Effects of Exogenous Caffeic Acid, L-Phenylalanine and NaCl Treatments on Main Active Components Content and In Vitro Digestion of Germinated Tartary Buckwheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Germination Conditions
2.3. Measurement of Soluble Protein Content
2.4. Measurement of Soluble Sugar and Reducing Sugar Contents
2.5. Measurement of Malondialdehyde (MDA) Content
2.6. Measurement of TP and TF Contents
2.7. Measurement of Antioxidant Capacities
2.8. In Vitro Digestion
2.9. Statistical Analysis
3. Results
3.1. Effect of Exogenous Additives on the Growth of Treated Sprouts
3.2. Changes in Soluble Protein Content in TB Sprouts
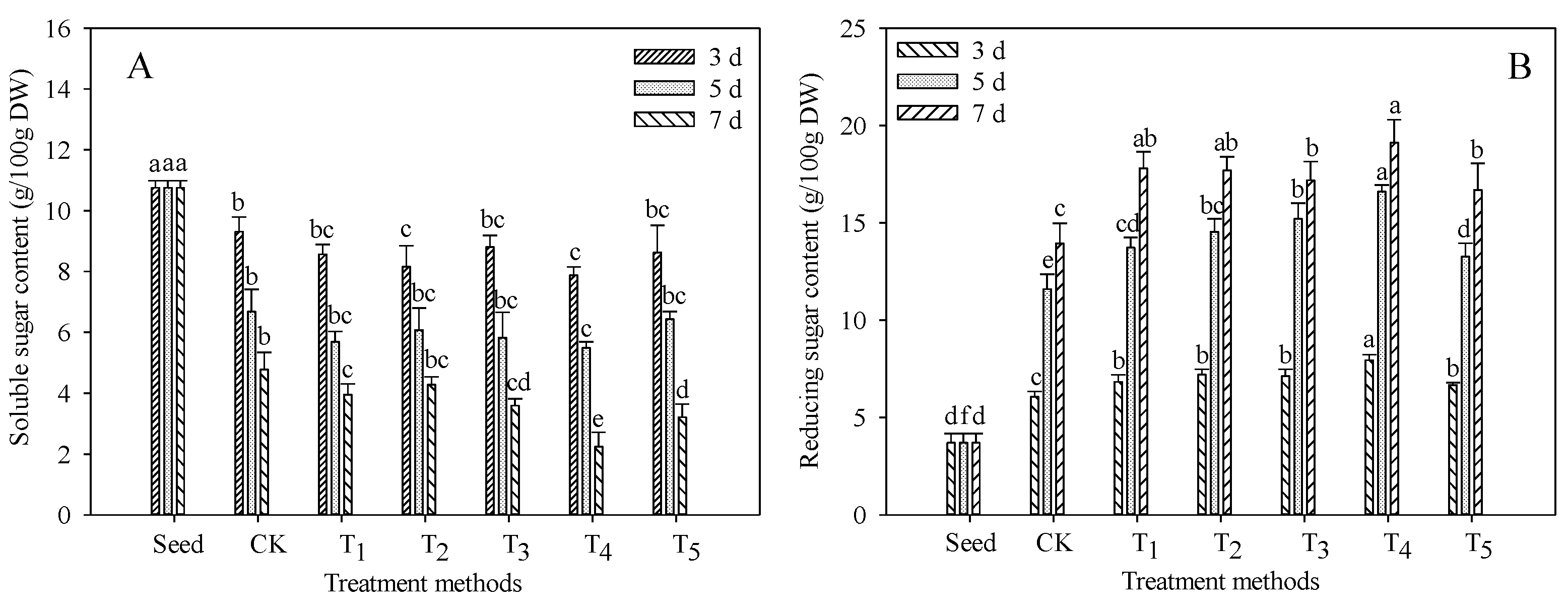
3.3. Changes in Soluble Sugar and Reducing Sugar Contents
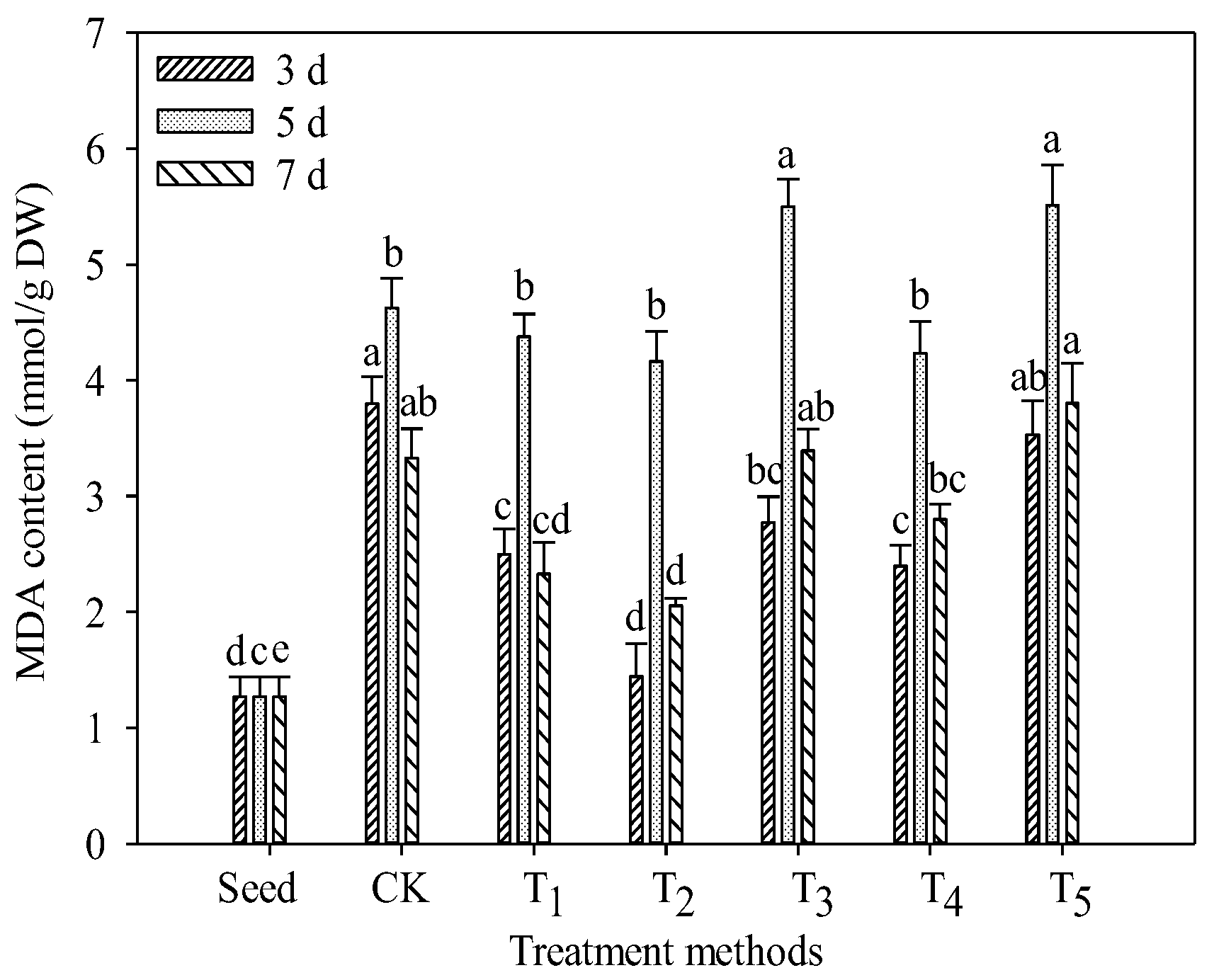
3.4. Changes in Malondialdehyde (MDA) Content
3.5. TF and TP Contents before and after In Vitro Digestion
3.6. DPPH, ABTS and FRAP Radical Scavenging Capacity before and after In Vitro Digestion
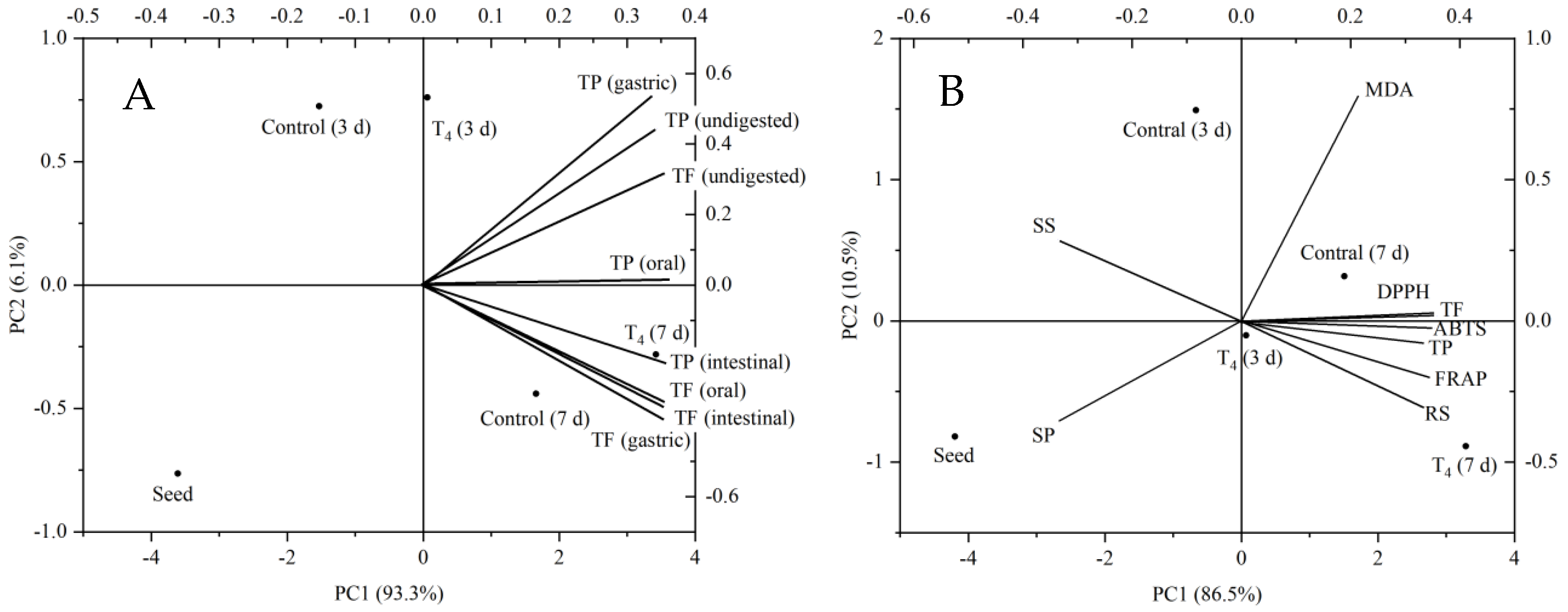
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar, F.A.; Pirzadah, T.B.; Malik, B.; Tahir, I.; Rehman, R.U. Molecular genetics of buckwheat and its role in crop improvement. In Buckwheat Germplasm in the World; Elsevier: Amsterdam, The Netherlands, 2018; pp. 271–286. [Google Scholar] [CrossRef]
- Morishita, T.; Hara, T.; Hara, T. Important agronomic characteristics of yielding ability in common buckwheat; ecotype and ecological differentiation, preharvest sprouting resistance, shattering resistance, and lodging resistance. Breed. Sci. 2020, 70, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Peñas, E.; García, M.d.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arouna, N.; Gabriele, M.; Pucci, L. The Impact of Germination on Sorghum Nutraceutical Properties. Foods 2020, 9, 1218. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W.; Kim, H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Ma, H.; Bian, Z.X.; Chen, X.Y.; Chu, Y.; Wang, S.M. Effects of Microwave combined with L-phe treatment on main nutritional components in germinated Tartary buckwheat. J. Anhui Poly. Univ. 2019, 34, 1–7. [Google Scholar]
