The Impact of Different Hydrocolloids on the Viscoelastic Properties and Microstructure of Processed Cheese Manufactured without Emulsifying Salts in Relation to Storage Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacture of Processed Cheese Samples
2.3. Basic Chemical Analysis
2.4. Rheological Measurements of Processed Cheese Samples
2.5. Scanning Electron Microscopy of Processed Cheese Samples
2.6. Statistical Analysis
3. Results and Discussion
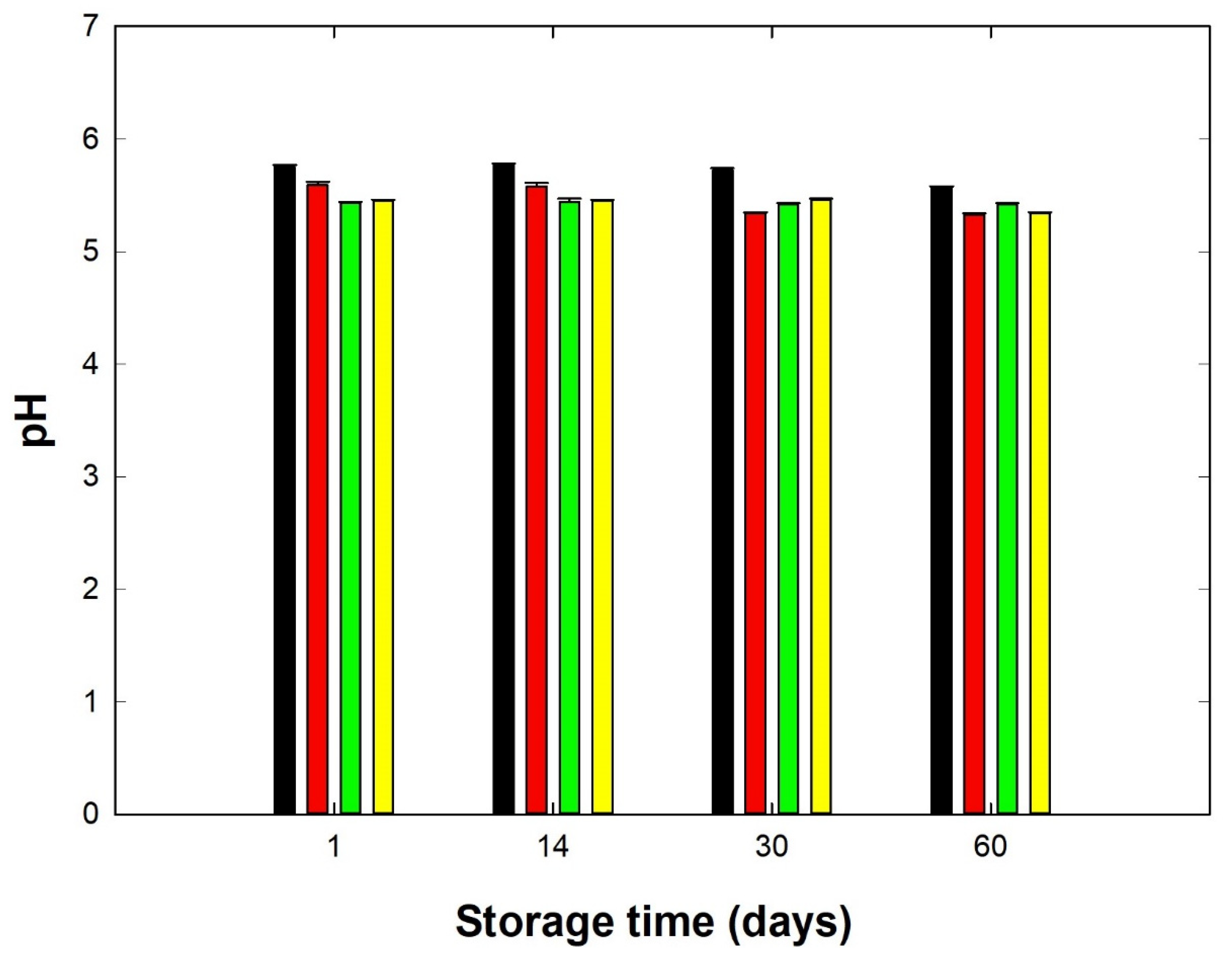
3.1. Basic Chemical Analysis of Processed Cheese Samples
3.2. Rheological Measurements
3.3. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini-Parvar, S.H.; Matia-Merino, L.; Golding, M. Effect of basil seed gum (BSG) on textural, rheological and microstructural properties of model processed cheese. Food Hydrocoll. 2015, 43, 557–567. [Google Scholar] [CrossRef]
- Hanaei, F.; Cuvelier, G.; Sieffermann, J.M. Consumer texture descriptions of a set of processed cheese. Food Qual. Prefer. 2015, 40, 316–325. [Google Scholar] [CrossRef]
- Salek, R.N.; Černíková, M.; Lorencová, E.; Pachlová, V.; Kůrová, V.; Šenkýřová, J.; Buňka, F. The impact of Cheddar or white brined cheese with various maturity degrees on the processed cheese consistency: A comparative study. Int. Dairy J. 2020, 111, 104816. [Google Scholar] [CrossRef]
- Salek, R.N.; Lorencová, E.; Míšková, Z.; Lazárková, Z.; Pachlová, V.; Adámek, R.; Bezděková, K.; Buňka, F. The impact of Chios mastic gum on textural, rheological and melting properties of spread-type processed cheese during storage. Int. Dairy J. 2020, 109, 104755. [Google Scholar] [CrossRef]
- Černíková, M.; Nebesářová, J.; Salek, R.N.; Popková, R.; Buňka, F. The effect of rework content addition on the microstructure and viscoelastic properties of processed cheese. J. Dairy Sci. 2018, 101, 2956–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, R.; Metzger, L.E. Process cheese: Scientific and technological aspects—A review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 194–214. [Google Scholar] [CrossRef]
- Salek, R.N.; Vašina, M.; Lapčík, L.; Černíková, M.; Lorencová, E.; Li, P.; Buňka, F. Evaluation of various emulsifying salts addition on selected properties of processed cheese sauce with the use of mechanical vibration damping and rheological methods. LWT 2019, 107, 178–184. [Google Scholar] [CrossRef]
- Salek, R.N.; Černíková, M.; Pachlová, V.; Bubelová, Z.; Konečná, V.; Buňka, F. Properties of spreadable processed Mozzarella cheese with divergent compositions of emulsifying salts in relation to the applied cheese storage period. LWT 2017, 77, 30–38. [Google Scholar] [CrossRef]
