Methylation, Hydroxylation, Glycosylation and Acylation Affect the Transport of Wine Anthocyanins in Caco-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Anthocyanin Samples
2.3. HPLC Analysis
2.4. Cultivation of Caco-2 Cells and Cytotoxicity Tests
2.4.1. Cultivation of Caco-2 Cells
2.4.2. Cytotoxicity Tests
2.5. Transmembrane Transport of Anthocyanins in Caco-2 Cells
2.6. Anthocyanin Stability Test
2.7. Experiments on the Influence of Phloridzin and Phloretin on the Transport of Mv-3-glc
2.8. RT-qPCR and Western Blotting Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicity Tests
3.2. Influence of Structure and Concentration on Transport
3.2.1. The Effect of Structure and Concentration on the Transport of Anthocyanin Extracts in Caco-2 Cells
Transport of Monoglycoside Mixture
Transport of Diglycoside Mixture
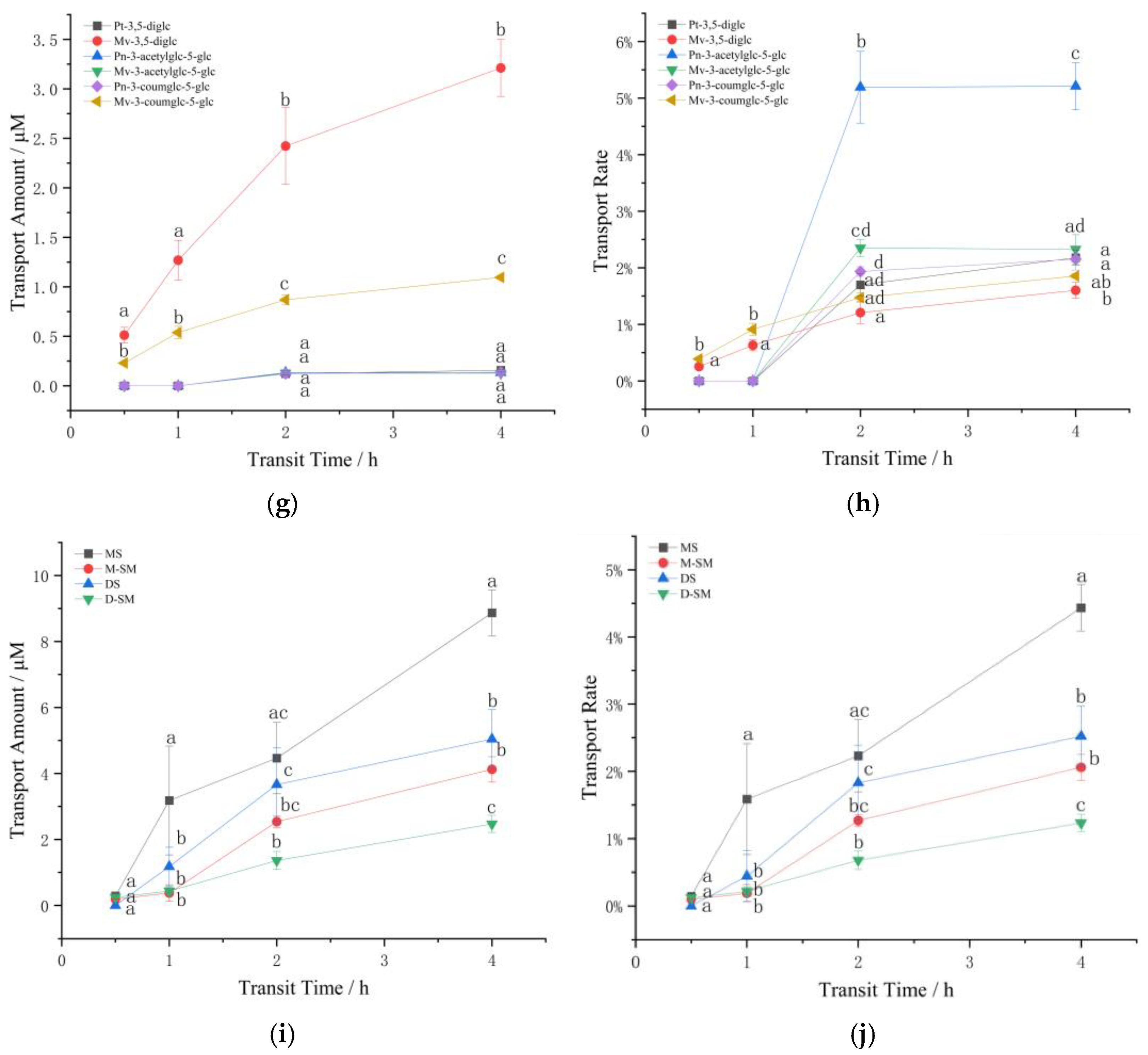
3.2.2. The Effect of Structure and Concentration on the Transport of Standard Anthocyanins in Caco-2
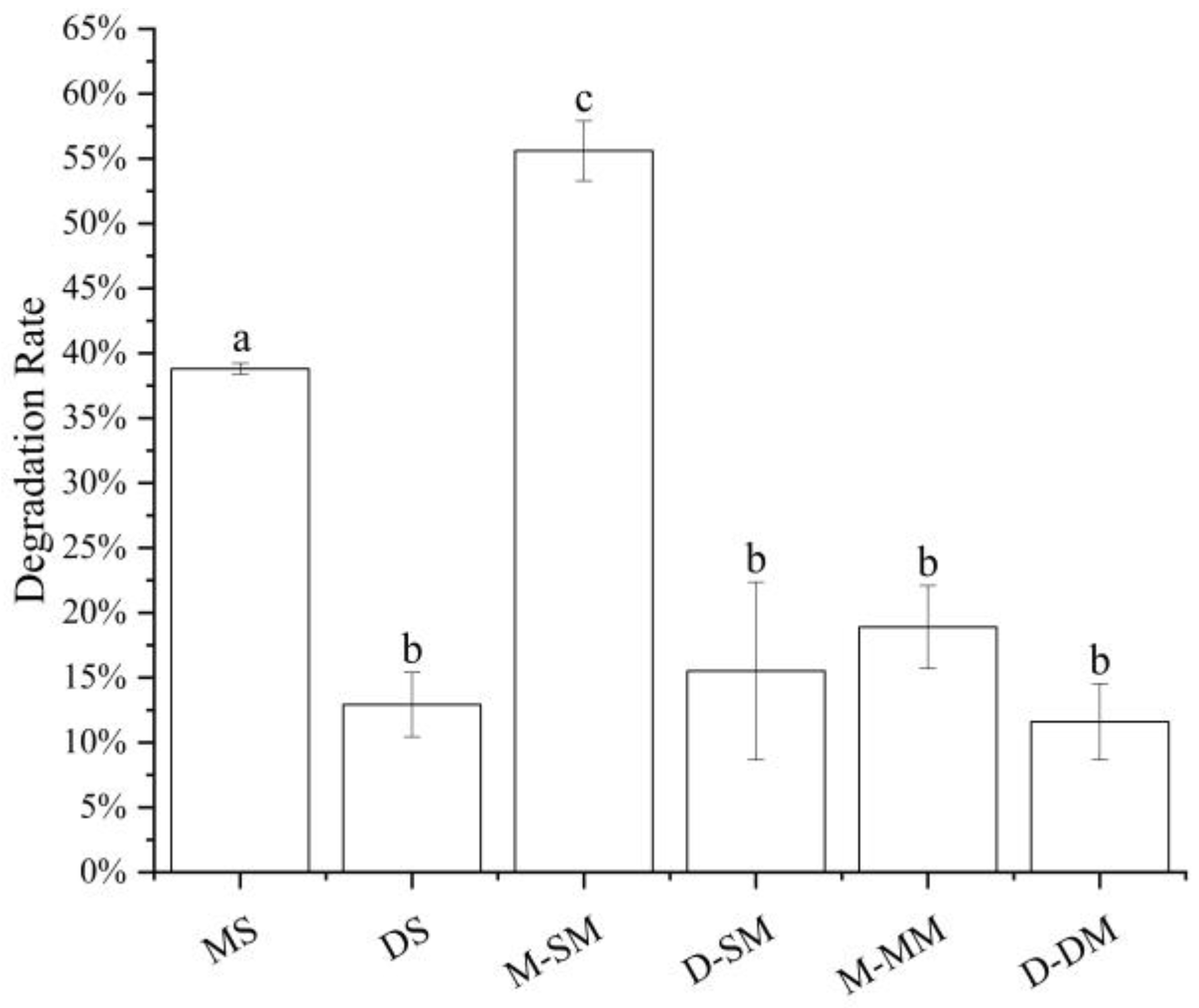
3.3. The Influence of Simulated Cell Transport Conditions on the Stability of Anthocyanins
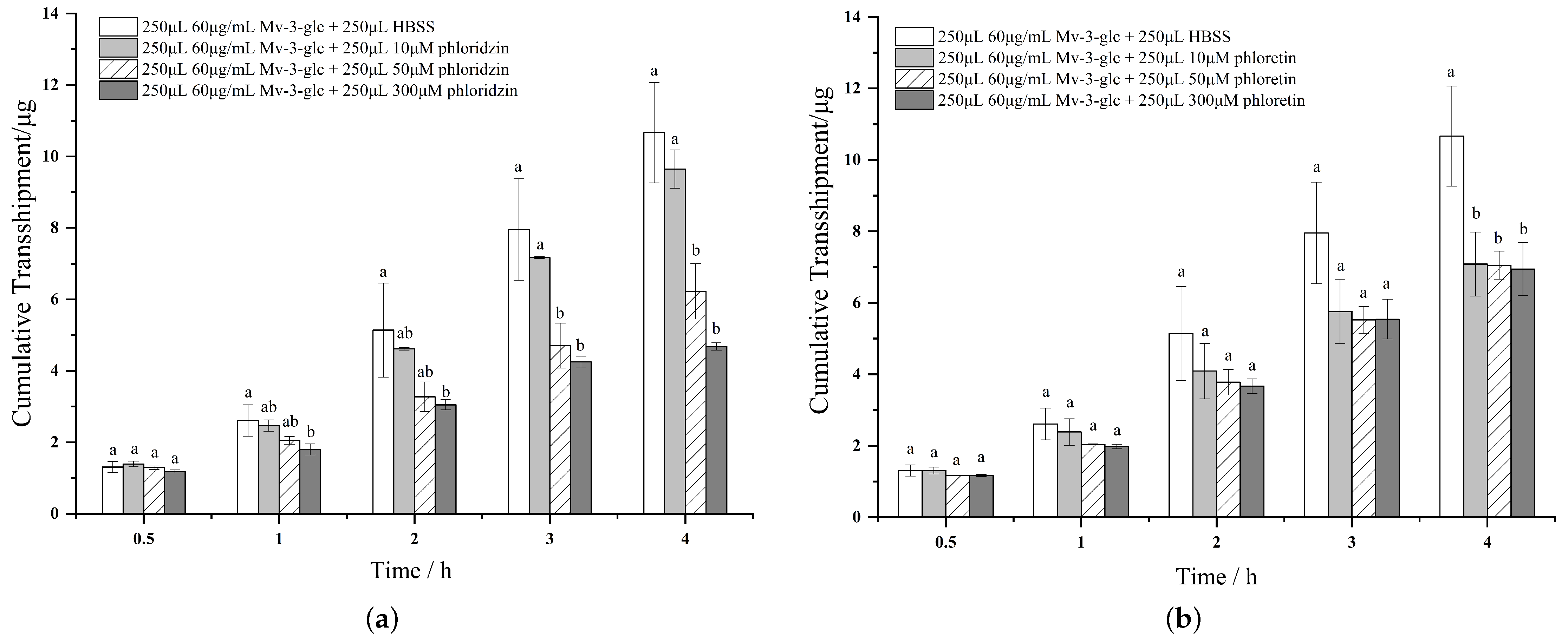
3.4. The Effect of Phloridzin and Phloretin on the Transport of Mv-3-glc
3.5. The Effect of Anthocyanin Transport on Cellular mRNA and Protein Expression
3.5.1. The Effect of Anthocyanin Transport on Cellular mRNA
3.5.2. The Effect of Anthocyanin on Cell Protein Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhang, J.; Zhang, L.F. Study on the mechanism of non-covalent interaction between rose anthocyanin extracts and whey protein isolate under different pH conditions. Food Chem. 2022, 384, 132492. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.Q.; Chu, M.J.; Yu, X.T.; Xie, Y.N.; Li, Y.L.; Du, Y.M.; Liu, X.M.; Zhang, Z.F.; Shi, O.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2022, 1–29. