Development of Flavor and Taste Components of Sous-Vide-Cooked Nile Tilapia (Oreochromis niloticus) Fillet as Affected by Various Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- (1)
- 50 °C, 30 min (50-30)
- (2)
- 50 °C, 45 min (50-45)
- (3)
- 50 °C, 60 min (50-60)
- (4)
- 60 °C, 30 min (60-30)
- (5)
- 60 °C, 45 min (60-45)
- (6)
- 60 °C, 60 min (60-60)
2.2. Total Sulfhydryl (-SH) and Carbonyl Content
2.3. Free Fatty Acid (FFA) Content
2.4. Peroxide Value (PV) and Thiobarbituric Acid Reactive Substance (TBARS) Value
2.5. Free Amino Acid Composition
2.6. Volatile Compounds
2.7. Adenosine Triphosphate (ATP)-Related Compounds
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
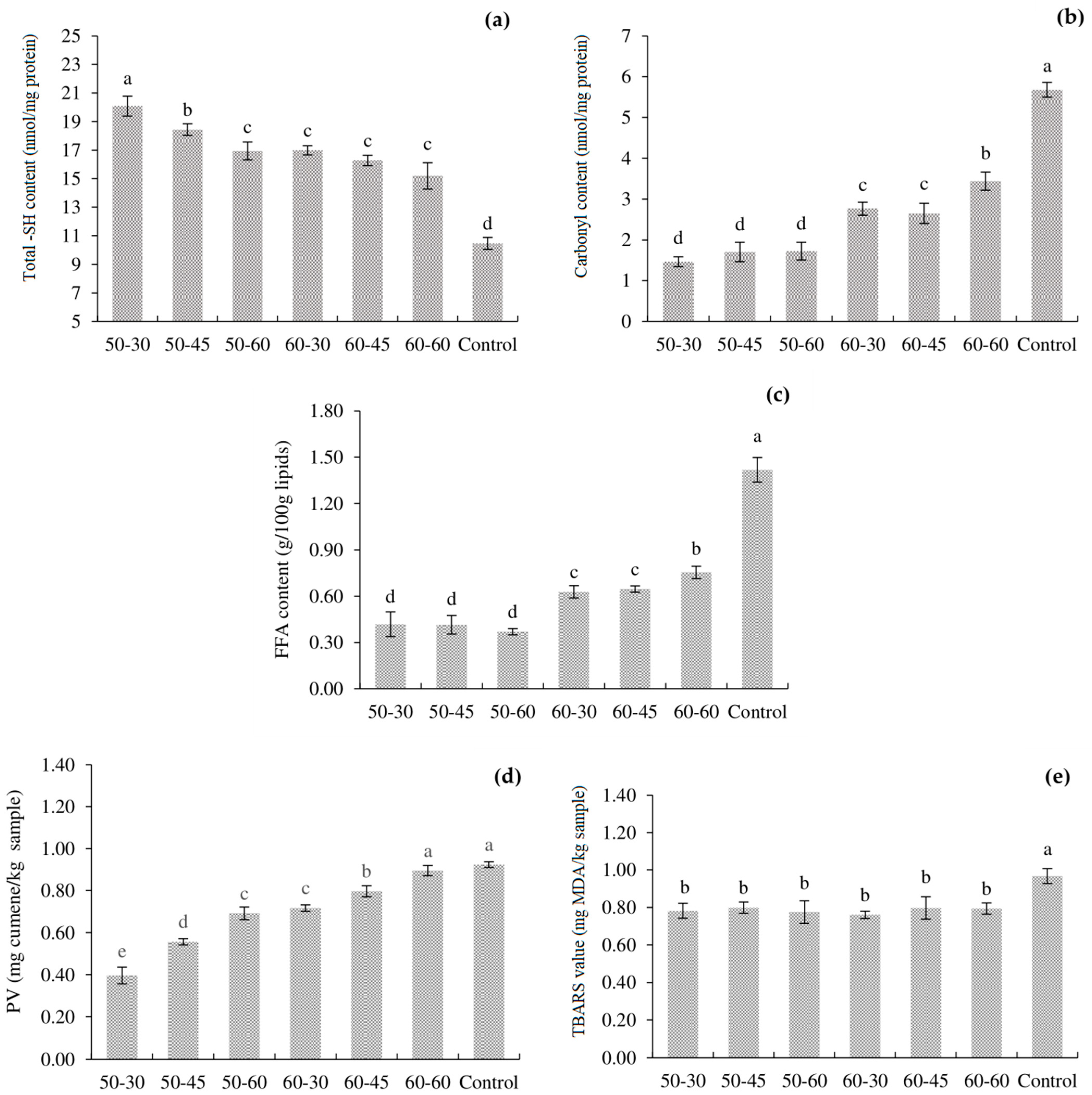
3.1. Total -SH and Carbonyl Content
3.2. FFA, PV and TBARS
3.3. Free Amino Acid Composition
3.4. Volatile Compounds
3.5. ATP-Related Compounds
3.6. Sensory Scores
3.7. PCA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, H.; Li, N.; Yan, L.; Xue, Y. The hydration characteristics, structural properties and volatile profile of squid (Symplectoteuthis oualaniensis) mantle muscle: Impacts of steaming, boiling, and sous vide cooking. Foods 2021, 10, 1646. [Google Scholar] [CrossRef] [PubMed]
- González-Fandos, E.; Villarino-Rodríguez, A.; García-Linares, M.C. Microbiological safety and sensory characteristics of salmon slices processed by sous vide method. Food Control 2005, 16, 75–85. [Google Scholar] [CrossRef]
- Llave, Y.; Shibata-Ishiwatari, N.; Watanabe, M.; Fukuoka, M.; Hamada-Sato, N.; Sakai, N. Analysis of the effects of thermal protein denaturation on the quality attributes of sous-vide cooked tuna. J. Food Process. Preserv. 2018, 42, e13347. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Garcia-Linares, M.C.; Rodrigez, A.V.; Garcia-Arias, M.T.; Garcia-Fernandes, M.C. Evaluation of the microbiological safety and sensory quality of rainbow trout (Oncorhynchus mykiss) processed by the sous-vide method. Food Microbiol. 2004, 21, 193–201. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Boonanuntanasarn, S.; Yongsawatdigul, J. Sensory characteristics and microbiological quality changes of Nile tilapia fillet processed by various sous-vide conditions during chilled storage. Turk. J. Fish. Aquat. Sci. 2022, 22, TRJFAS21079. [Google Scholar] [CrossRef]
