Shedding Light on Metals Release from Chestnut Wood to Wine Spirit Using ICP-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Wine Spirit Samples
2.2. Multi-Elemental Analysis of WSs by ICP-MS
2.3. Statistical Analysis
3. Results
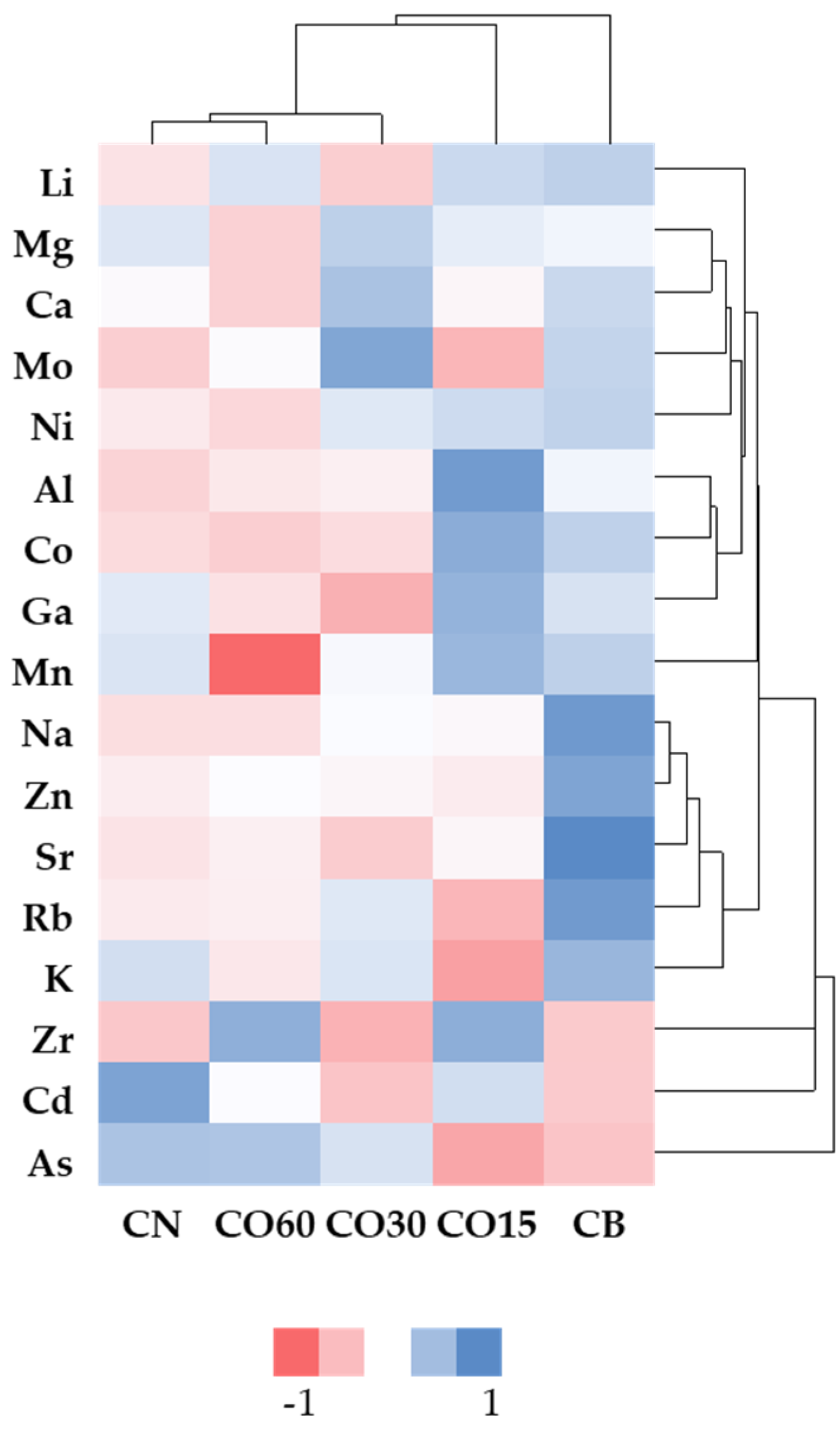
3.1. Mineral Elements Concentration during Ageing Experiment
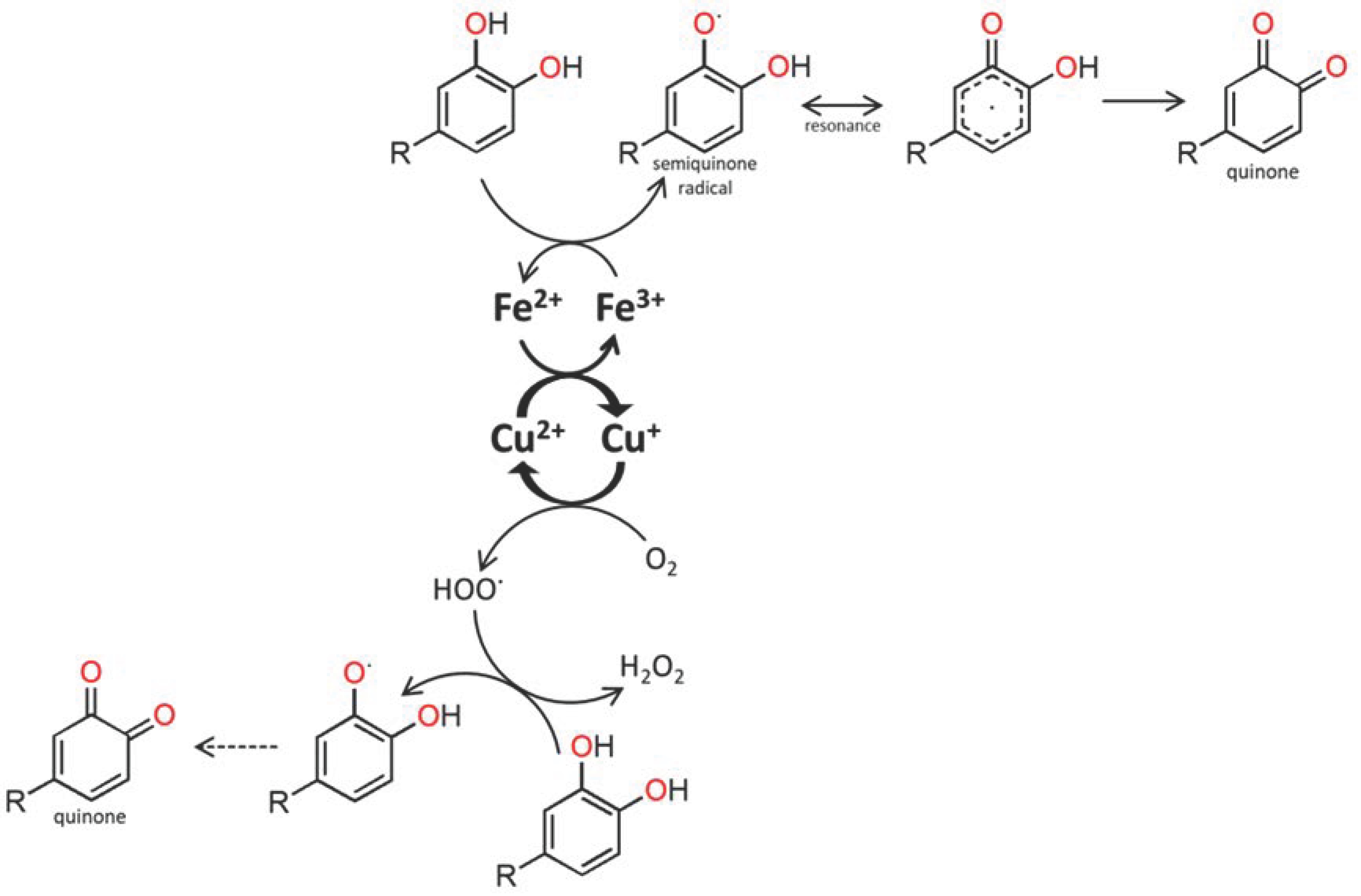
3.1.1. Solubility Properties to Consider
3.1.2. Alkaline and Alkaline Earth Elements
3.1.3. Heavy Metals and Other Elements
3.2. Global Behaviour of Mineral Elements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catarino, S.; Curvelo-Garcia, A.S.; Bruno de Sousa, R. Revisão: Elementos contaminantes nos vinhos. Ciência Téc. Vitiv. 2008, 23, 3–19. [Google Scholar]
- Lafon, R.; Lafon, J.; Couillaud, P. Le Cognac: Sa Distillation; J.B. Baillière et Fils Editeures: Paris, France, 1964; p. 270. [Google Scholar]
- Canas, S.; Danalache, F.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Santos, N.; Fargeton, L.; Boissier, B.; Catarino, S. Behaviour of low molecular weight compounds, iron and copper of wine spirit aged with chestnut staves under different levels of micro-oxygenation. Molecules 2020, 25, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bonić, M.; Tešević, V.; Nikićević, N.; Cvejić, J.; Milosavljević, S.; Vajs, V.; Mandić, B.; Urošević, I.; Veličković, M.; Jovanić, S. Heavy metals content in Serbian old plum brandies. J. Serb. Chem. Soc. 2013, 78, 1–19. [Google Scholar] [CrossRef]
- Szymczycha-Madeja, A.; Welna, M.; Jamroz, P.; Lesniewicz, A.; Pohl, P. Advances in assessing the elemental composition of distilled spirits using atomic spectrometry. Trends Anal. Chem. 2015, 64, 127–135. [Google Scholar] [CrossRef]
- Buglass, A.J. Chemical Composition of Beverages and Drinks. In Handbook of Food Chemistry; Cheung, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–62. [Google Scholar]
- Clark, A.C.; Wilkes, E.N.; Scollary, G.R. Chemistry of copper in white wine: A review. Aust. J. Grape Wine Res. 2015, 21, 339–350. [Google Scholar] [CrossRef]
- Cameán, A.M.; Moreno, I.; López-Artíguez, M.; Repetto, M.; González, A.G. Differentiation of Spanish brandies according to their metal content. Talanta 2001, 54, 53–59. [Google Scholar] [CrossRef]
- Kokkinofta, R.; Petrakis, P.V.; Mavromoustakos, T.; Theocharis, C.R. Authenticity of the traditional Cypriot spirit “Zivania” on the basis of metal content using a combination of coupled plasma spectroscopy and statistical analysis. J. Agric. Food Chem. 2003, 51, 6233–6239. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N. Metals in alcoholic beverages: A review of sources, effects, concentrations, removal, speciation, and analysis. J. Food Compos. Anal. 2008, 21, 672–683. [Google Scholar] [CrossRef]
