a | 53.53 ± 0.25 b | 49.11 ± 0.79 c |
| λmax (nm) | 338.7 ± 0.5 a | 338.2 ± 0.2 a | 337.8 ± 0.7 a |
| Sample | |||
|---|---|---|---|
| T3 | T5 | T7 | |
| Mean particle diameter (nm) | 214.5 ± 0.9 b | 5384.7 ± 1007.3 a | 236.5 ± 0.6 b |
| ζ-potential (mV) | 12.18 ± 1.11 a | −4.62 ± 0.69 b | −21.99 ± 0.67 c |
| H0 (×106) | 98.93 ± 5.79 b | 272.33 ± 0.32 a | 19.82 ± 0.07 c |
| Imax (×106) | 65.46 ± 1.88 b | 106.81 ± 1.13 a | 59.89 ± 2.79 c |
| λmax (nm) | 338.8 ± 0.2 c | 344.5 ± 0.2 a | 340.3 ± 0.0 b |
| Sample | |||
|---|---|---|---|
| P7T3 | P7T5 | P7T7 | |
| Mean particle diameter (nm) | 178.1 ± 0.6 b | 3045.0 ± 2062.0 a | 178.1 ± 0.8 b |
| ζ-potential (mV) | 9.07 ± 0.89 a | −5.82 ± 1.37 b | −21.07 ± 1.55 c |
| H0 (×106) | 114.40 ± 1.34 b | 233.34 ± 6.09 a | 20.30 ± 1.26 c |
| Imax (×106) | 68.56 ± 0.80 b | 109.07 ± 4.98 a | 58.51 ± 0.83 c |
| λmax (nm) | 339.0 ± 0.0 c | 344.3 ± 0.0 a | 340.2 ± 0.2 b |
| Sample | |||
|---|---|---|---|
| T7P3 | T7P5 | T7P7 | |
| Mean particle diameter (nm) | 169.1 ± 1.8 b | 2114.8 ± 115.6 a | 170.8 ± 1.9 b |
| ζ-potential (mV) | 9.14 ± 1.27 a | −4.39 ± 0.85 b | −19.41 ± 1.03 c |
| H0 (×106) | 223.43 ± 2.28 a | 78.62 ± 1.92 b | 20.62 ± 0.12 c |
| Imax (×106) | 64.82 ± 1.77 b | 70.73 ± 0.86 a | 59.62 ± 2.32 c |
| λmax (nm) | 339.8 ± 0.2 a | 340.3 ± 0.5 a | 340.3 ± 0.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, E.H.; Kwon, C.W.; Choi, S.J. Impacts of the Dynamic High-Pressure Pre-Treatment and Post-Treatment of Whey Protein Aggregates on Their Physicochemical Properties and Emulsifying Activities. Foods 2022, 11, 3588. https://doi.org/10.3390/foods11223588
Yoo EH, Kwon CW, Choi SJ. Impacts of the Dynamic High-Pressure Pre-Treatment and Post-Treatment of Whey Protein Aggregates on Their Physicochemical Properties and Emulsifying Activities. Foods. 2022; 11(22):3588. https://doi.org/10.3390/foods11223588
Chicago/Turabian StyleYoo, Eun Hee, Chang Woo Kwon, and Seung Jun Choi. 2022. "Impacts of the Dynamic High-Pressure Pre-Treatment and Post-Treatment of Whey Protein Aggregates on Their Physicochemical Properties and Emulsifying Activities" Foods 11, no. 22: 3588. https://doi.org/10.3390/foods11223588