- Sanchez del Pulgar, J.; Roldán, M.; Ruiz-Carrascal, J. Volatile compounds profile of sous-vide cooked pork cheeks as affected by cooking conditions (vacuum packaging, temperature and time). Molecules 2013, 18, 12538–12547. [Google Scholar] [CrossRef] [PubMed]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K. Changes in lipids of shrimp (Acetes vulgaris) during salting and fermentation. Eur. J. Lipid Sci. Technol. 2017, 119, 1700253. [Google Scholar] [CrossRef]
- He, J.; Zhou, G.H.; Bai, Y.; Wang, C.; Zhu, S.R.; Xu, X.L.; Li, C.B. The effect of meat processing methods on changes in disulfide bonding and alteration of protein structures: Impact on protein digestion products. RSC Adv. 2018, 8, 17595–17605. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chem. 2020, 320, 126656. [Google Scholar] [CrossRef]
- Pegg, R.B.; Shahidi, F. Heat effects on meat. In Flavour Development, 1st ed.; Encyclopedia of Meat Sciences; Academic Press: Oxford, UK, 2004. [Google Scholar]
- Niu, J.; Zhao, B.; Guo, X.; Yin, T. Effects of vacuum freeze-drying and vacuum spray-drying on biochemical properties and functionalities of myofibrillar proteins from silver carp. J. Food Qual. 2019, 19, 9457835. [Google Scholar] [CrossRef]
- Ellman, G.E. Tissue sulfhydryl samples. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; Xiong, Y.L. Physicochemical changes of myosin and gelling properties of washed tilapia mince as influenced by oxidative stress and microbial transglutaminase. J. Food Sci. Technol. 2015, 52, 3824–3836. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Lowry, R.R.; Tinsley, I.J. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Takeungwongtrakul, S.; Benjakul, S. Oxidative stability of shrimp oil-in-water emulsions as affected by antioxidant incorporation. Int. Aquat. Res. 2013, 5, 14. [Google Scholar] [CrossRef]
- Minh-Thuy, L.T.; Okazaki, E.; Osako, K. Isolation and characterization of acid soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014, 149, 264–270. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Sumpavapol, P.; Kazufumi, O.; Faithong, N. Chemical compositions, sensory and antioxidative properties of salted shrimp paste (Ka-pi) in Thailand. Int. Food Res. J. 2015, 22, 1454–1465. [Google Scholar]
- Kuda, T.; Fujita, M.; Goto, H.; Yano, T. Effects of freshness on ATP-related compounds in retorted chub mackerel Scomber japonicus. LWT-Food Sci. Technol. 2007, 40, 1186–1190. [Google Scholar] [CrossRef]