- Ceballos-Magaña, S.G.; Jurado, J.M.; Martín, M.J.; Pablos, F. Quantitation of twelve metals in tequila and mezcal spirits as authenticity parameters. J. Agric. Food Chem. 2009, 57, 1372–1376. [Google Scholar] [CrossRef]
- Hopfer, H.; Gilleland, G.; Ebeler, S.E.; Nelson, J. Elemental profiles of whisk(e)y allow differentiation by type and region. Beverages 2017, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Pawlaczyk, A.; Gajek, M.; Jozwik, K.; Szynkowska, M.I. Multielemental analysis of various kinds of whisky. Molecules 2019, 24, 1193. [Google Scholar] [CrossRef] [PubMed]
- Magdas, D.A.; Cristea, G.; Pîrnau, A.; Feher, I.; Hategan, A.R.; Dehelean, A. Authentication of Transylvanian spirits based on isotope and elemental signatures in conjunction with statistical methods. Foods 2021, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Wyk, T.N.; van Jaarsveld, F.; Caleb, O.J. Metal concentrations in grape spirits. S. Afr. J. Enol. Vitic. 2021, 42, 36–43. [Google Scholar] [CrossRef]
- Grembecka, M.; Szefer, P. Metals and metalloids in foods: Essentiality, toxicity, applicability. In Food Quality: Control, Analysis and Consumers Concerns; Medina, D.A., Laine, A.M., Eds.; Nova Science Publishers, Inc.: Hauppauge, New York, USA, 2011; pp. 1–60. [Google Scholar]
- Flora, S.J.S.; Agrawal, S. Arsenic, cadmium, and lead. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, Massachusetts, USA, 2017; pp. 537–566. [Google Scholar] [CrossRef]
- Cameán, A.M.; Moreno, I.M.; López-Artíguez, M.; Repetto, M.; González, A.G. Metallic profiles of Sherry brandies. Sci. Aliment. 2000, 20, 433–440. [Google Scholar] [CrossRef]
- Bettin, S.M.; Isique, W.; Franco, D.W.; Andersen, M.L.; Knudsen, S.; Skibsted, L.H. Phenols and metals in sugar-cane spirits. Quantitative analysis and effect on radical formation and radical scavenging. Eur. Food Res. Technol. 2002, 215, 169–175. [Google Scholar] [CrossRef]
- Catarino, S.; Pinto, D.; Curvelo-Garcia, A.S. Validação e comparação de métodos de análise em espectrofotometria de absorção atómica com chama para doseamento de cobre e ferro em vinhos e aguardentes. Ciência Téc. Vitiv. 2003, 18, 65–76. [Google Scholar]
- Ivanova-Petropulos, V.; Balabanova, B.; Bogeva, E.; Frentiu, T.; Ponta, M.; Senila, M.; Gulaboski, R.; Irimie, F.D. Rapid determination of trace elements in Macedonian grape brandies for their characterization and safety evaluation. Food Anal. Methods 2017, 10, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Pál, L.; Muhollari, T.; Bujdosó, O.; Baranyai, E.; Nagy, A.; Árnyas, E.; Ádány, R.; Sándor, J.; McKee, M.; Szűcs, S. Heavy metal contamination in recorded and unrecorded spirits. Should we worry? Regul. Toxicol. Pharmacol. 2020, 116, 1–10. [Google Scholar] [CrossRef]
