Domestic Cooking Affects the Prebiotic Performances of Chinese Yam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cooked Yam
2.2. Preparation and Purification of Yam Polysaccharides from Cooked Yams
2.3. In Vitro Fermentation of Cooked Yams and Yam Polysaccharides
2.4. Gut Microbiota Analysis
2.5. Determination of Polysaccharide Content in Yams
2.6. Determination of Polysaccharide Molecular Weight
2.7. Evaluation of Probiotic Effects of Yam Powder
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Household Cooking Process on Yam Polysaccharides
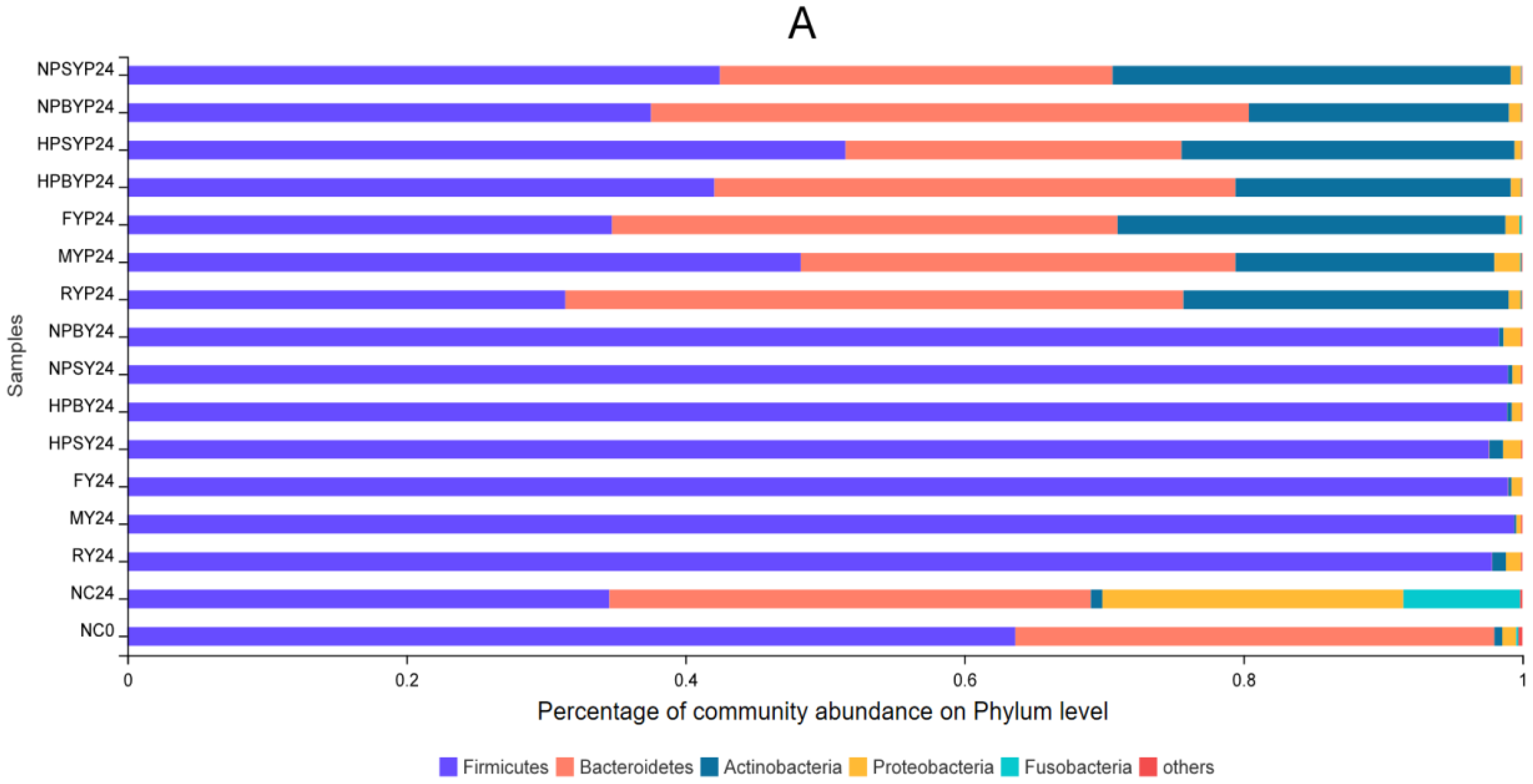
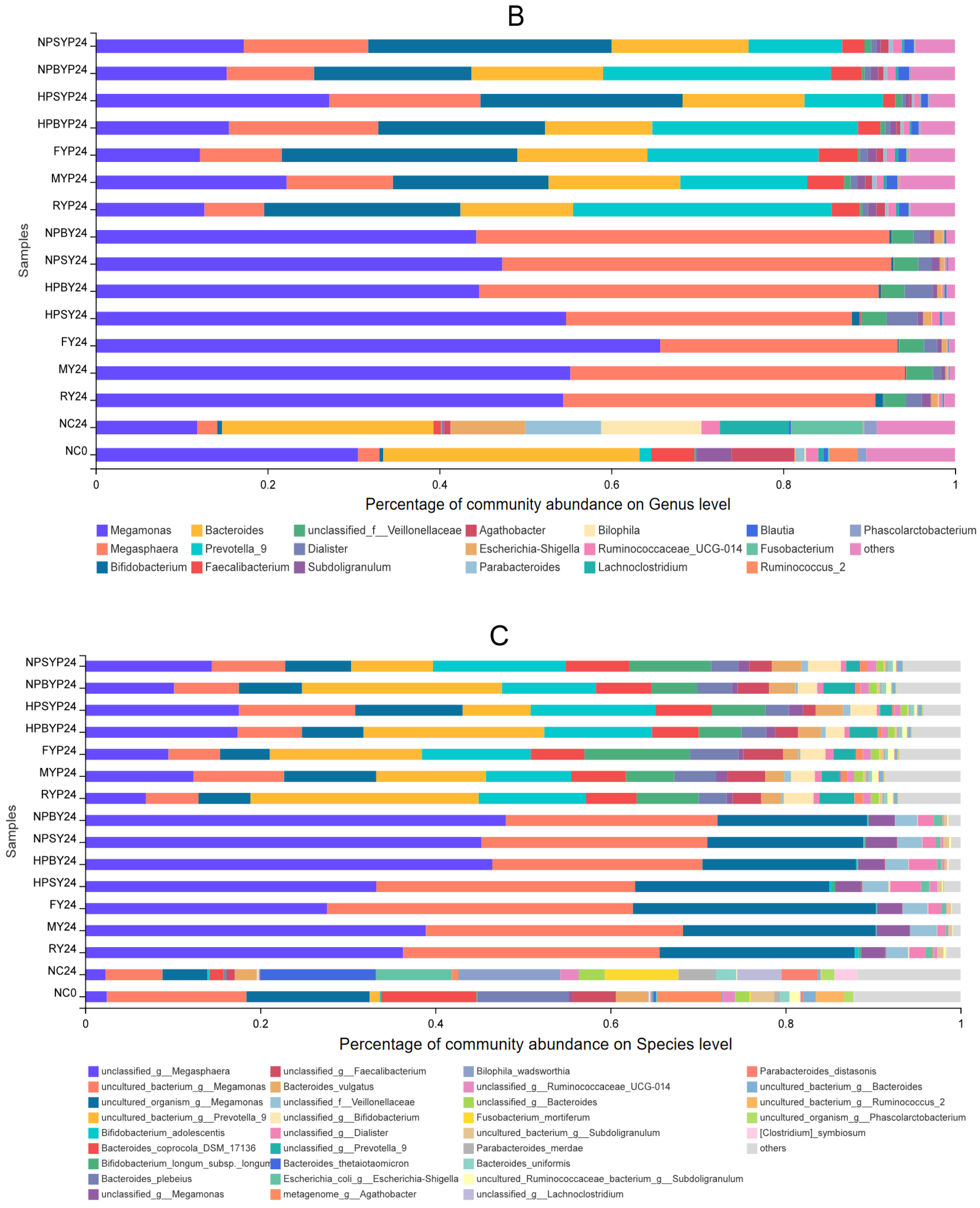
3.2. Effect of Yams and Yam Polysaccharides on Gut Microbiota Communities
3.3. Correlation Analysis