- Lucey, J.A.; Johnson, M.E.; Horne, D.S. Invited Review: Perspectives on the Basis of the Rheology and Texture Properties of Cheese. J. Dairy Sci. 2003, 86, 2725–2743. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, Y. Influence of “creaming” on the properties of processed cheese and changes in the structure of casein during cheese making. Milchwissenschaft 2008, 63, 149–153. [Google Scholar]
- Schäffer, B.; Lőrinczy, D.; Belágyi, J. DSC and electronmicroscopic investigation of dispersion-type processed cheese made without peptization. J. Therm. Anal. Calorim. 1999, 56, 1211–1216. [Google Scholar] [CrossRef]
- Schäffer, B.; Szakály, S.; Lőrinczy, D. Processed cheeses made with and without peptization. J. Therm. Anal. Calorim. 2001, 64, 671–679. [Google Scholar] [CrossRef]
- Kůrová, V.; Salek, R.N.; Černíková, M.; Lorencová, E.; Zalešáková, L.; Buňka, F. Furcellaran as a substitute for emulsifying salts in processed cheese spread and the resultant storage changes. Int. J. Dairy Technol. 2022, 75, 679–689. [Google Scholar] [CrossRef]
- Černíková, M.; Buňka, F.; Pospiech, M.; Tremlová, B.; Hladká, K.; Pavlínek, V.; Březina, P. Replacement of traditional emulsifying salts by selected hydrocolloids in processed cheese production. Int. Dairy J. 2010, 20, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Hladká, K.; Randulová, Z.; Tremlová, B.; Ponížil, P.; Mančík, P.; Černíková, M.; Buňka, F. The effect of cheese maturity on selected properties of processed cheese without traditional emulsifying agents. Food Sci. Technol. 2014, 55, 650–656. [Google Scholar] [CrossRef]
- Černíková, M.; Nebesářová, J.; Salek, R.N.; Řiháčková, L.; Buňka, F. Microstructure and textural and viscoelastic properties of model processed cheese with different dry matter and fat in dry matter content. J. Dairy Sci. 2017, 100, 4300–4307. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, E.; Eliot, C. Defining the conditions for heat-induced gelation of a caseinate-stabilized emulsion. Colloids Surf. B 2003, 29, 89–97. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Pluta-Kubica, A.; Černíková, M.; Dimitreli, G.; Nebesářová, J.; Exarhopoulos, S.A.; Thomareis, S.; Salek, R.N.; Buňka, F. Influence of the melt holding time on fat droplet size and the viscoelastic properties of model spreadable processed cheeses with different compositions. Int. Dairy J. 2021, 113, 104880. [Google Scholar] [CrossRef]
- ISO Standard No. 5534; Cheese and Processed Cheese—Determination of the Total Solid Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO Standard No. 1735; Cheese and Processed Cheese Products—Determination of Fat Content—Gravimetric Method (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- Černíková, M.; Salek, R.N.; Kozáčková, D.; Běhalová, H.; Luňáková, L.; Buňka, F. The effect of selected processing parameters on viscoelastic properties of model processed cheese spreads. Int. Dairy J. 2017, 66, 84–90. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, S. Strain and strain rate dependence of gellan, agar and agar–gellan gels as model systems. J. Food Eng. 2014, 141, 93–98. [Google Scholar] [CrossRef]