- Tamasi, G.; Donati, A.; Leone, G.; Magnani, A.; Cini, R.; Macchia, E.; Rossi, C.; Bonechi, C. Grappa quality from the Chianti and Montepulciano areas (Tuscany, Italy): Monitoring the leaching of copper from distillation columns. Int. J. Food Sci. Technol. 2018, 53, 1558–1565. [Google Scholar] [CrossRef]
- Cacho, J.; Castells, J.E.; Esteban, A.; Laguna, B.; Sagristá, N. Iron, copper, and manganese influence on wine oxidation. Am. J. Enol. Vitic. 1995, 46, 380–384. [Google Scholar]
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar]
- Elias, R.J.; Waterhouse, A.L. Controlling the Fenton reaction in wine. J. Agric. Food Chem. 2010, 58, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wine. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Chemistry of manganese and interaction with iron and copper in wine. Am. J. Enol. Vitic. 2016, 67, 377–384. [Google Scholar] [CrossRef]
- Lima, C.M.G.; Benoso, P.; Pierezan, M.D.; Santana, R.F.; De Souza Hassemer, G.; da Rocha, R.A.; Nora, F.M.D.; Verruck, S.; Caetano, D.; Simal-Gandara, J. A state-of-the-art review of the chemical composition of sugarcane spirits and current advances in quality control. J. Food Compos. Anal. 2022, 106, 1–10. [Google Scholar] [CrossRef]
- Cruz, S.; Canas, S.; Belchior, A. Effect of ageing system and time on the quality of wine brandy aged at industrial-scale. Ciência Téc. Vitiv. 2012, 27, 83–93. [Google Scholar]
- Canas, S.; Anjos, O.; Caldeira, I.; Fernandes, T.A.; Santos, N.; Lourenço, S.; Granja-Soares, J.; Fargeton, L.; Boissier, B.; Catarino, S. Micro-oxygenation level as a key to explain the variation in the colour and chemical composition of wine spirits aged with chestnut wood staves. LWT Food Sci. Technol. 2022, 154, 1–8. [Google Scholar] [CrossRef]
- Canas, S. Phenolic composition and related properties of aged wine spirits: Influence of barrel characteristics. A review. Beverages 2017, 3, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Canas, S.; Caldeira, I.; Belchior, A.P. Comparison of alternative systems for the ageing of wine brandy. Wood shape and wood botanical species effect. Ciência Téc. Vitiv. 2009, 24, 91–99. [Google Scholar]
- Caldeira, I.; Anjos, O.; Portal, V.; Belchior, A.P.; Canas, S. Sensory and chemical modifications of wine-brandy aged with chestnut and oak wood fragments in comparison to wooden barrels. Anal. Chim. Acta 2010, 660, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Caldeira, I.; Belchior, A.P.; Spranger, M.I.; Clímaco, M.C.; Bruno-de-Sousa, R. Chestnut Wooden Barrels for the Ageing of Wine Spirits. International Organization of Vine and Wine. 2018. Available online: http://www.oiv.int/en/technical-standards-and-documents/collective-expertise/spirit-beverages (accessed on 15 August 2022).