- Benjakul, S.; Sungsri-in, R.; Kijroongrojana, K. Effect of treating of squid with sodium chloride in combination with oxidising agent on bleaching, physical and chemical changes during frozen storage. Food Bioprocess Technol. 2012, 5, 2077–2084. [Google Scholar] [CrossRef]
- Shui, S.S.; Yao, H.; Jiang, Z.D.; Benjakul, S.; Aubourg, S.P.; Zhang, B. The differences of muscle proteins between neon flying squid (Ommastrephes bartramii) and jumbo squid (Dosidicus gigas) mantles via physicochemical and proteomic analyses. Food Chem. 2021, 264, 130374. [Google Scholar] [CrossRef]
- Dominguez, R.; Purrinos, L.; Pérez-Santaescolastica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Prevention of quality loss and melanosis of Pacific white shrimp by cashew leaf extracts. Food Control. 2019, 95, 257–266. [Google Scholar] [CrossRef]
- Senapati, S.R.; Kumar, G.P.; Singh, C.B.; Xavier, K.A.M.; Chouksey, M.K.; Nayak, B.B.; Balange, A.K. Melanosis and quality attributes of chill stored farm raised white leg shrimp (Litopenaeus vannamei). J. Appl. Nat. Sci. 2017, 9, 626–631. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, J.; Ayisi, C.L.; Cao, X. Effects of salinity and alkalinity on fatty acids, free amino acids and related substance anabolic metabolism of Nile tilapia. Aquac. Fish. 2022, 7, 389–395. [Google Scholar] [CrossRef]
- Herath, S.S.; Haga, Y.; Satoh, S. Effects of long-term feeding of corn co-product-based diets on growth, fillet color, and fatty acid and amino acid composition of Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 464, 205–212. [Google Scholar] [CrossRef]
- Bermudez, R.; Franco, D.; Carballo, J.; Sentandreu, M.A.; Lorenzo, J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014, 56, 226–235. [Google Scholar] [CrossRef]
- Zhang, D.; Ayed, C.; Fisk, I.D.; Liu, Y. Effect of cooking processes on tilapia aroma and potential umami perception. Food Sci. Hum. Wellness 2023, 12, 35–44. [Google Scholar] [CrossRef]
- Li, M.; Guan, Z.; Ge, Y.; Zhang, X.; Ling, C. Effect of pretreatment on water migration and volatile components of heat pump dried tilapia fillets. Dry. Technol. 2019, 38, 1828–1842. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; Zhang, Y.; Song, H.; Raza, A.; Pang, W.; Gong, L.; Jiang, C. Comparison of different volatile extraction methods for the identification of fishy off-odor in fish by-products. Animals 2022, 27, 6177. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, X.; Zhao, M.; Liu, X.; Pang, Y.; Zhang, M. Characterization of the key aroma constituents in fried tilapia through the sensorics concept. Foods 2022, 11, 494. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Richards, M.P. Effect of myoglobin from Eastern little tuna muscle on lipid oxidation of washed Asian seabass mince at different pH conditions. J. Food Sci. 2011, 76, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Roldan, M.; Ruiz, J.; Pulgar, J.S.; Perez-Palacios, T.; Antequera, T. Volatile compound profile of sous-vide cooked lamb loins at different temperature-time combinations. Meat Sci. 2015, 100, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; Zheng, X.; Chen, Z.H.; Zhang, Y.Y. Fresh and grilled eel volatile fingerprinting by e-Nose, GC-O, GC-MS and GC x GC-QTOF combined with purge and trap and solvent-assisted flavor. Food Res. Int. 2019, 115, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Liu, Q.; Bao, H.; Wang, X.; Miao, S. Effects of different freshness on the quality of cooked tuna steak. Innov. Food Sci. Emerg. Technol. 2017, 44, 67–73. [Google Scholar] [CrossRef]
- Massa, A.E.; Palacios, D.L.; Paredi, M.; Crupin, M. Postmortem changes in quality indices of ice-stored flounder (Paralichithys patagonicus). J. Food Biochem. 2005, 29, 570–590. [Google Scholar] [CrossRef]
- Everitt, M. Consumer-targeted sensory quality. In Global Issues in Food Science and Technology; Barbosa-Canovas, G.V., Mortimer, A., Lineback, D., Spiess, W., Buckle, K., Colonna, P., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 117–128. [Google Scholar]
- Yao, W.; Cai, Y.; Liu, D.; Chen, Y.; Li, J.; Zhang, M.; Chen, N.; Zhang, H. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Food Chem. 2022, 370, 130989. [Google Scholar] [CrossRef]
- Min, D.B.; Callison, A.L.; Lee, H.O. Singlet oxygen oxidation for 2-pentylfuranand 2-pentenyfuran formation in soybean oil. JFS Food Chem. Toxicol. 2003, 68, 1175–1178. [Google Scholar]
- Hu, G.; Zhu, Y.; Hernandez, M.; Koutchma, T.; Shao, S. An efficient method for the simultaneous determination of furan, 2-methylfuran and 2-pentylfuran in fruit juices by headspace solid phase microextraction and gas chromatography–flame ionisation detector. Food Chem. 2016, 192, 9–14. [Google Scholar] [CrossRef] [PubMed]