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: Its relationship to phenolic profile. J. Funct. Food. 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, N.; He, F.; Duan, C.Q. Reactivity comparison of three malvidin-type anthocyanins forming derived pigments in model wine solutions. Food Chem. 2022, 384, 132534. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, C.L.; Wang, H.; Han, F.L.; Liu, Y.J.; Wang, L.; Liu, Y. Stability of anthocyanins and their degradation products from cabernet sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef] [Green Version]
- Casedas, G.; Les, F.; Gomez-Serranillos, M.P.; Smith, C.; Lopez, V. Anthocyanin profile, antioxidant activity and enzyme inhibiting properties of blueberry and cranberry juices: A comparative study. Food Funct. 2017, 8, 4187–4193. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, Y.; Jin, S.G.; Kim, J.Y. Acai berry extract as a regulator of intestinal inflammation pathways in a Caco-2 and RAW 264.7 co-culture model. J. Food Biochem. 2021, 45, e13848. [Google Scholar] [CrossRef]
- Verediano, T.A.; Martino, H.S.D.; Kolba, N.; Fu, Y.M.; Paes, M.C.D.; Tako, E. Black corn (Zea mays L.) soluble extract showed anti-inflammatory effects and improved the intestinal barrier integrity in vivo (Gallus gallus). Food Res. Int. 2022, 157, 111227. [Google Scholar] [CrossRef]
- Huang, W.Y.; Yan, Z.; Li, D.J.; Ma, Y.H.; Zhou, J.Z.; Sui, Z.Q. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.F.D.; Sun, X.F.; Peluzio, M.D.G.; Zhu, M.J. Anthocyanins/anthocyanidins and colorectal cancer: What is behind the scenes? Crit. Rev. Food Sci. 2017, 59, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wei, X.T.; Kortesniemi, M.; Pariyani, R.; Zhang, Y.M.; Yang, B.R. Effects of acylated and nonacylated anthocyanins extracts on gut metabolites and microbiota in diabetic Zucker rats: A metabolomic and metagenomic study. Food Res. Int. 2022, 153, 110978. [Google Scholar] [CrossRef]
