Evaluation of the Volatile Composition and Sensory Behavior of Habanero Pepper during Lactic Acid Fermentation by L. plantarum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Matter: Capsicum chinense
2.2. Bacterial Growth Conditions
2.3. Habanero Pepper Fermentation
2.4. Biomass Quantification and Lactic Acid Measurement
2.4.1. Quantification of Biomass
2.4.2. Lactic Acid and pH Measurement
2.5. Volatile Compound Extraction
2.6. Volatile Compound Quantification by Gas Chromatography
2.7. Hedonic Sensory Analysis
2.7.1. Preparation of the Samples
2.7.2. Sensory Evaluation
2.8. Statistical Analysis
3. Results
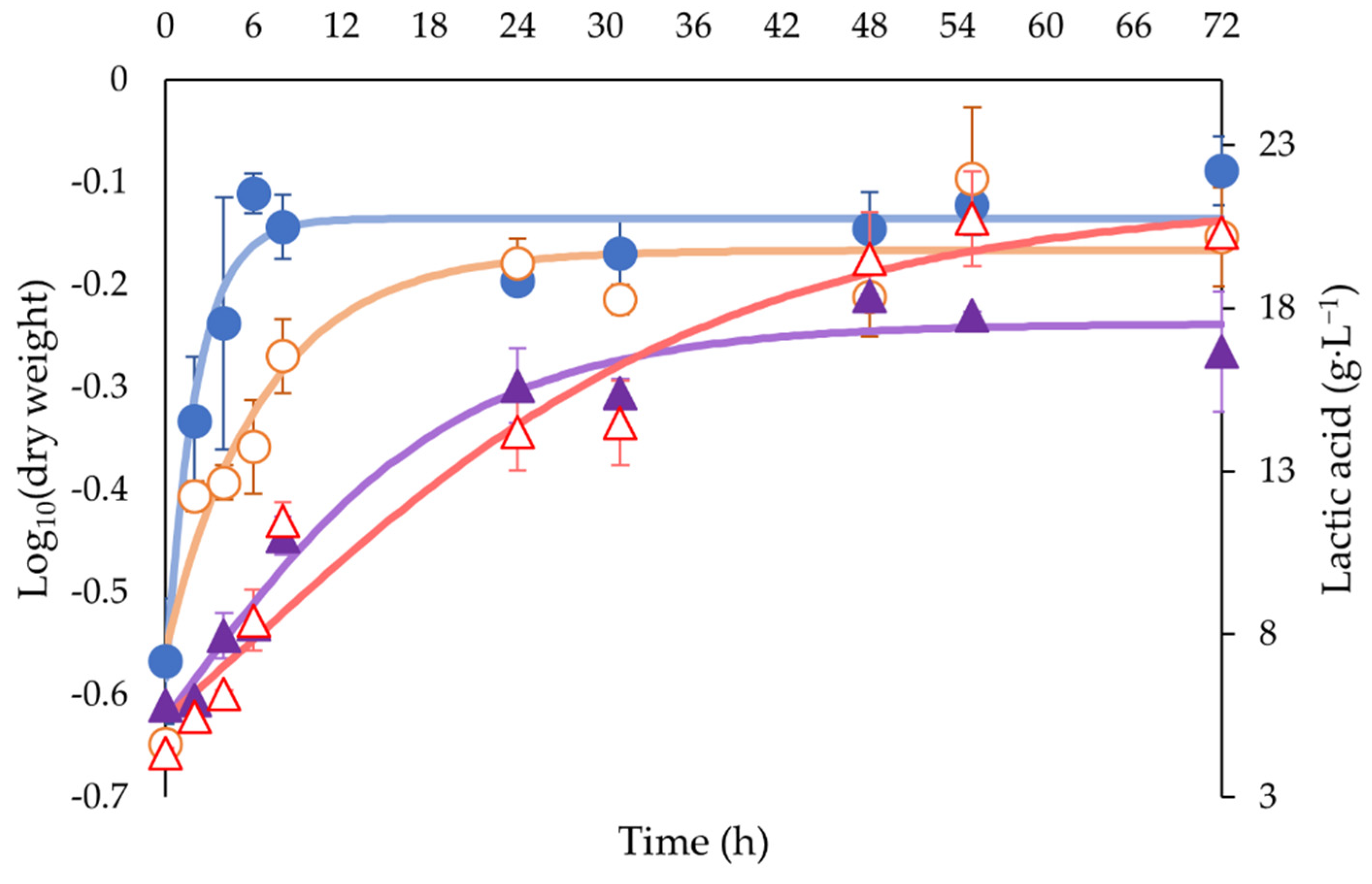
3.1. Bacterial Growth during Fermentation and Lactic Acid Production
3.2. Kinetic Parameter Analysis
3.3. Volatile Compound Production during Fermentation
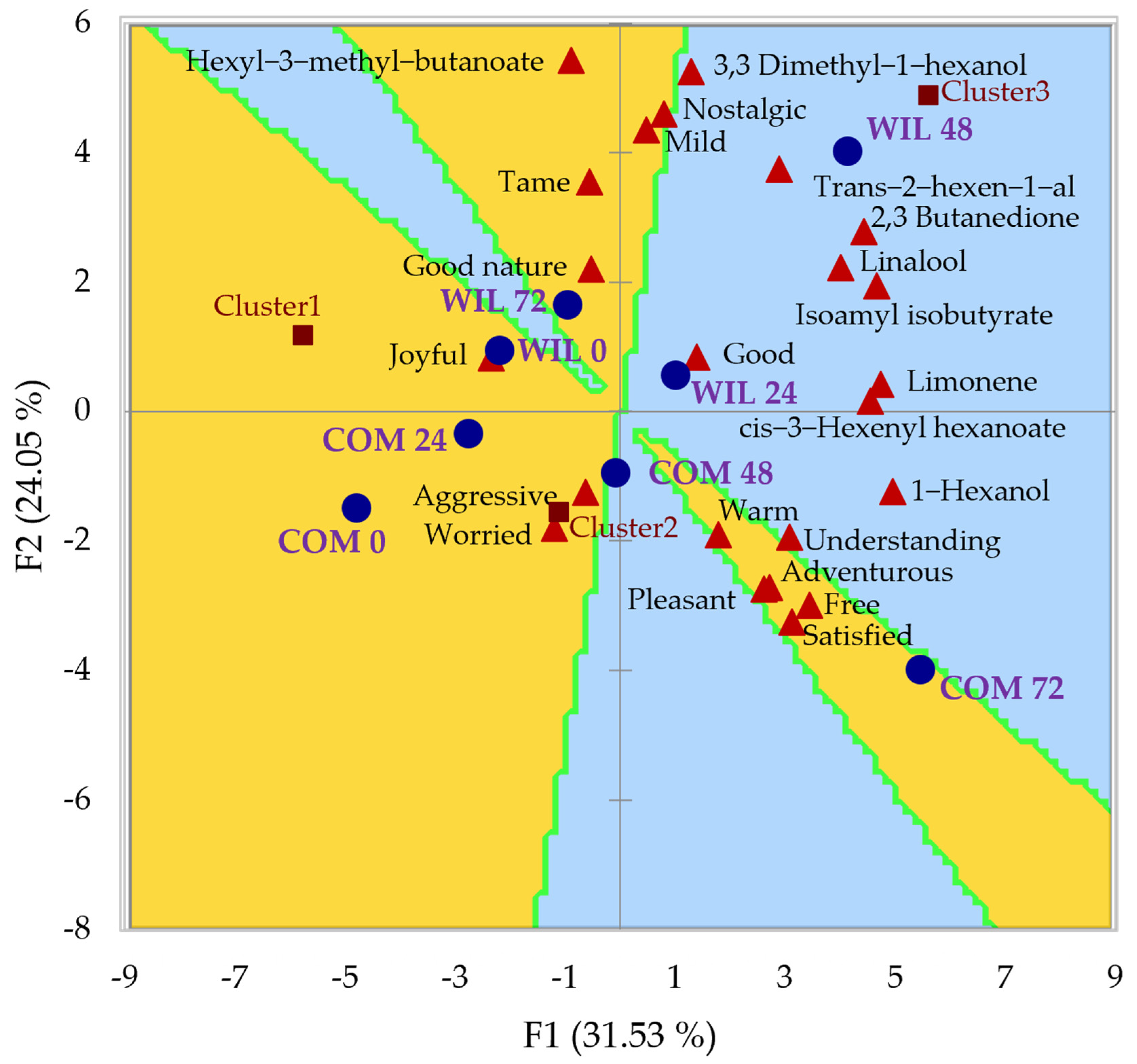
3.4. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Time (h) | pH | |
|---|---|---|
| COM | WIL | |
| 0 | 4.95 ± 0.01 | 4.92 ± 0.01 |
| 2 | 4.48 ± 0.01 | 4.39 ± 0.00 |
| 4 | 4.13 ± 0.00 | 4.07 ± 0.01 |
| 6 | 3.94 ± 0.01 | 3.88 ± 0.01 |
| 8 | 3.79 ± 0.00 | 3.77 ± 0.00 |
| 24 | 3.56 ± 0.00 | 3.42 ± 0.00 |
| 31 | 3.59 ± 0.00 | 3.42 ± 0.00 |
| 48 | 3.55 ± 0.02 | 3.39 ± 0.00 |
| 55 | 3.61 ± 0.00 | 3.49 ± 0.00 |
| 72 | 3.62 ± 0.01 | 3.49 ± 0.00 |
Appendix B

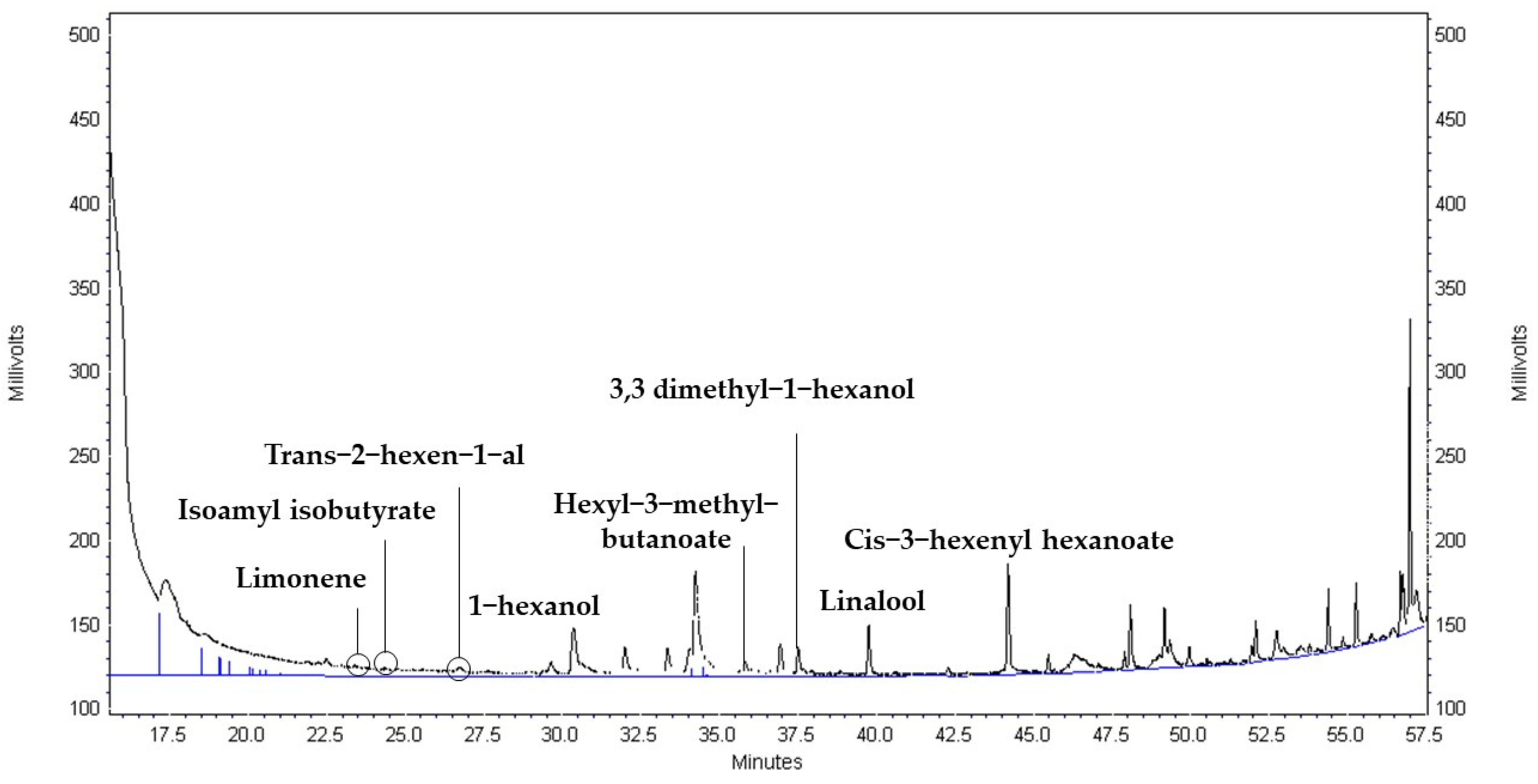
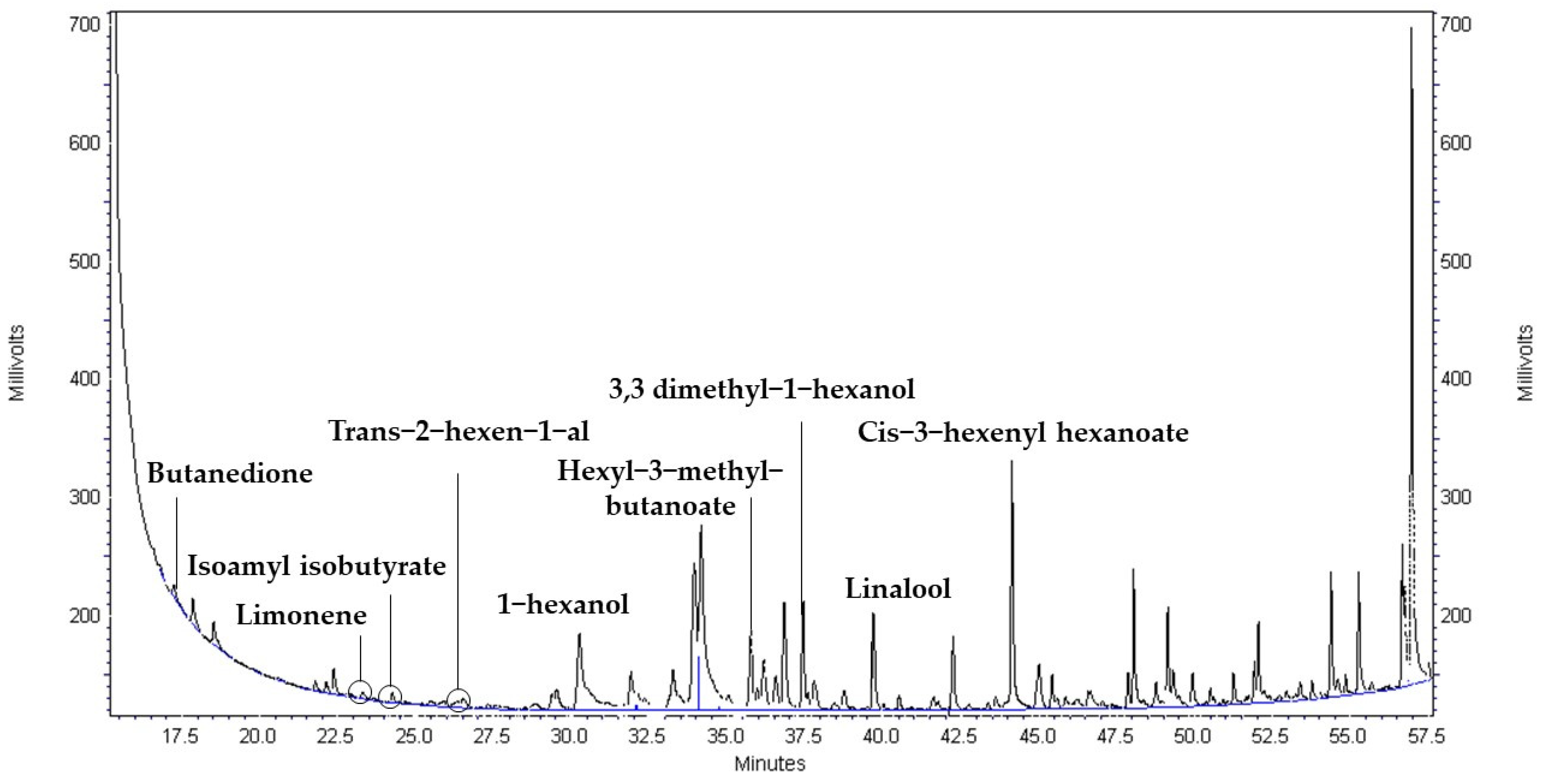
References