- Fengel, D.; Wegner, G. Wood. Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989; p. 612. [Google Scholar]
- Kaya, A.D.; Bruno de Sousa, R.; Curvelo-Garcia, A.S.; Ricardo-da-Silva, J.M.; Catarino, S. Effect of wood aging on wine mineral composition and 87Sr/86Sr isotopic ratio. J. Agric. Food Chem. 2017, 65, 4766–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilet, A.; De Sousa, R.B.; Ricardo-da-Silva, J.M.; Catarino, S. Barrel-to-barrel variation of phenolic and mineral composition of red wine. BIO Web Conf. 2019, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Smailagić, A.; Zagorac, D.D.; Veljović, S.; Sredojević, M.; Relić, D.; Akšić, M.F.; Roglić, G.; Natić, M. Release of wood extractable elements in experimental spirit model: Health risk assessment of the wood species generated in Balkan cooperage. Food Chem. 2021, 338, 127804. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Caldeira, I.; Belchior, A.P. Extraction/oxidation kinetics of low molecular weight compounds in wine brandy resulting from different ageing technologies. Food Chem. 2013, 138, 2460–2467. [Google Scholar] [CrossRef]
- Caldeira, I.; Santos, R.; Ricardo-da-Silva, J.; Anjos, O.; Belchior, A.P.; Canas, S. Kinetics of odorant compounds in wine brandies aged in different systems. Food Chem. 2016, 211, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés, S. Assessment of minerals in aged grape marc distillates by FAAS/FAES and ICP-MS. Characterization and safety evaluation. Food Control 2014, 35, 49–55. [Google Scholar] [CrossRef]
- Granja-Soares, J.; Roque, R.; Cabrita, M.J.; Anjos, O.; Belchior, A.P.; Caldeira, I.; Canas, S. Effect of innovative technology using staves and micro-oxygenation on the sensory and odorant profile of aged wine spirit. Food Chem. 2020, 333, 127450. [Google Scholar] [CrossRef]
- Caldeira, I.; Vitória, C.; Anjos, O.; Fernandes, T.A.; Gallardo, E.; Fargeton, L.; Boissier, B.; Catarino, S.; Canas, S. Wine spirit ageing with chestnut staves under different micro-oxygenation strategies: Effects on the volatile compounds and sensory profile. Appl. Sci. 2021, 11, 2–15. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Antunes, A.M.M.; Caldeira, I.; Anjos, O.; de Freitas, V.; Fargeton, L.; Boissier, B.; Catarino, S.; Canas, S. Identification of gallotannins and ellagitannins in aged wine spirits: A new perspective using alternative ageing technology and high-resolution mass spectrometry. Food Chem. 2022, 382, 132322. [Google Scholar] [CrossRef]
- Regulation EU 2019/787. Definition, description, presentation and labelling of spirit drinks, the use of the names of spirit drinks in the presentation and labelling of other foodstuffs, the protection of geographical indications for spirit drinks, the use of ethyl alcohol and distillates of agricultural origin in alcoholic beverages, and repealing Regulation (EC) No 110/2008. Off. J. Eur. Union 2019, L130, 1–54. [Google Scholar]
- Canas, S.; Anjos, O.; Caldeira, I.; Belchior, A.P. Phenolic profile and colour acquired by the wine spirit in the beginning of ageing: Alternative technology using micro-oxygenation vs traditional technology. LWT Food Sci. Technol. 2019, 11, 260–269. [Google Scholar] [CrossRef]