| Category | Name | 50-30 | 50-45 | 50-60 | 60-30 | 60-45 | 60-60 | Control |
|---|---|---|---|---|---|---|---|---|
| Umami | Aspartic acid (Asp) | 5.45 ± 1.01 b | 7.78 ± 1.22 a | 8.99 ± 1.66 a | 9.33 ± 2.02 a | 7.12 ± 1.63 ab | 8.59 ± 1.67 a | 7.14 ± 1.44 a |
| Glutamic acid (Glu) | 30.66 ± 2.99 b | 30.33 ± 2.05 b | 36.06 ± 3.66 a | 33.21 ± 3.41 ab | 36.77 ± 2.06 a | 38.02 ± 1.99 a | 34.34 ± 3.06 ab | |
| Total | 36.11 | 38.11 | 45.05 | 42.54 | 43.89 | 46.61 | 41.48 | |
| Sweet | Alanine (Ala) | 25.51 ± 2.68 b | 25.08 ± 4.55 b | 23.23 ± 2.16 b | 27.12 ± 2.44 b | 26.66 ± 2.19 b | 33.99 ± 3.03 a | 23.03 ± 4.11 b |
| Glycine (Gly) | 53.22 ± 4.15 ab | 46.77 ± 8.02 b | 50.11 ± 7.81 ab | 54.54 ± 5.55 ab | 66.02 ± 8.08 a | 60.09 ± 9.63 ab | 56.66 ± 8.95 ab | |
| Proline (Pro) | 10.03 ± 1.02 | 12.15 ± 1.45 | 12.66 ± 2.02 | 10.99 ± 2.33 | 11.52 ± 2.05 | 13.13 ± 2.01 | 13.06 ± 2.12 | |
| Serine (Ser) | 0.89 ± 0.21 | 1.03 ± 0.16 | 0.88 ± 0.17 | 0.89 ± 0.20 | 0.92 ± 0.21 | 0.90 ± 0.19 | 0.97 ± 0.23 | |
| Threonine (Thr) | 2.56 ± 0.29 | 2.33 ± 0.18 | 2.54 ± 0.20 | 2.61 ± 0.25 | 2.30 ± 0.20 | 2.45 ± 0.16 | 2.22 ± 0.21 | |
| Total | 92.21 | 87.36 | 89.42 | 96.15 | 107.42 | 97.43 | 95.28 | |
| Bitter | Arginine (Arg) | 2.33 ± 0.26 b | 2.65 ± 0.33 ab | 3.03 ± 0.34 a | 2.33 ± 0.29 b | 1.47 ± 0.32 c | 1.35 ± 0.32 c | 1.33 ± 0.35 c |
| Histidine (His) | 4.65 ± 0.23 a | 4.44 ± 0.19 a | 4.54 ± 0.15 a | 4.71 ± 0.15 a | 4.11 ± 0.20 b | 3.72 ± 0.29 b | 4.04 ± 0.17 b | |
| Isoleucine (Ile) | 0.55 ± 0.09 | 0.50 ± 0.10 | 0.47 ± 0.07 | 0.47 ± 0.07 | 0.42 ± 0.08 | 0.42 ± 0.06 | 0.49 ± 0.10 | |
| Leucine (Leu) | 5.62 ± 0.32 a | 5.23 ± 0.26 a | 5.05 ± 0.26 a | 3.99 ± 0.19 b | 3.90 ± 0.21 b | 4.02 ± 0.15 b | 3.62 ± 0.15 c | |
| Methionine (Met) | 0.36 ± 0.08 | 0.35 ± 0.09 | 0.30 ± 0.06 | 0.34 ± 0.09 | 0.36 ± 0.07 | 0.36 ± 0.05 | 0.33 ± 0.08 | |
| Phenylalanine (Phe) | 0.29 ± 0.05 | 0.30 ± 0.06 | 0.34 ± 0.06 | 0.29 ± 0.05 | 0.33 ± 0.06 | 0.32 ± 0.06 | 0.33 ± 0.05 | |
| Tryptophan (Trp) | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.03 | 0.12 ± 0.04 | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.10 ± 0.03 | |
| Valine (Val) | 0.13 ± 0.03 a | 0.16 ± 0.02 a | 0.16 ± 0.03 a | 0.09 ± 0.02 ab | 0.07 ± 0.02 b | 0.09 ± 0.02 ab | 0.06 ± 0.02 b | |
| Total | 14.01 | 13.72 | 13.98 | 12.34 | 10.75 | 10.37 | 10.30 | |