- Muñoz-Ramírez, L.S.; Peña-Yam, L.P.; Álvarez-Gil, M.A.; Iglesias-Andreu, L.G.; Avilés-Viñas, S.A.; Canto-Flick, A.; Guzmán-Antonio, A.; Santana-Buzzy, N. Selection of habanero pepper f1 hybrids (Capsicum chinense jacq.) at the Yucatan peninsula, Mexico with a high potential for different markets. Agriculture 2020, 10, 478. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.; Sosa-Moguel, O.; Pino, J.; Sauri-Duch, E. GC-MS Characterization of Volatile Compounds in Habanero Pepper (Capsicum chinense Jacq.) by Optimization of Headspace Solid-Phase Microextraction Conditions. Food Anal. Methods. 2015, 8, 1005–1013. [Google Scholar] [CrossRef]
- Sosa-Moguel, O.; Pino, J.A.; Ayora-Talavera, G.; Sauri-Duch, E.; Cuevas-Glory, L. Biological activities of volatile extracts from two varieties of Habanero pepper (Capsicum chinense Jacq.). Int. J. Food Prop. 2018, 20, S3042–S3051. [Google Scholar] [CrossRef] [Green Version]
- Joshi, D.D.; Somkuwar, B.G.; Kharkwal, H.; Chander, S. Aroma based varieties of Capsicum Chinense Jacq., geographical distribution and scope for expansion of the species. J. Appl. Res. Med. Aromat. Plants 2022, 29, 100379. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Aromagrams—Aromatic profiles in the appreciation of food quality. Food Chem. 2007, 101, 845–872. [Google Scholar] [CrossRef]
- Saa, D.L.T.; Nissen, L.; Gianotti, A. Metabolomic approach to study the impact of flour type and fermentation process on volatile profile of bakery products. Food Res. Int. 2019, 119, 510–516. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G. Rapid quantification of major volatile metabolites in fermented food and beverages using gas chromatography-mass spectrometry. Metabolites 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Li, Y.; Zhu, H.; Liu, Y.; Quan, K. Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung beans. LWT 2021, 146, 111434. [Google Scholar] [CrossRef]
- Acin-Albiac, M.; Filannino, P.; Arora, K.; Da Ros, A.; Gobbetti, M.; Di Cagno, R. Role of lactic acid bacteria phos-pho-β-glucosidases during the fermentation of cereal by-products. Foods 2021, 10, 97. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Mozzi, F.; Ortiz, M.E.; Bleckwedel, J.; De Vuyst, L.; Pescuma, M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013, 54, 1152–1161. [Google Scholar] [CrossRef]
- Jiang, Y.; King, J.M.; Prinyawiwatkul, W. A review of measurement and relationships between food, eating behavior and emotion. Trends Food Sci. Technol. 2014, 36, 15–28. [Google Scholar] [CrossRef]
- Stankovic, M.; Veljovic, K.; Popovic, N.; Kojic, S.; Manevski, S.D.; Radojkovic, D.; Golic, N. Lactobacillus brevis BGZLS10-17 and Lb. plantarum BGPKM22 exhibit anti-inflammatory effect by attenuation of NF-kB and MAPK signaling in Human Bronchial Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 5547. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Cruz, C.; Rodríguez-Buenfil, I.M.; Cabal-Prieto, A.; Cuervo-Osorio, V.D.; Oney-Montalvo, J.E.; Herre-ra-Corredor, J.A.; Ramírez-Sucre, M.O.; de Jesús Ramírez-Rivera, E. Modeling consumer satisfaction to identify drivers for liking: An online survey based on images of Habanero pepper (Capsicum chinense Jacq.). J. Sens. Stud. 2021, 36, 1–10. [Google Scholar] [CrossRef]