- Montaser, A. Inductively Coupled Plasma Mass Spectrometry; Wiley-WCH: New York, NY, USA, 1998; p. 964. [Google Scholar]
- Catarino, S.; Curvelo-Garcia, A.S.; Bruno de Sousa, R. Evaluation of contaminant elements in Portuguese wines and original musts by inductively coupled plasma mass spectrometry. Talanta 2006, 70, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, R.H.; Herring, F.H.; Madura, J.D.; Bissonnette, C. General Chemistry: Principles and Modern Applications, 10th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Suponik, T.; Blanco, B. Removal of heavy metals from groundwater affected by acid mine drainage. Physicochem. Probl. Miner. Process. 2014, 50, 359–372. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. The Chemistry of Wine. Stabilization and Treatments, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006; p. 441. [Google Scholar]
- Catarino, S.; Madeira, M.; Monteiro, F.; Caldeira, I.; Bruno de Sousa, R.; Curvelo-Garcia, A.S. Mineral composition through soil-wine system of Portuguese vineyards and its potential for wine traceability. Beverages 2018, 4, 85. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, D.R.; Andrade-Sobrinho, L.G.; Leite-Neto, A.F.; Reche, R.V.; Isique, W.D.; Ferreira, M.M.C.; Lima-Neto, B.S.; Franco, D.W. Comparison between cachaça and rum using pattern recognition methods. J. Agric. Food Chem. 2004, 52, 3429–3433. [Google Scholar] [CrossRef]
- Nascimento, R.F.; Bezerra, C.W.B.; Furuya, S.M.B.; Schultz, M.S.; Polastro, L.R.; Lima Neto, B.S.; Franco, D.W. Mineral profile of Brazilian cachaças and other international spirits. J. Food Compos. Anal. 1999, 12, 17–25. [Google Scholar] [CrossRef]
- McKinnon, A.J.; Scollary, G.R.; Solomon, D.H.; Williams, P.J. The influence of wine components on the spontaneous precipitation of calcium L(+)—Tartrate in a model wine solution. Am. J. Enol. Vitic. 1995, 46, 509–517. [Google Scholar]
- Lopez, F.F.; Cabrera, C.; Lorenzo, M.L.; Lopez, M.L. Aluminium levels in wine, beer and other alcoholic beverages consumed in Spain. Sci. Total Environ. 1998, 220, 1–9. [Google Scholar] [CrossRef]
- Catarino, S.; Curvelo-Garcia, A.S.; Bruno de Sousa, R. Determination of aluminum in wine by graphite furnace AAS: Validation of analytical method. At. Spectrosc. 2002, 23, 196–200. [Google Scholar]
- Flores, C.R.; Figueroa, J.A.L.; Wrobel, K.; Wrobel, K. ICP-MS multi-element profiles and HPLC determination of furanic compounds in commercial tequila. Eur. Food Res. Technol. 2009, 228, 951–958. [Google Scholar] [CrossRef]
- Pfahl, L.; Catarino, S.; Fontes, N.; Graça, A.; Ricardo-da-Silva, J. Effect of barrel-to-barrel variation on color and phenolic composition of a red wine. Foods 2021, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2021; Volume 2. [Google Scholar]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.; Ferraria, A.M.; Galvão, A.M.; Botelho do Rego, A.M.; Suárez, A.C.M.; Carvalho, M.F.N.N. Synthesis, characterization and study of properties of camphor Zn(II) complexes. J. Organomet. Chem. 2014, 760, 186–196. [Google Scholar] [CrossRef]
- OIV. OIV Collective Expertise: Arsenic and Wine: A Review; International Organisation of Vine and Wine: Paris, France, 2021. [Google Scholar]