| Tasteless | Cysteine (Cys) | ND | ND | ND | ND | ND | 0.02 ± 0.00 | 0.03 ± 0.01 |
| Lysine (Lys) | 32.32 ± 2.42 a | 30.16 ± 2.15 a | 30.50 ± 3.50 a | 26.24 ± 2.22 b | 22.01 ± 2.04 c | 20.99 ± 2.22 cd | 17.16 ± 3.08 d | |
| Tyrosine (Tyr) | 3.05 ± 0.19 a | 2.88 ± 0.13 a | 3.10 ± 0.16 a | 3.04 ± 0.11 a | 3.02 ± 0.09 a | 2.96 ± 0.12 a | 2.56 ± 0.15 b | |
| Total | 35.37 | 33.04 | 33.60 | 29.28 | 25.03 | 23.95 | 19.72 | |
| Total amino acids | 177.70 | 172.23 | 182.05 | 180.31 | 187.09 | 178.36 | 166.78 | |
| Volatile Compounds 1 | Aroma Description 2 | RI 3 | 50-30 | 50-45 | 50-60 | 60-30 | 60-45 | 60-60 | Control |
|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||
| Butanal | Pungent, fatty | 891 | 0.23 | 0.22 | 0.15 | 0.28 | 0.13 | 0.21 | 0.30 |
| 2,3-Methylbutanal | Nutty, chocolate | 902 | 0.55 | 1.08 | 0.26 | 0.35 | 0.24 | 0.16 | 0.21 |
| Hexanal | Green, grassy | 1050 | 5.42 | 3.66 | 3.02 | 1.67 | 1.62 | 1.04 | 0.67 |
| Heptanal | Green, fatty | 1180 | 1.05 | 1.23 | 1.22 | 1.02 | 1.43 | 1.33 | 1.45 |
| 2,4-Heptadienal | Fatty taste, fishy smell | 1128 | 0.66 | 0.42 | 0.51 | 0.25 | 0.22 | 0.12 | 0.22 |
| Nonanal | Green, citrusy | 1374 | 0.44 | 0.40 | 0.36 | 0.36 | 0.21 | 0.33 | 0.29 |
| 2-Octenal | Oily, nutty | 977 | 1.03 | 1.28 | 1.20 | 0.41 | 0.23 | 0.35 | 0.61 |
| Benzaldehyde | Pleasant almond, nutty | 1563 | 0.23 | 0.16 | 0.44 | 0.69 | 1.02 | 1.55 | 2.06 |
| Total aldehydes | 9.61 | 8.45 | 7.16 | 5.03 | 5.10 | 5.09 | 5.81 | ||
| Ketones | |||||||||
| 2-Butanone | Ethereal, acetone-like | 902 | 0.66 | 1.25 | 1.03 | 1.22 | 0.39 | 0.23 | 0.28 |
| 2-Heptanone | Green taste, citrus flavor | 1145 | 0.31 | 0.26 | 0.55 | 0.34 | 0.40 | 1.03 | 2.25 |
| 5-Hepten-2-one, 6-methyl- | Fatty, metallic | 1025 | 0.23 | 0.19 | 0.22 | 0.24 | 0.20 | ND | ND |
| 2-Nonanone | Oily, plastic | 1388 | 0.14 | 0.33 | 0.36 | ND | ND | ND | ND |
| 3-Octanone | Mushroom | 983 | 0.21 | 0.17 | 0.17 | 0.12 | 0.22 | 0.14 | 0.15 |
| 2-Decanone | Floral, orange, fatty | 1492 | 1.02 | 1.37 | 1.34 | 1.21 | 0.44 | 0.38 | 0.46 |
| 2-Undecanone | Orange, fatty | 1201 | 0.34 | 0.41 | 0.55 | 0.21 | 0.12 | 0.16 | 0.12 |
| Acetoin | Buttery, sweet, cream | 1296 | 7.03 | 6.78 | 8.88 | 10.08 | 10.15 | 12.49 | 8.16 |
| Total ketones | 9.94 | 10.76 | 13.10 | 13.42 | 11.92 | 14.43 | 11.42 | ||
| Alcohols | |||||||||
| 1-Penten-3-ol | Balsam | 882 | 0.98 | 0.66 | 2.02 | 1.64 | 2.69 | 2.70 | 1.08 |