- Verbeken, D.; Thas, O.; Dewettinck, K. Textural properties of gelled dairy desserts containing κ-carrageenan and starch. Food Hydrocoll. 2004, 18, 817–823. [Google Scholar] [CrossRef]
- Piska, I.; Štětina, J. Influence of cheese ripening and rate of cooling of the processed cheese mixture on rheological properties of processed cheese. J. Food Eng. 2004, 61, 551–555. [Google Scholar] [CrossRef]
- Černíková, M.; Buňka, F.; Pavlínek, V.; Březina, P.; Hrabě, J.; Valášek, P. Effect of carrageenan type on viscoelastic properties of processe cheese. Food Hydrocoll. 2008, 22, 1054–1061. [Google Scholar] [CrossRef]
- Imeson, A. (Ed.) Agar. In Food Stabilisers, Thickeners and Gelling Agents, 1st ed.; Wiley-Blackwell Pub.: Chichester, UK, 2010; pp. 31–49. [Google Scholar]
- Venugopal, V. Carrageenans. In Marine Polysaccharides: Food Applications; Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 341–344. [Google Scholar]
- Laneuville, S.I.; Turgeon, S.L. Microstructure and stability of skim milk acid gels containing an anionic bacterial exopolysaccharide and commercial polysaccharides. Int. Dairy J. 2014, 37, 5–15. [Google Scholar] [CrossRef]
- Stevens, P. Gelatine. In Food Stabilisers, Thickeners and Gelling Agents; Imeson, A., Ed.; Wiley-Blackwell Publisher: Chichester, UK, 2010; pp. 116–144. [Google Scholar]
- Barth, A.P.; Tormena, C.F.; Viotto, W.H. PH influences hydrolysis of sodium polyphosphate in dairy matrices and the structure of processed cheese. J. Dairy Sci. 2017, 100, 8735–8743. [Google Scholar] [CrossRef] [Green Version]
- Awad, R.A.; Abdel-Hamid, L.B.; El-Shabrawy, S.A.; Singh, R.K. Texture and microstructure of block type processed cheese with formulated emulsifying salt mixtures. LWT 2002, 35, 54–61. [Google Scholar] [CrossRef]
- Imeson, A.P. Carrageenan and furcellaran. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Elsevier, Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 164–185. [Google Scholar]
- Noronha, N.; O’Riordan, E.D.; O’Sullivan, M. Influence of processing parameters on the texture and microstructure of imitation cheese. Eur. Food Res. Technol. 2008, 226, 385–393. [Google Scholar] [CrossRef]





| Raw Materials Processing Parameters | Composition % (w/w) | |||
|---|---|---|---|---|
| CS * | AG * | CR * | PG * | |
| Raw materials | ||||
| Water | 35.4 | 33.2 | 33.2 | 33.2 |
| Edam cheese | 43.1 | 47.7 | 47.7 | 47.7 |
| Butter | 19.0 | 18.1 | 18.1 | 18.1 |
| Emulsifying salts ** | 2.5 | none | none | none |
| Hydrocolloid | none | 1.0 | 1.0 | 1.0 |