- Reis, P.M.; Romão, C.C.; Royo, B. Dioxomolybdenum(VI) complexes as catalysts for the hydrosilylation of aldehydes and ketones. Dalton Trans. 2006, 15, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.; Romão, C.C. Silane/MoO2Cl2 as an efficient system for the reduction of esters. J. Mol. Catal. A 2006, 253, 96–98. [Google Scholar] [CrossRef]
- OIV. OIV Collective Expertise: Lead and Wine: A Review; International Organisation of Vine and Wine: Paris, France, 2020. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.Z.; Sadegh, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]


| Time (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Modality | 0 | 21 | 60 | 180 | 270 | 365 | |||
| Li (µg/L) |  | CB | 1.40 ± 0.05 | 1.2 ± 0.3 | 1.5 ± 0.3 | 2.5 ± 0.8 | 3 ± 1 | 3.2 ± 0.8 |  |
 | O15 | 1.40 ± 0.05 a | 1.8 ± 0.2 a | 1.9 ± 0.5 a | 3.2 ± 0.4 b | 4.4 ± 0.2 c | 3.0 ± 0.5 b | ||
 | O30 | 1.40 ± 0.05 a | 1.1 ± 0.3 a | 1.2 ± 0.1 a | 1.8 ± 0.2 b | 2.6 ± 0.1 c | 2.1 ± 0.3 b | ||
 | C60 | 1.40 ± 0.05 | 1.5 ± 0.9 | 1.6 ± 0.8 | 3 ± 1 | 3 ± 2 | 3 ± 2 | ||
 | N | 1.40 ± 0.05 | 1.1 ± 0.5 | 1.2 ± 0.5 | 2 ± 1 | 2 ± 1 | 2 ± 1 | ||
| Na (µg/L) |  | CB | 698 ± 51 ab | 611 ± 6 a C | 406 ± 143 a | 920 ± 154 abc | 1249 ± 154 bc | 1459 ± 371 c | 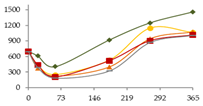 |
 | O15 | 698 ± 51 c | 425 ± 5 ab B | 249 ± 72 a | 529 ± 7 bc | 1146 ± 200 d | 1061 ± 123 d | ||
 | O30 | 698 ± 51 b | 379 ± 11 a A | 222 ± 59 a | 400 ± 41 a | 943 ± 159 bc | 1075 ± 13 c | ||
 | C60 | 698 ± 51 bc | 437 ± 9 ab B | 207 ± 20 a | 519 ± 19 b | 903 ± 254 cd | 1013 ± 38 d | ||
 | N | 698 ± 51 b | 392 ± 10 a A | 185 ± 69 a | 324 ± 39 a | 872 ± 235 bc | 1012 ± 69 c | ||
| Mg (µg/L) |  | CB | 128 ± 11 a | 603 ± 133 bc C | 389 ± 3 ab B | 799 ± 42 cd | 1017 ± 147 d | 1413 ± 248 e |  |
 | O15 | 128 ± 11 a | 351 ± 63 a AB | 234 ± 2 a A | 731 ± 122 b | 1368 ± 254 c | 1440 ± 99 c | ||
 | O30 | 128 ± 11 a | 264 ± 79 a A | 248 ± 8 a A | 668 ± 112 b | 1097 ± 16 c | 1544 ± 374 d | ||
 | C60 | 128 ± 11 a | 286 ± 19 a AB | 235 ± 19 a A | 749 ± 242 b | 852 ± 306 b | 1310 ± 202 c | ||
 | N | 128 ± 11 a | 470 ± 16 ab BC | 275 ± 83 ab A | 724 ± 70 bc | 1069 ± 413 cd | 1464 ± 252 d | ||
| K (µg/L) |  | CB | 6 ± 1 a | 306 ± 110 bc | 173 ± 26 ab | 420 ± 99 bc | 524 ± 166 c | 506 ± 109 c | 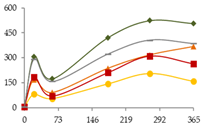 |
 | O15 | 6 ± 1 a | 81 ± 3 bc | 56 ± 27 ab | 144 ± 19 cd | 206 ± 28 d | 159 ± 48 d | ||
 | O30 | 6 ± 1 a | 167 ± 54 abc | 92 ± 51 ab | 236 ± 111 bcd | 317 ± 137 cd | 367 ± 17 d | ||
 | C60 | 6 ± 1 | 185 ± 148 | 71 ± 55 | 211 ± 154 | 310 ± 158 | 264 ± 142 | ||
 | N | 6 ± 1 | 292 ± 299 | 158 ± 157 | 322 ± 258 | 406 ± 314 | 386 ± 303 | ||
| Ca (µg/L) |  | CB | nd | 433 ± 195 a | 936 ± 96 ab | 4218 ± 5 c | 1517 ± 478 ab | 1966 ± 1122 b | 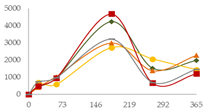 |
 | O15 | nd | 677 ± 76 a | 572 ± 53 a | 2714 ± 154 b | 2053 ± 241 b | 1438 ± 1070 ab | ||
 | O30 | nd | 405 ± 33 a | 1031 ± 306 ab | 2985 ± 38 c | 1372 ± 594 b | 2262 ± 48 c | ||
 | C60 | nd | 490 ± 117 a | 941 ± 426 a | 4674 ± 16 b | 652 ± 568 a | 1198 ± 1099 a | ||
 | N | nd | 732 ± 675 | 964 ± 361 | 3216 ± 1973 | 695 ± 821 | 1464 ± 302 | ||
| Rb (ng/L) |  | CB | 151 ± 28 a | 433 ± 146 bc B | 352 ± 41 abB | 507 ± 116 bcd | 689 ± 116 d | 643 ± 114 cd | 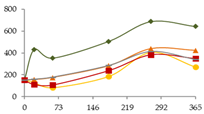 |