- Zhang, M.; Wang, D.; Gao, X.; Yue, Z.; Zhou, H. Exogenous caffeic acid and epicatechin enhance resistance against Botrytis cinerea through activation of the phenylpropanoid pathway in apples. Sci. Hortic. 2020, 268, 109348. [Google Scholar] [CrossRef]
- Alegria, A.; Garcia-Llatas, G.; Cilla, A. Static Digestion Models: General Introduction. Impact Food Bioact. Health 2015, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Menchaca-Armenta, M.; Frutos, M.J.; Ramírez-Wong, B.; Valero-Cases, E.; Muelas-Domingo, R.; Quintero-Ramos, A.; Torres-Chávez, P.I.; Carbonell-Barrachina, A.; Ledesma-Osuna, A.I.; Campas-Baypoli, O.N. Changes in phytochemical content, bioaccesibility and antioxidant capacity of corn tortillas during simulated in vitro gastrointestinal digestion. Food Chem. 2022, 134223. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Pucci, L. Diet bioactive compounds: Implications for oxidative stress and inflammation in the vascular system. Endocr. Metab Immune Disord. Drug Targets 2017, 17, 264–275. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Wang, S.; Wang, J.; Peng, W. Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein. Foods 2022, 11, 1373. [Google Scholar] [CrossRef]
- Hasan, M.T.; AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Artington, VA, USA, 1990. [Google Scholar]
- Zhang, S.W.; Zong, Y.J.; Fang, C.Y.; Huang, S.H.; Li, J.; Xu, J.H.; Wang, Y.F.; Liu, C.H. Optimization of Anthrone Colorimetric Method for Rapid Determination of Soluble Sugar in Barley Leaves. Food Res. Dev. 2020, 41, 196–200. [Google Scholar]
- Zhuang, L.; Xu, K.; Zhu, Y.; Wang, F.; Xiao, J.; Guo, L. Calcium affects glucoraphanin metabolism in broccoli sprouts under ZnSO4 stress. Food Chem. 2021, 334, 127520. [Google Scholar] [CrossRef]