- Solverson, P. Anthocyanin bioactivity in obesity and diabetes: The essential role of glucose transporters in the gut and periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.K.; Bai, X.L.; Yuan, H.; Zhang, Y.; Ayeni, E.A.; Liao, X. Polyphenolic glycosides from the fruits extract of Lycium ruthenicum Murr and their monoamine oxidase B inhibitory and neuroprotective activities. J. Agric. Food Chem. 2022, 70, 7968–7980. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.L.; Simon, J.E.; Lila, M.A.; Rochet, J. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson׳s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef] [Green Version]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef] [Green Version]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef]
- Elferink, H.; Bruekers, J.P.J.; Veeneman, G.H.; Boltje, T.J. A comprehensive overview of substrate specificity of glycoside hydrolases and transporters in the small intestine: “A gut feeling”. Cell. Mol. Life Sci. 2020, 77, 4799–4826. [Google Scholar] [CrossRef]
- Kellett, G.L.; Helliwell, P.A. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem. J. 2000, 350, 155–162. [Google Scholar] [CrossRef]
- Koop, B.L.; da Silva, M.N.; da Silva, F.D.; Lima, K.T.D.; Soares, L.S.; de Andrade, C.J.; Valencia, G.A.; Monteiro, A.R. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: Sources, classification and enhanced stabilization by encapsulation and adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional uses, structural and functional variations, approaches to increase yields and products’ quality, hepatoprotection, liver longevity, and commercial products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef] [PubMed]