 | O15 | 151 ± 28 a b | 128 ± 15 ab A | 81 ± 20 a A | 185 ± 23 ab | 405 ± 76 c | 270 ± 143 bc | ||
 | O30 | 151 ± 28 | 156 ± 20 A | 175 ± 90 A | 283 ± 81 | 440 ± 123 | 424 ± 139 | ||
 | C60 | 151 ± 28 a | 111 ± 13 a A | 106 ± 34 a A | 238 ± 131 ab | 383 ± 71 b | 348 ± 93 b | ||
 | N | 151 ± 28 | 160 ± 44 A | 178 ± 47 A | 286 ± 129 | 416 ± 214 | 342 ± 59 | ||
| Sr (ng/L) |  | CB | 544 ± 40 a | 3531 ± 680 bc B | 2586 ± 170 b B | 3607 ± 108 bc B | 5177 ± 1416 c B | 3726 ± 739 bc B | 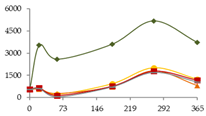 |
 | O15 | 544 ± 40 | 623 ± 119 A | 218 ± 153 A | 928 ± 127 A | 2006 ± 929 A | 1238 ± 820 A | ||
 | O30 | 544 ± 40 a | 549 ± 107 a A | 259 ± 12 a A | 725 ± 222 aA | 1802 ± 376 b A | 804 ± 343 a A | ||
 | C60 | 544 ± 40 ab | 645 ± 116 b A | 139 ± 46 a A | 765 ± 144 bc A | 1777 ± 63 d A | 1169 ± 409 c A | ||
 | N | 544 ± 40 b | 609 ± 190 bc A | 52 ± 25 a A | 720 ± 48 bc A | 1688 ± 320 d A | 1048 ± 249 c A | ||
| Time (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Modality | 0 | 21 | 60 | 180 | 270 | 365 | |||
| Al (µg/L) |  | CB | 5.5 ± 0.8 a | 8 ± 2 a | - | 21 ± 2 b | 24 ± 3 b A | 32 ± 2 c | 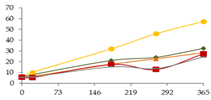 |
 | O15 | 5.5 ± 0.8 | 10 ± 2 | - | 32 ± 7 | 46 ± 14 B | 57 ± 27 | ||
 | O30 | 5.5 ± 0.8 a | 6 ± 3 a | - | 18 ± 3 b | 22.7 ± 0.5 bc A | 28 ± 4 c | ||
 | C60 | 5.5 ± 0.8 a | 5.3 ± 0.5 a | - | 18 ± 1 b | 13 ± 1 b A | 27 ± 4 c | ||
 | N | 5.5 ± 0.8 a | 6.0 ± 0.3 a | - | 15 ± 4 ab | 14 ± 5 ab A | 25 ± 8 b | ||
| Mn (µg/L) |  | CB | 1.5 ± 0.1 a | 13 ± 1 b C | 18 ± 2 b D | 37 ± 3 c | 42 ± 4 cd | 47 ± 4 d | 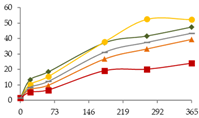 |
 | O15 | 1.5 ± 0.1 a | 10 ± 1 ab BC | 15.4 ± 0.2 b CD | 38 ± 6 c | 52 ± 9 d | 52 ± 5 d | ||
 | O30 | 1.5 ± 0.1 a | 7 ± 2 a AB | 10 ± 2 a AB | 27 ± 6 b | 33 ± 6 b | 39 ± 13 b | ||
 | C60 | 1.5 ± 0.1 a | 5 ± 2 a A | 7 ± 2 a A | 19 ± 6 b | 20 ± 2 b | 24 ± 5 b | ||
 | N | 1.5 ± 0.1 a | 8.4 ± 0.1 b AB | 12.2 ± 0.6 b BC | 31 ± 5 c | 37 ± 2 d | 43 ± 1 e | ||
| Co (ng/L) |  | CB | 149 ± 7 c | 23 ± 30 a | 64 ± 11 ab BC | 122 ± 15 bc B | 153 ± 43 c A | 149 ± 20 c | 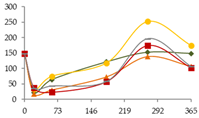 |
 | O15 | 149 ± 7 bc | 34 ± 15 a | 75 ± 20 a C | 118 ± 22 b B | 253 ± 20 d B | 174 ± 12 c | ||
 | O30 | 149 ± 7 c | 18 ± 14 a | 30 ± 4 a A | 73 ± 1 ab A | 139.0 ± 0.3 c A | 107 ± 63 bc | ||
 | C60 | 149 ± 7 c | 38 ± 5 a | 23 ± 10 a A | 58 ± 24 a A | 174 ± 21 c A | 102 ± 25 b | ||
 | N | 149 ± 7 bc | 37 ± 20 a | 44 ± 6 a AB | 58.4 ± 0.1 a A | 197 ± 15 c AB | 107 ± 33 b | ||
| Ni (µg/L) |  | CB | 5.4 ± 0.3 d | 2.323 ± 0.004 bc | 1.49 ± 0.08 a B | 1.49 ± 0.08 a B | 2.6 ± 0.2 c | 1.6 ± 0.5 ab | 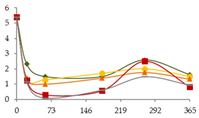 |
 | O15 | 5.4 ± 0.3 c | 1.25 ± 0.06 a | 1.3 ± 0.2 b B | 1.70 ± 0.06 ab B | 2.0 ± 0.4 ab | 1.5 ± 0.4 a | ||
 | O30 | 5.4 ± 0.3 b | 1.39 ± 0.06 a | 1.012 a B | 1.39 ± 0.01 a B | 1.8 ± 0.7 a | 1.3 ± 1.5 a | ||
 | C60 | 5.4 ± 0.3 b | 1 ± 1 a | 0.289 a A | 0.5 ± 0.2 a A | 2.5 ± 0.9 a | 0.8 ± 0.5 a | ||
 | N | 5.4 ± 0.3 c | 1.2 ± 0.6 ab | 0.088±0.088 a A | 0.61 ± 0.02 ab A | 1.5 ± 0.2 b | 0.9 ± 1.0 ab | ||
| Zn (µg/L) |  | CB | 86 ± 3 | 229 ± 262 | 137 ± 161 | 224 ± 234 | 218 ± 247 | 256 ± 311 | 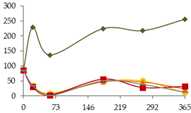 |