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric determination of lactic acid. J. Anal. Chem. 2016, 71, 755–758. [Google Scholar] [CrossRef]
- Pino, J.; Sauri-Duch, E.; Marbot, R. Changes in volatile compounds of Habanero chile pepper (Capsicum chinense Jack. cv. Habanero) at two ripening stages. Food Chem. 2006, 94, 394–398. [Google Scholar] [CrossRef]
- Bogusz Junior, S.; Tavares, A.M.; Filho, J.T.; Zini, C.A.; Godoy, H.T. Analysis of the volatile compounds of Brazilian chilli peppers (Capsicum spp.) at two stages of maturity by solid phase micro-extraction and gas chromatography-mass spectrometry. Food Res. Int. 2012, 48, 98–107. [Google Scholar] [CrossRef]
- de Jesús Ramírez-Rivera, E.; Rodriguez-Buenfil, I.M.; Pérez-Robles, M.; Oney-Montalvo, J.E.; Prinyawiwatkul, W.; Ca-bal-Prieto, A.; Ramírez-Sucre, M.O.; Herrera-Corredor, J.A. Development and validation of a methodology for the sensometric characterisation of high-pungency peppers: A case study of habanero pepper (Capsicum chinense Jacq.). Int. J. Food Sci. Technol. 2021, 56, 573–586. [Google Scholar] [CrossRef]
- Oluwajoba, S.O.; Akinyosoye, F.A.; Oyetayo, O.V. Comparative sensory and proximate evaluation of spontaneously fermenting kunuzaki made from germinated and ungerminated composite cereal grains. Food Sci. Nutr. 2013, 1, 336–349. [Google Scholar] [CrossRef]