| 1-Hexanol | Green, herbal | 1360 | 0.66 | 2.01 | 1.69 | 1.33 | 1.45 | 1.92 | 0.54 |
| 1-Hexanol, 2-ethyl- | Citrus | 960 | 0.31 | 0.26 | 0.75 | 0.38 | 0.44 | 0.56 | 1.02 |
| 1-Heptanol | Oily, balsam | 1114 | 0.21 | 0.66 | 0.82 | 0.43 | 0.18 | 0.21 | 0.22 |
| 2-Nonen-1-ol | Cucumber, oily | 1206 | ND | 0.44 | 0.67 | ND | ND | ND | ND |
| 1-Octen-3-ol | Mushroom, earthy | 1438 | 16.55 | 17.06 | 14.05 | 14.62 | 14.21 | 13.22 | 10.12 |
| 1-Octanol | Mushroom, waxy | 1556 | ND | 0.33 | 1.01 | 0.67 | 1.35 | 1.02 | 1.44 |
| Total alcohols | 18.71 | 21.42 | 21.01 | 19.07 | 20.32 | 19.63 | 14.42 | ||
| N-containing compounds | |||||||||
| Trimethylamine | Ammonia, fishy, pungent | 587 | 28.42 | 28.21 | 24.68 | 25.05 | 23.95 | 16.15 | 30.25 |
| 2,6-Dimethylpyrazine | Nutty, roasted, popcorn | 1309 | 4.06 | 4.55 | 3.02 | 2.55 | 4.45 | 5.03 | 7.02 |
| Trimethylpyrazine | Nutty, roasted, earthy | 1401 | 1.42 | 1.03 | 1.48 | 1.65 | 1.78 | 4.26 | 3.11 |
| 2-Ethyl-3,5-dimethylpyrazine | Nutty, roasted, burnt | 1438 | ND | ND | ND | ND | 0.11 | ND | 0.31 |
| Total N-containing compounds | 33.90 | 33.79 | 29.18 | 29.25 | 30.29 | 25.44 | 40.69 | ||
| S-containing compounds | |||||||||
| Methanethiol | Pungent, sulphury | 622 | 13.22 | 12.23 | 12.66 | 15.15 | 13.02 | 10.92 | 4.11 |
| 4-Methylthiazole | Cooked meat | 1298 | 1.02 | 2.22 | 2.09 | 1.51 | 2.67 | 2.33 | 1.02 |
| 2-Methylthiazoline | Cooked meat | 1436 | ND | ND | ND | ND | 0.21 | 0.33 | 0.09 |
| 4,5-Dimethylthiazole | Cooked meat | 1380 | 3.45 | 3.05 | 3.03 | 2.99 | 4.87 | 3.05 | 1.05 |
| Dimethyl trisulphide | Meaty, onion | 970 | 0.41 | 0.63 | 0.95 | 0.66 | 0.60 | 1.12 | 0.65 |
| Dimethyl tetrasulphide | Meaty | 1015 | 0.25 | 0.65 | 0.99 | 0.56 | 0.24 | 0.32 | 0.41 |
| Total S-containing compounds | 18.35 | 18.78 | 19.72 | 20.87 | 21.61 | 18.07 | 7.33 | ||
| Others | |||||||||
| Limonene | Citrus, orange | 1190 | 1.73 | 2.02 | 1.44 | 0.54 | 0.50 | 0.56 | ND |
| 2-Pentylfuran | fruity | 1229 | 4.03 | 2.65 | 4.44 | 5.27 | 6.08 | 6.22 | 10.15 |
| 2-Methylfuran | fruity | 1027 | 3.52 | 1.86 | 3.65 | 5.43 | 4.08 | 10.02 | 10.11 |
| Total others | 9.28 | 6.53 | 9.53 | 11.24 | 10.66 | 16.80 | 20.26 | ||
| Total peak abundance | 99.79 | 99.73 | 99.70 | 98.88 | 99.90 | 99.46 | 99.93 |
| Parameters | 50-30 | 50-45 | 50-60 | 60-30 | 60-45 | 60-60 | Control |
|---|---|---|---|---|---|---|---|