| Processing parameters | ||||
| Stirring speed (rpm) | 3000 | 3000 | 3000 | 3000 |
| Holding time (s) | 60 | 60 | 60 | 60 |
| Target temperature (°C) | 90 | 90 | 90 | 90 |
| Total time (min) | 11 | 11 | 11 | 11 |
| Storage Time (days) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 30 | 60 | |||||||||
| Hydrocolloid | G* (Pa) | ŋ (Pa∙s) | tan δ | G* (Pa) | ŋ (Pa∙s) | tan δ | G* (Pa) | ŋ (Pa∙s) | tan δ | G* (Pa) | ŋ (Pa∙s) | tan δ |
| CS * | 7445 ± 197 a,A | 1335 ± 2 a,A | 0.6 a,A | 8866 ± 213 a,B | 1257 ± 2 a,B | 0.6 a,A | 9441 ± 268 a,C | 1075 ± 3 a,C | 0.5 a,B | 10778 ± 314 a,D | 148 ± 1 a,D | 0.5 a,B |
| AG * | 44159 ± 576 b,A | 1624 ± 3 b,A | 0.2 b,A | 39817 ± 512 b,B | 2758 ± 3 b,B | 0.2 b,A | 38523 ± 451 b,B | 3437 ± 3 b,C | 0.2 b,A | 36950 ± 425 b,C | 4129 ± 3 b,D | 0.1 b,B |
| CR * | 85682 ± 931 c,A | 6286 ± 4 c,A | 0.2 b,A | 80343 ± 889 c,B | 7450 ± 3 c,B | 0.2 b,A | 77249 ± 817 c,C | 10692 ± 4 c,C | 0.2 b,A | 75521 ± 756 c,D | 12016 ± 4 c,D | 0.1 b,B |
| PG * | 10504 ± 278 d,A | 1011 ± 2 d,A | 0.3 c,A | 8090 ± 195 d,B | 1081 ± 2 d,B | 0.4 c,B | 7687 ± 187 d,B | 1242 ± 2 d,C | 0.4 c,B | 5369 ± 138 d,C | 2016 ± 2 d,D | 0.5 c,C |
| Size of Fat Globules | ||||
|---|---|---|---|---|
| Hydrocolloid | Mean (μm) | Minimum Size (μm) | Maximum Size (μm) | Difference (μm) |
| CS * | 0.199 ± 0.015 a | 0.029 ± 0.034 a | 0.790 ± 0.052 a | 0.761 ± 0.018 a |
| AG * | 0.756 ± 0.084 b | 0.135 ± 0.071 b | 4.583 ± 0.098 b | 4.448 ± 0.027 b |
| CR * | 0.761 ± 0.056 b | 0.145 ± 0.028 b | 2.507 ± 0.067 c | 2.362 ± 0.039 c |
| PG * | 0.490 ± 0.062 c | 0.052 ± 0.049 c | 3.890 ± 0.073 d | 3.838 ± 0.024 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratochvílová, A.; Salek, R.N.; Vašina, M.; Lorencová, E.; Kůrová, V.; Lazárková, Z.; Dostálová, J.; Šenkýřová, J. The Impact of Different Hydrocolloids on the Viscoelastic Properties and Microstructure of Processed Cheese Manufactured without Emulsifying Salts in Relation to Storage Time. Foods 2022, 11, 3605. https://doi.org/10.3390/foods11223605
Kratochvílová A, Salek RN, Vašina M, Lorencová E, Kůrová V, Lazárková Z, Dostálová J, Šenkýřová J. The Impact of Different Hydrocolloids on the Viscoelastic Properties and Microstructure of Processed Cheese Manufactured without Emulsifying Salts in Relation to Storage Time. Foods. 2022; 11(22):3605. https://doi.org/10.3390/foods11223605
Chicago/Turabian StyleKratochvílová, Alena, Richardos Nikolaos Salek, Martin Vašina, Eva Lorencová, Vendula Kůrová, Zuzana Lazárková, Jolana Dostálová, and Jana Šenkýřová. 2022. "The Impact of Different Hydrocolloids on the Viscoelastic Properties and Microstructure of Processed Cheese Manufactured without Emulsifying Salts in Relation to Storage Time" Foods 11, no. 22: 3605. https://doi.org/10.3390/foods11223605