- Ji, H.; Tang, W.; Zhou, X.; Wu, Y. Combined Effects of Blue and Ultraviolet Lights on the Accumulation of Flavonoids in Tartary Buckwheat Sprouts. Pol. J. Food Nutr. Sci. 2016, 66, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Živković, A.; Polak, T.; Cigić, B.; Požrl, T. Germinated Buckwheat: Effects of Dehulling on Phenolics Profile and Antioxidant Activity of Buckwheat Seeds. Foods 2021, 10, 740. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Lv, X.; Meng, G.; Li, W.; Fan, D.; Wang, X.; Espinoza-Pinochet, C.A.; Cespedes-Acuña, C.L. Sulforaphane and its antioxidative effects in broccoli seeds and sprouts of different cultivars. Food Chem. 2020, 316, 126216. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Vidal, J.; Ruiz-Riaguas, A.; Córdova, M.F.-D.; Ortega-Barrales, P.; Llorent-Martínez, E. Phenolic profile and antioxidant activity of Jasonia glutinosa herbal tea. Influence of simulated gastrointestinal in vitro digestion. Food Chem. 2019, 287, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Corona-Leo, L.S.; Meza-Márquez, O.G.; Hernández-Martínez, D.M. Effect of in vitro digestion on phenolic compounds and antioxidant capacity of different apple (Malus domestica) varieties harvested in Mexico. Food Biosci. 2021, 43, 101311. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, H.M.; Zeng, W.J.; Li, Q.; Wang, Y.; Wang, S.Q. Physiological responses of five plants in northwest China arid area under drought stress. Chin. J. Appl. Ecol. 2017, 28, 1455–1463. [Google Scholar] [CrossRef]
- Shi, R.G.; Zhao, H.Y.; Qi, M.Y.; Dang, C.X.; Li, W.; Huang, H.Y.; Lin, H. Effects of exogenous Choline chloride and calcium chloride on germination and physiological characteristics of wheat under salt stress. J. Anhui Ag. Sci. 2020, 48, 22–26. [Google Scholar]
- Yin, Y.; Tian, X.; He, X.; Yang, J.; Yang, Z.; Fang, W. Exogenous melatonin stimulated isoflavone biosynthesis in NaCl-stressed germinating soybean (Glycine max L.). Plant Physiol Bioch. 2022, 185, 123–131. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and Antioxidant Activity of Caffeic Acid Derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.Y.; Chwn, C.L.; He, G.P.; Liu, S.Q.; Lv, Y.P. Antioxidant Capacity of Mango Fermented Beverage in Simulated Digestion in Vitro. Food Sci. Tech.-Brazil. 2021, 46, 110–115. [Google Scholar] [CrossRef]