| ATP-related compounds (μmol/g sample) | |||||||
| ATP | 0.25 ± 0.04 | 0.21 ± 0.03 | 0.19 ± 0.06 | 0.25 ± 0.03 | 0.24 ± 0.05 | 0.22 ± 0.04 | 0.25 ± 0.03 |
| ADP | 0.87 ± 0.05 a | 0.86 ± 0.03 a | 0.64 ± 0.03 b | 0.85 ± 0.02 a | 0.55 ± 0.04 c | 0.56 ± 0.03 c | 0.48 ± 0.05 c |
| AMP | 5.30 ± 0.12 a | 5.43 ± 0.09 a | 4.29 ± 0.10 c | 5.02 ± 0.12 b | 4.18 ± 0.27 c | 4.26 ± 0.22 c | 4.07 ± 0.06 c |
| IMP | 12.59 ± 0.85 c | 13.09 ± 0.69 bc | 13.17 ± 0.21 b | 13.14 ± 0.35 b | 14.31 ± 0.47 a | 14.25 ± 0.70 a | 13.30 ± 0.38 b |
| HxR | 0.88 ± 0.03 b | 0.91 ± 0.04 b | 0.91 ± 0.05 b | 0.90 ± 0.06 b | 0.94 ± 0.07 b | 0.92 ± 0.04 b | 2.89 ± 0.06 a |
| Hx | 0.27 ± 0.05 | 0.28 ± 0.04 | 0.25 ± 0.02 | 0.25 ± 0.03 | 0.27 ± 0.03 | 0.25 ± 0.03 | 0.26 ± 0.03 |
| Sensory scores | |||||||
| Odor liking score | 6.58 ± 0.43 | 6.82 ± 0.28 | 6.86 ± 0.29 | 6.90 ± 0.31 | 6.76 ± 0.27 | 6.88 ± 0.32 | 6.78 ± 0.29 |
| Flavor liking score | 6.20 ± 0.25 d | 6.52 ± 0.34 cd | 6.60 ± 0.23 cd | 6.70 ± 0.22 c | 7.88 ± 0.31 a | 7.72 ± 0.25 a | 7.16 ± 0.25 b |
| Overall liking score | 6.36 ± 0.31 c | 6.74 ± 0.28 c | 6.60 ± 0.30 c | 7.04 ± 0.22 b | 7.46 ± 0.21 a | 7.62 ± 0.37 a | 6.56 ± 0.24 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongsetkul, J.; Yongsawatdigul, J.; Boonanuntanasarn, S.; Benjakul, S. Development of Flavor and Taste Components of Sous-Vide-Cooked Nile Tilapia (Oreochromis niloticus) Fillet as Affected by Various Conditions. Foods 2022, 11, 3681. https://doi.org/10.3390/foods11223681
Pongsetkul J, Yongsawatdigul J, Boonanuntanasarn S, Benjakul S. Development of Flavor and Taste Components of Sous-Vide-Cooked Nile Tilapia (Oreochromis niloticus) Fillet as Affected by Various Conditions. Foods. 2022; 11(22):3681. https://doi.org/10.3390/foods11223681
Chicago/Turabian StylePongsetkul, Jaksuma, Jirawat Yongsawatdigul, Surintorn Boonanuntanasarn, and Soottawat Benjakul. 2022. "Development of Flavor and Taste Components of Sous-Vide-Cooked Nile Tilapia (Oreochromis niloticus) Fillet as Affected by Various Conditions" Foods 11, no. 22: 3681. https://doi.org/10.3390/foods11223681
APA StylePongsetkul, J., Yongsawatdigul, J., Boonanuntanasarn, S., & Benjakul, S. (2022). Development of Flavor and Taste Components of Sous-Vide-Cooked Nile Tilapia (Oreochromis niloticus) Fillet as Affected by Various Conditions. Foods, 11(22), 3681. https://doi.org/10.3390/foods11223681