- Hahm, T.H.; Tanaka, M.; Matsui, T. Current knowledge on intestinal absorption of anthocyanins. J. Agric. Food Chem. 2022, 70, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Victoria-Campos, C.I.; Ornelas-Paz, J.D.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Failla, M.L.; Pérez-Martínez, J.D.; Rios-Velasco, C.; Ibarra-Junquera, V. Gastrointestinal metabolism and bioaccessibility of selected anthocyanins isolated from commonly consumed fruits. Food Chem. 2022, 383, 132451. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Rompp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef] [Green Version]
- Steinert, R.E.; Ditscheid, B.; Netzel, M.; Jahreis, G. Absorption of black currant anthocyanins by monolayers of human intestinal epithelial Caco-2 cells mounted in ussing type chambers. J. Agric. Food Chem. 2008, 56, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.G.; Akoh, C.C.; Fischer, J.; Krewer, G. Absorption of anthocyanins from blueberry extracts by Caco-2 human intestinal cell monolayers. J. Agric. Food Chem. 2006, 54, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Atnip, A.; Bomser, J.; Giusti, M.M. Aglycone structures and glycosylations affect anthocyanin transport and uptake in human gastric epithelial (NCI-N87) cells. J. Food Compos. Anal. 2018, 65, 33–39. [Google Scholar] [CrossRef]
- Novotny, J.A.; Clevidence, B.A.; Kurilich, A.C. Anthocyanin kinetics are dependent on anthocyanin structure. Br. J. Nutr. 2012, 107, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Liu, J.; Li, Z.; Wang, L.Q.; Gao, W.N.; Zhang, Z.Q.; Guo, C.J. Sodium-dependent glucose transporter 1 and glucose transporter 2 mediate intestinal transport of quercetrin in Caco-2 cells. Food Nutr. Res. 2020, 64, 3745. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; Feng, D.; Song, G.; Li, H.W.; Tang, H.W.; Ling, W.H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of Cyanidin-3-O-β-glucoside in Caco-2 cells. Nutrients 2014, 6, 4165–4177. [Google Scholar] [CrossRef] [PubMed]