- Sengul, H.; Surek, E.; Nilufer-Erdil, D. Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in-vitro gastrointestinal digestion model. Food Res. Int. 2014, 62, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.C.; Souza, M.C.; Sumere, B.R.; Silva, L.G.; da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.; Rostagno, M.A. Simultaneous extraction and separation of bioactive compounds from apple pomace using pressurized liquids coupled on-line with solid-phase extraction. Food Chem. 2020, 318, 126450. [Google Scholar] [CrossRef]
- Pastor, F.T.; Šegan, D.M.; Gorjanović, S.; Kalušević, A.M.; Sužnjević, D. Development of voltammetric methods for antioxidant activity determination based on Fe(III) reduction. Microchem. J. 2020, 155, 104721. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.; Wang, S.; Wang, J.; Wang, S. Effects of microwave irradiation of Fagopyrum tataricum seeds on the physicochemical and functional attributes of sprouts. LWT 2022, 165, 113738. [Google Scholar] [CrossRef]
- Liao, X.; Zheng, S.J.; Lu, K.K.; Xiao, X.N.; Wu, A.R.; Ming, J. Plant Polyphenols Exert Antioxidant Activity of by Nrf2/ARE Signaling Pathway: A Review. Food Sci. 2016, 37, 227–232. [Google Scholar]
- Yang, R.L.; Guo, Z.Z.; Shi, Y.Q.; Guo, M.Y.; Huang, W. Changes in bioaccessibility and antioxidant activity of phenolics in Prunus mume during simulated digestion in vitro. Food FI 2022, 1–8. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Tang, N.; Liu, R.; Nie, R.; Guo, Y.; Liu, R.; Chang, M. Effects of different processing methods on bioactive substances and antioxidation properties of Lycium barbarum (goji berry) from China. Food Biosci. 2021, 42, 101048. [Google Scholar] [CrossRef]