- Santiago-Cruz, I.A.; de Jesús Ramírez-Rivera, E.; López-Espíndola, M.; Hidalgo-Contreras, J.V.; Prinyawiwatkul, W.; Herrera-Corredor, J.A. Use of online questionnaires to identify emotions elicited by different types of corn tortilla in consumers of different gender and age groups. J. Sens. Stud. 2021, 36, e12638. [Google Scholar] [CrossRef]
- Van de Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 2002, 82, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Parlindungan, E.; Dekiwadia, C.; Jones, O.A.H. Factors that influence growth and bacteriocin production in Lactiplantibacillus plantarum B21. Process Biochem. 2021, 107, 18–26. [Google Scholar] [CrossRef]
- Aranha, B.C.; Hoffmann, J.F.; Barbieri, R.L.; Rombaldi, C.V.; Chaves, F.C. Untargeted Metabolomic Analysis of Capsicum spp. by GC–MS. Phytochem. Anal. 2017, 28, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas-Glory, L.; Pino, J.; Lopez-Sauri, D.; Novelo-Torres, B.; Sauri-Duch, E. Characterization of odor-contributing volatiles in two Habanero pepper varieties by gas chromatography–olfactometry. Chem. Pap. 2020, 74, 2239–2246. [Google Scholar] [CrossRef]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile organic compounds profile during milk fermentation by Lactobacillus pentosus and correlations between volatiles flavor and carbohydrate metabolism. J. Dairy Sci. 2014, 97, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Lugtenberg, B.J.; Kravchenko, L.V.; Simons, M. Tomato seed and root exudate sugars: Composition, utilization by Pseudomonas biocontrol strains and role in rhCizosphere colonization. Environ. Microbiol. 1999, 1, 439–446. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Sabatini, N.; Mucciarella, M.R.; Marsilio, V. Volatile compounds in uninoculated and inoculated table olives with Lactobacillus plantarum (Olea europaea L.; cv. Moresca and Kalamata). LWT—Food Sci. Technol. 2008, 41, 2017–2022. [Google Scholar] [CrossRef]
- Salmeron, I.; Fuciños, P.; Charalampopoulos, D.; Pandiella, S.S. Volatile compounds produced by the pro-biotic strain Lactobacillus plantarum NCIMB 8826 in cereal-based substrates. Food Chem. 2009, 117, 265–271. [Google Scholar] [CrossRef]
- Yu, A.O.; Goldman, E.A.; Brooks, J.T.; Golomb, B.L.; Yim, I.S.; Gotcheva, V.; Angelov, A.; Kim, E.B.; Marco, M.L. Strain diversity of plant-associated Lactiplantibacillus plantarum. Microb. Biotechnol. 2021, 14, 1990–2008. [Google Scholar] [CrossRef]
- Bianchi, G.; Lo Scalzo, R. Characterization of hot pepper spice phytochemicals, taste compounds content and volatile profiles in relation to the drying temperature. J. Food Biochem. 2018, 42, e12675. [Google Scholar] [CrossRef]
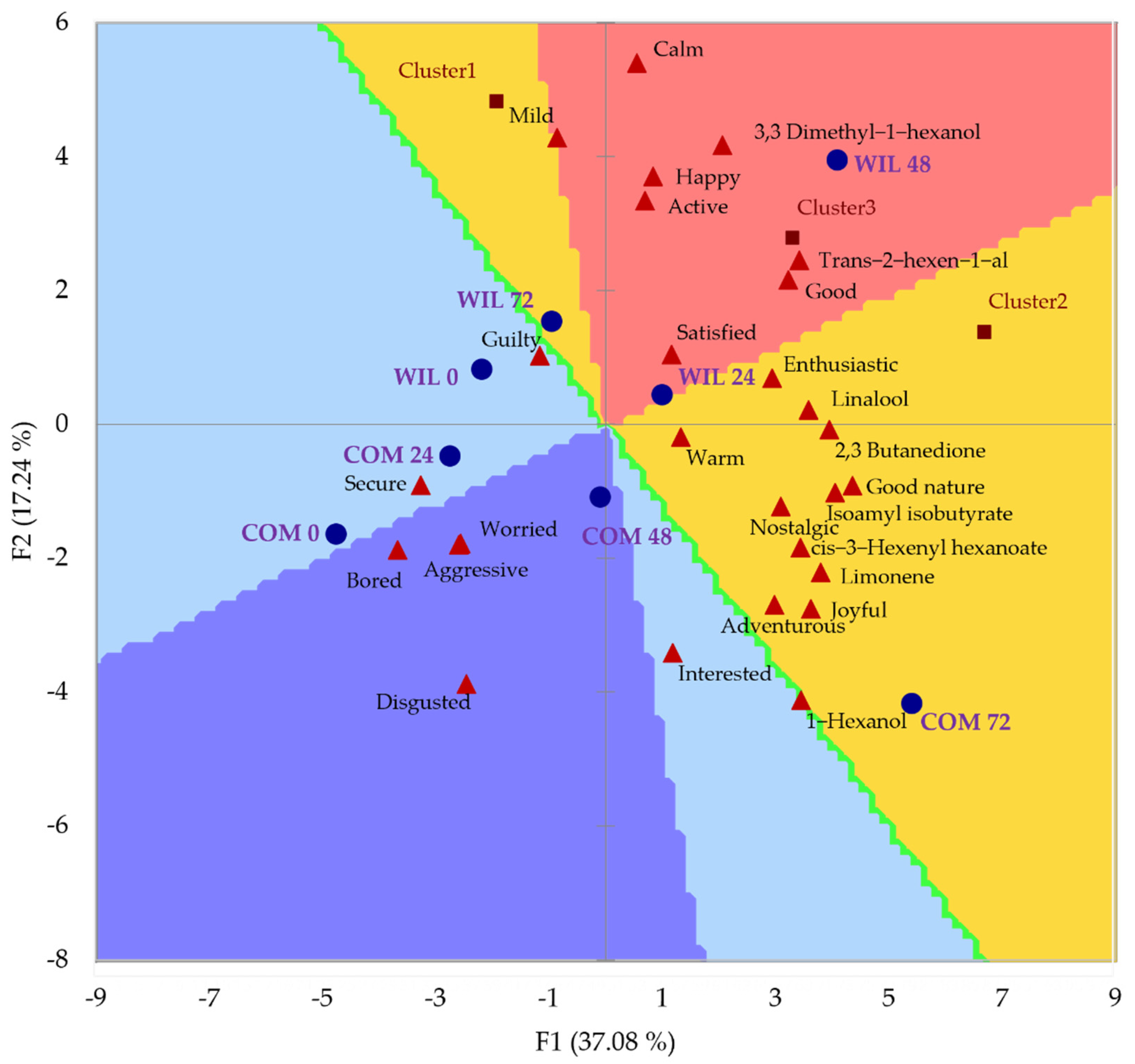
| Terms Used for Emotion Evaluation | |
|---|---|
| Active | Active |
| Joyful | Joyful |
| Adventurous | Adventurous |
| Loving | Loving |
| Aggressive | Aggressive |
| Mild | Mild |
| Bored | Bored |
| Nostalgic | Nostalgic |
| Calm | Calm |
| Pleasant | Pleasant |
| Disgusted | Disgusted |
| Satisfied | Satisfied |
| Enthusiastic | Enthusiastic |
| Response Variable | Strain | |
|---|---|---|
| COM | WIL | |
| μ (h−1) | 0.0680 ± 0.0025 b | 0.0336 ± 0.0074 a |
| ∆X (g L−1) | 0.54 ± 0.03 a | 0.58 ± 0.06 a |