- Han, F.L.; Oliveira, H.; Brás, N.F.; Fernandes, I.; Cruz, L.; De Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins—Impact of structural complexity and phase II metabolization. Food Chem. 2020, 317, 126398. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.P.; Chan, L.K.Y.; Leung, P.S. Involvement of the niacin receptor GPR109a in the local control of glucose uptake in small intestine of type 2 diabetic mice. Nutrients 2015, 7, 7543–7561. [Google Scholar] [CrossRef] [Green Version]
- Asenstorfer, R.E.; Lee, D.F.; Jones, G.P. Influence of structure on the ionisation constants of anthocyanin and anthocyanin-like wine pigments. Anal. Chim. Acta 2006, 563, 10–14. [Google Scholar] [CrossRef]
- Zhang, J.J.; Giampieri, F.; Afrin, S.; Battino, M.; Zheng, X.D.; Reboredo-Rodriguez, P. Structure-stability relationship of anthocyanins under cell culture condition. Int. J. Food Sci. Nutr. 2019, 70, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, Y.N.; Li, S.L.; Wu, C.Q.; Wang, J.Q.; Zheng, N. Modulation of mucin (MUC2, MUC5AC and MUC5B) mRNA expression and protein production and secretion in Caco-2/HT29-MTX Co-Cultures following exposure to individual and combined aflatoxin M1 and ochratoxin A. Toxins 2019, 11, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Hassan, Y.I.; Renaud, J.; Liu, R.H.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono- and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef]



| Anthocyanins | Substitution Pattern | ||||||
|---|---|---|---|---|---|---|---|
| 3 | 5 | 6 | 7 | 3′ | 4′ | 5′ | |
| Delphinidin (Dp) | OH | OH | H | OH | OH | OH | OH |
| Cyanidin (Cy) | OH | OH | H | OH | H | OH | H |
| Petunidin (Pt) | OH | OH | H | OH | OMe | OH | OH |
| Peonidin (Pn) | OH | OH | H | OH | OMe | OH | H |
| Malvidin (Mv) | OH | OH | H | OH | OMe | OH | OMe |
| Pelargonidin (Pg) | OH | OH | H | OH | H | OH | H |