- GUO, X.H.; Yan, H.; ZHANG, Y.; Yi, W.; HUANG, S.X.; LIU, Y.S.; Wei, L. Kiwifruit (Actinidia chinensis ‘Hongyang’) cytosolic ascorbate peroxidases (AcAPX1 and AcAPX2) enhance salinity tolerance in Arabidopsis thaliana. J. Integr. Agr. 2022, 21, 1058–1070. [Google Scholar] [CrossRef]
- Gueta-Dahan, Y.; Yaniv, Z.; Zilinskas, B.A.; Ben-Hayyim, G. Salt and oxidative stress: Similar and specific responses and their relation to salt tolerance in Citrus. Planta 1997, 203, 460–469. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.L.; Wang, L.; Qin, C.P.; Ma, J.Z. Research progress on pharmacological action of caffeic acid and its derivatives. Prog. Veter. Med. 2021, 42, 103–106. [Google Scholar] [CrossRef]
- Wu, W.M.; Liang, L.; Yuan, L.; Wang, T.; Lei, L.; Qiang, C.; Rui, W. Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: A structure–activity insight. Food Chem. 2007, 105, 107–115. [Google Scholar] [CrossRef]



| Treatment Methods | Treatment Conditions | ||
|---|---|---|---|
| CA (mg/L) | L-Phe (mmol/L) | NaCl (mmol/L) | |
| CK | 0 | 0 | 0 |
| T1 | 17 | 0 | 0 |
| T2 | 0 | 2.7 | 0 |
| T3 | 0 | 0 | 2.7 |
| T4 | 17 | 2.7 | 2.7 |
| T5 | 30 | 2.7 | 2.7 |
| Chemical Extraction | Oral Phase | Gastric Phase | Intestinal Phase | Bioaccessibility/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | |
| Seed | 55.37 ± 1.51 d | 78.03 ± 20.65 d | 12.35 ± 0.52 f | 3.09 ± 0.31 e | 6.57 ± 0.29 e | 3.06 ± 0.16 f | 8.98 ± 0.25 e | 12.64 ± 0.33 f | 5.87 ± 0.12 e | 9.07 ± 0.55 f | 16.53 ± 0.10 f | 6.54 ± 0.21 f | 16.39 | 21.18 | 52.96 |
| T1 | 159.37 ± 6.24 bc | 235.64 ± 5.97 c | 40.65 ± 1.80 c | 42.11 ± 1.49 b | 68.97 ± 0.69 b | 12.09 ± 0.38 b | 92.63 ± 3.22 c | 89.41 ± 1.49 c | 17.96 ± 0.71 c | 123.55 ± 8.23 d | 183.91 ± 2.45 c | 25.40 ± 0.25 c | 77.53 | 78.05 | 62.48 |
| T2 | 166.09 ± 5.26 a | 267.22 ± 6.19 ab | 43.06 ± 1.90 b | 42.92 ± 0.69 b | 70.18 ± 0.66 ab | 12.42 ± 0.36 b | 99.06 ± 3.90 b | 94.01 ± 2.11 b | 19.20 ± 0.27 b | 142.19 ± 5.08 b | 189.34 ± 1.47 b | 26.90 ± 0.58 b | 85.61 | 70.85 | 62.46 |