 | O15 | 86 ± 3 c | 35 ± 7 b | 9 ± 11 a | 48 ± 0.8 b | 50 ± 7 b | 11 ± 4 a | ||
 | O30 | 86 ± 3 e | 32 ± 1 bc | 6 ± 2 a | 47 ± 10 d | 46 ± 6 cd | 23 ± 9 b | ||
 | C60 | 86 ± 3 d | 30 ± 2 b | 2.0 a | 56 ± 9 c | 27.8 ± 0.8 b | 31.5 ± 0.6 b | ||
 | N | 86 ± 3 c | 35 ± 3 b | 1.2 ± 0.3 a | 51 ± 15 b | 38 ± 2 b | 12 ± 3 a | ||
| As (ng/L) |  | CB | 26 ± 4 a | 188 ± 35 b | 187 ± 41 b B | 317 ± 14 c | 232 ± 15 b D | 353 ± 38 c |  |
 | O15 | 26 ± 4 a | 138 ± 21 b | 152 ± 41 b B | 196 ± 39 bc | 201 ± 4 bc CD | 234 ± 48 c | ||
 | O30 | 26 ± 4 a | 132 ± 29 c | 60 ± 17 ab A | 117 ± 8 bc | 113 ± 18 bc A | 786 ± 44 d | ||
 | C60 | 26 ± 4 a | 126 ± 59 ab | 67 ± 20 ab A | 107 ± 4 ab | 172 ± 29 b BC | 1020 ± 106 c | ||
 | N | 26 ± 4 | 155 ± 27 | 53 ± 7 A | 198 ± 133 | 126 ± 22 AB | 1041 ± 872 | ||
| Cd (ng/L) |  | CB | 7.94 a | - | 38 ± 13 a | 312 ± 33 c B | 107 ± 12 b | 78 ± 23 ab |  |
 | O15 | 7.94 | - | 44 ± 17 | 252 ± 38 B | 135 ± 101 | 107 | ||
 | O30 | 7.94 a | - | 53 ± 16 ab | 138 ± 12 cA | 53 ± 14 ab | 76.6 b | ||
 | C60 | 7.94 | - | 71 ± 12 | 137 ± 62 A | 58 ± 11 | 92 ± 39 | ||
 | N | 7.94 | - | 86 ± 92 | 48 ± 23 A | 118 ± 30 | 138 ± 28 | ||
| Mo (ng/L) |  | CB | 246 ± 18 c | - | 54 ± 3 a D | 64 ± 10 a | 102 ± 4 b | 62 ± 5 a | 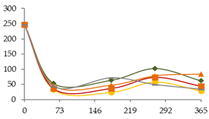 |
 | O15 | 246 ± 18 c | - | 34 ± 3 ab A | 24 ± 8 a | 58 ± 8 ab | 28 ± 1 ab | ||
 | O30 | 246 ± 18 c | - | 46 ± 4 a CD | 47 ± 12 a | 78 ± 10 a | 83 ± 4 ab | ||
 | C60 | 246 ± 18 b | - | 36 ± 4 a AB | 36 ± 22 a | 73 ± 20 a | 44 ± 20 a | ||
 | N | 246 ± 18 b | - | 43 ± 2 a BC | 71 ± 41 a | 49 ± 25 a | 34 ± 39 a | ||
| Pb (ng/L) |  | CB | 8025 ± 181 c | 1654 ± 155 b B | 583 ± 100 a | 817 ± 76 a | 462 a | - | 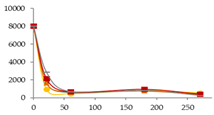 |
 | O15 | 8025 ± 181 b | 931 ± 44 a A | 447 ± 172 a | 804 ± 400 a | 540 ± 287 a | - | ||
 | O30 | 8025 ± 181 d | 1628 ± 111 c B | 689 ± 64 ab | 755.3 ± 0.4 b | 370 ± 137 a | - | ||
 | C60 | 8025 ± 181 c | 2156 ± 5 b C | 663 ± 500 a | 956 ± 63 a | 407 ± 52 a | - | ||
 | N | 8025 ± 181 d | 2879 ± 213 c D | 686 ± 167 ab | 829 ± 239 b | 273 ± 10 a | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catarino, S.; Thanasi, V.; Morin, G.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Fargeton, L.; Boissier, B.; Canas, S. Shedding Light on Metals Release from Chestnut Wood to Wine Spirit Using ICP-MS. Foods 2022, 11, 3617. https://doi.org/10.3390/foods11223617
Catarino S, Thanasi V, Morin G, Anjos O, Fernandes TA, Caldeira I, Fargeton L, Boissier B, Canas S. Shedding Light on Metals Release from Chestnut Wood to Wine Spirit Using ICP-MS. Foods. 2022; 11(22):3617. https://doi.org/10.3390/foods11223617
Chicago/Turabian StyleCatarino, Sofia, Vasiliki Thanasi, Gael Morin, Ofélia Anjos, Tiago A. Fernandes, Ilda Caldeira, Laurent Fargeton, Benjamin Boissier, and Sara Canas. 2022. "Shedding Light on Metals Release from Chestnut Wood to Wine Spirit Using ICP-MS" Foods 11, no. 22: 3617. https://doi.org/10.3390/foods11223617
APA StyleCatarino, S., Thanasi, V., Morin, G., Anjos, O., Fernandes, T. A., Caldeira, I., Fargeton, L., Boissier, B., & Canas, S. (2022). Shedding Light on Metals Release from Chestnut Wood to Wine Spirit Using ICP-MS. Foods, 11(22), 3617. https://doi.org/10.3390/foods11223617