| Qp (g L−1 h−1) | 0.7751 ± 0.1160 a | 0.8596 ± 0.1014 a |
| ∆P (g L−1) | 9.91 ± 0.09 a | 10.81 ± 1.03 b |
| Strain | Time (h) | 2,3 Butanedione (×102 µg mL−1) | Limonene (µg mL−1) | Isoamyl isobutyrate (µg mL−1) | Trans-2- hexen-1-al (µg mL−1) | Hexyl-3-methyl-butanoate (µg mL−1) | 1-Hexanol (µg mL−1) | Linalool (µg mL−1) | 3,3 Dimethyl-1-hexanol (µg mL−1) | Cis-3-hexenyl hexanoate (µg mL−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| COM | 0 | Nd | 1.01 ± 0.00 a | 0.04 ± 0.00 a | 0.83 ± 0.01 a | 0.73 ± 0.00 a | 2.10 ± 0.09 a | 1.56 ± 0.04 a | 1.45 ± 0.03 a | 1.34 ± 0.53 a |
| 24 | Nd | 1.01 ± 0.01 a | 0.05 ± 0.01 a | 0.82 ± 0.00 a | 0.73 ± 0.00 a | 2.46 ± 0.02 b | 1.80 ± 0.01 b | 1.65 ± 0.12 b | 2.38 ± 0.00 d | |
| 48 | Nd | 1.03 ± 0.01 a | 0.05 ± 0.00 a | 0.88 ± 0.02 a | 0.73 ± 0.00 a | 3.35 ± 0.67 d | 2.00 ± 0.12 c | 2.19 ± 0.24 c | 3.00 ± 0.22 f | |
| 72 | 8.16 ± 9.13 a | 1.07 ± 0.07 b | 0.11 ± 0.07 a | 0.89 ± 0.10 a | 0.73 ± 0.00 a | 6.01 ± 0.43 e | 2.14 ± 0.01 d | 1.85 ± 0.01 b | 3.40 ± 0.03 g | |
| WIL | 0 | Nd | 1.03 ± 0.02 a | 0.06 ± 0.00 a | 0.81 ± 0.03 a | 0.73 ± 0.00 a | 2.32 ± 0.20 b | 1.65 ± 0.05 a | 1.40 ± 0.10 a | 1.46 ± 0.04 a |
| 24 | Nd | 1.01 ± 0.01 a | 0.05 ± 0.00 a | 0.86 ± 0.01 a | 0.73 ± 0.00 a | 2.30 ± 0.03 b | 1.62 ± 0.01 a | 1.72 ± 0.06 b | 1.69 ± 0.04 b | |
| 48 | 8.11 ± 9.15 a | 1.04 ± 0.03 a | 0.09 ± 0.06 a | 1.00 ± 0.06 b | 0.74 ± 0.01 a | 2.95 ± 0.22 c | 2.14 ± 0.00 d | 3.19 ± 0.05 d | 2.75 ± 0.04 e | |
| 72 | Nd | 1.01 ± 0.00 a | 0.05 ± 0.01 a | 0.82 ± 0.00 a | 0.73 ± 0.00 a | 2.41 ± 0.10 b | 1.95 ± 0.04 c | 3.10 ± 0.70 d | 2.01 ± 0.01 c |