| T3 | 160.81 ± 7.83 b | 244.31 ± 10.19 bc | 37.72 ± 0.72 d | 39.46 ± 1.08 c | 67.63 ± 1.89 c | 10.70 ± 0.50 d | 103.04 ± 4.91 b | 89.32 ± 2.02 c | 18.58 ± 1.01 c | 131.86 ± 5.40 c | 180.93 ± 4.31 c | 25.89 ± 0.34 c | 82.00 | 74.06 | 68.63 |
| T4 | 174.74 ± 3.21 a | 278.99 ± 6.50 a | 45.25 ± 2.08 a | 51.05 ± 1.62 a | 73.43 ± 1.55 a | 13.44 ± 0.38 a | 125.99 ± 6.72 a | 109.54 ± 1.62 a | 20.27 ± 0.57 a | 155.21 ± 2.72 a | 196.26 ± 4.29 a | 28.14 ± 0.60 a | 88.82 | 70.35 | 62.19 |
| T5 | 159.94 ± 3.17 c | 236.87 ± 22.48 c | 37.75 ± 2.16 d | 44.13 ± 1.65 b | 67.49 ± 0.84 c | 11.20 ± 0.62 c | 98.60 ± 4.87 bc | 84.39 ± 3.59 d | 19.12 ± 0.61 b | 141.74 ± 7.86 b | 167.37 ± 2.54 d | 24.17 ± 0.58 d | 88.62 | 70.66 | 64.04 |
| CK | 147.07 ± 5.28 c | 218.30 ± 17.14 c | 32.84 ± 1.07 e | 34.50 ± 1.97 d | 54.95 ± 0.56 d | 9.89 ± 0.24 e | 79.78 ± 5.28 d | 70.59 ± 2.11 e | 15.89 ± 0.54 d | 107.39 ± 5.73 e | 148.92 ± 6.76 e | 21.24 ± 0.76 e | 73.02 | 68.22 | 64.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Wang, N.; Wang, S.; Wang, J.; Dong, Y. Effects of Exogenous Caffeic Acid, L-Phenylalanine and NaCl Treatments on Main Active Components Content and In Vitro Digestion of Germinated Tartary Buckwheat. Foods 2022, 11, 3682. https://doi.org/10.3390/foods11223682
Peng W, Wang N, Wang S, Wang J, Dong Y. Effects of Exogenous Caffeic Acid, L-Phenylalanine and NaCl Treatments on Main Active Components Content and In Vitro Digestion of Germinated Tartary Buckwheat. Foods. 2022; 11(22):3682. https://doi.org/10.3390/foods11223682
Chicago/Turabian StylePeng, Wenping, Nan Wang, Shunmin Wang, Junzhen Wang, and Yulu Dong. 2022. "Effects of Exogenous Caffeic Acid, L-Phenylalanine and NaCl Treatments on Main Active Components Content and In Vitro Digestion of Germinated Tartary Buckwheat" Foods 11, no. 22: 3682. https://doi.org/10.3390/foods11223682
APA StylePeng, W., Wang, N., Wang, S., Wang, J., & Dong, Y. (2022). Effects of Exogenous Caffeic Acid, L-Phenylalanine and NaCl Treatments on Main Active Components Content and In Vitro Digestion of Germinated Tartary Buckwheat. Foods, 11(22), 3682. https://doi.org/10.3390/foods11223682