| Gene | Primer | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| Human SLC5A1 (AF070544.1) | CAGATGATGCGGGAGAAGAA | CGAAGATGCTCGTGGAGTAATA |
| Human SLC2A2 (J03810.1) | ATGAACTGCCCACAATCTCATA | GGACCAGAGCATGGTGATTAG |
| Human β-Actin (NM_001101.5) | CCTTCCTGGGCATGGAGTC | TGATCTTCATTGTGCTGGGTG |
| Concentration (μM) | Cell Survival Rate (%) | ||||
|---|---|---|---|---|---|
| MM | DM | MS | DS | SM | |
| 0 | 100 ± 3.78 a,b | 100 ± 3.78 b | 100 ± 3.78 b | 100 ± 3.78 a,b | 100 ± 3.78 a |
| 25 | 118.57 ± 5.66 a | 103.00 ± 1.38 a | 117.14 ± 8.83 a | 108.14 ± 13.10 a | 95.00 ± 20.54 a |
| 50 | 100.71 ± 3.74 a,b | 99.29 ± 3.16 b | 95.71 ± 8.83 b | 93.71 ± 8.79 a,b | 92.57 ± 2.39 a,b |
| 100 | 84.00 ± 8.60 b | 92.71 ± 1.93 c | 86.57 ± 5.60 c | 88.29 ± 16.97 b,c | 81.43 ± 7.13 b,c |
| 200 | 81.71 ± 7.96 c | 85.71 ± 3.93 c | 86.29 ± 5.43 c | 75.29 ± 8.24 c | 73.71 ± 4.52 c |
| 400 | 63.71 ± 6.93 d | 46.71 ± 9.65 d | 74.71 ± 5.94 d | 57.57 ± 20.85 d | 52.43 ± 3.44 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lin, J.; Cheng, T.; Liu, Y.; Han, F. Methylation, Hydroxylation, Glycosylation and Acylation Affect the Transport of Wine Anthocyanins in Caco-2 Cells. Foods 2022, 11, 3793. https://doi.org/10.3390/foods11233793
Liu Y, Lin J, Cheng T, Liu Y, Han F. Methylation, Hydroxylation, Glycosylation and Acylation Affect the Transport of Wine Anthocyanins in Caco-2 Cells. Foods. 2022; 11(23):3793. https://doi.org/10.3390/foods11233793
Chicago/Turabian StyleLiu, Yang, Jiali Lin, Tiantian Cheng, Yangjie Liu, and Fuliang Han. 2022. "Methylation, Hydroxylation, Glycosylation and Acylation Affect the Transport of Wine Anthocyanins in Caco-2 Cells" Foods 11, no. 23: 3793. https://doi.org/10.3390/foods11233793
APA StyleLiu, Y., Lin, J., Cheng, T., Liu, Y., & Han, F. (2022). Methylation, Hydroxylation, Glycosylation and Acylation Affect the Transport of Wine Anthocyanins in Caco-2 Cells. Foods, 11(23), 3793. https://doi.org/10.3390/foods11233793