| Factor | 2, 3 Butanedione | Limonene | Isoamyl isobutyrate | Trans-2-hexen-1-al | Hexyl-3-methyl-butanoate | 1-Hexanol | Linalool | 3,3 Dimethyl-1-hexanol | Cis-3-hexenyl hexanoate |
|---|---|---|---|---|---|---|---|---|---|
| A: Strain | 0.9955 | 0.7008 | 0.9937 | 0.4223 | 0.1606 | 0.0002 * | 0.1458 | 0.0029 * | 0.0007 * |
| B: Time | 0.4202 | 0.4346 | 0.4995 | 0.0164 * | 0.8433 | 0.0001 * | 0.0000 * | 0.0004 * | 0.0000 * |
| A*B | 0.1173 | 0.1976 | 0.2565 | 0.0666 | 0.5202 | 0.0001 * | 0.0025 * | 0.0201 * | 0.0043 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Salas, D.; Oney-Montalvo, J.E.; Ramírez-Rivera, E.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Evaluation of the Volatile Composition and Sensory Behavior of Habanero Pepper during Lactic Acid Fermentation by L. plantarum. Foods 2022, 11, 3618. https://doi.org/10.3390/foods11223618
López-Salas D, Oney-Montalvo JE, Ramírez-Rivera E, Ramírez-Sucre MO, Rodríguez-Buenfil IM. Evaluation of the Volatile Composition and Sensory Behavior of Habanero Pepper during Lactic Acid Fermentation by L. plantarum. Foods. 2022; 11(22):3618. https://doi.org/10.3390/foods11223618
Chicago/Turabian StyleLópez-Salas, Diego, Julio Enrique Oney-Montalvo, Emmanuel Ramírez-Rivera, Manuel Octavio Ramírez-Sucre, and Ingrid Mayanin Rodríguez-Buenfil. 2022. "Evaluation of the Volatile Composition and Sensory Behavior of Habanero Pepper during Lactic Acid Fermentation by L. plantarum" Foods 11, no. 22: 3618. https://doi.org/10.3390/foods11223618