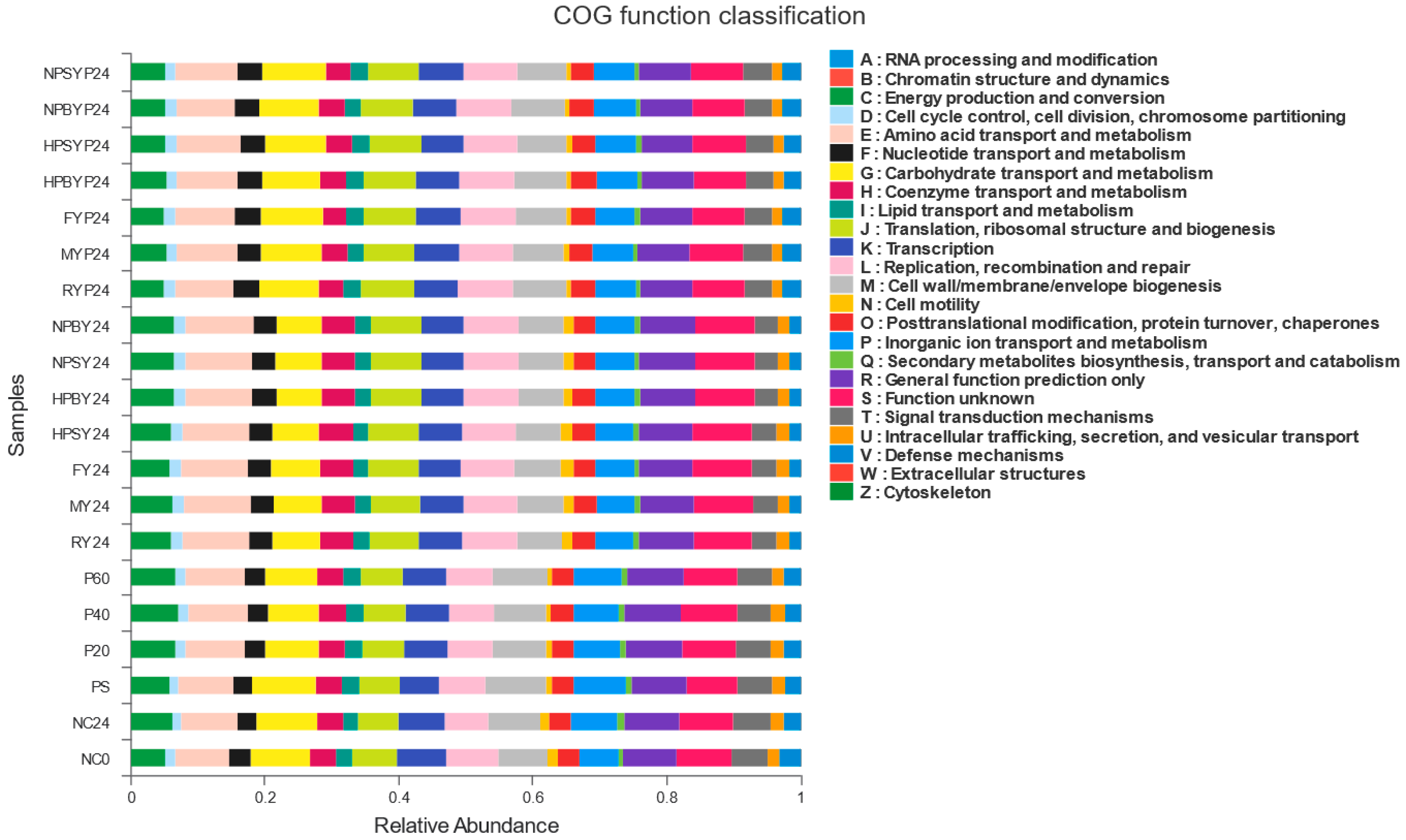
3.4. Function Prediction of PICRUSt
3.5. Analysis of the Probiotic Effect of Yam Powder with Different Processing Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, B.; Yu, L.; Liu, J.; Wang, T.T.Y.; Luo, Y.; Yu, L.L.; Zhang, H.; Gong, L.; Wang, J. Home-based preparation approaches altered the availability of health beneficial components from carrot and blueberry. Food Sci. Nutr. 2017, 5, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Hu, M.; Tao, J.; Yang, H.; Yan, P.; An, G.; Wang, H. The protective effects of Chinese yam polysaccharide against obesity-induced insulin resistance. J. Funct. Foods 2019, 55, 238–247. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Yu, Y.; Shen, M. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Zhang, Y.Z.; Wu, X.; Yin, Y.L.; Tan, Z.L.; Feng, Y.; Yan, F.Y.; Bo, M.J.; Huang, R.L.; Li, T.J. Fermentation characterization of chinese yam polysaccharide and its effects on the gut microbiota of rats. Int. J. Microbiol. 2009, 2009, 598152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Gong, L.; Wen, T.; Wang, J. Role of the Microbiome in Mediating Health Effects of Dietary Components. J. Agric. Food Chem. 2020, 68, 12820–12835. [Google Scholar] [CrossRef]
- Zhang, N.; Liang, T.; Jin, Q.; Shen, C.; Zhang, Y.; Jing, P. Chinese yam (Dioscorea opposita Thunb.) alleviates antibiotic-associated diarrhea, modifies intestinal microbiota, and increases the level of short-chain fatty acids in mice. Food Res. Int. 2019, 122, 191–198. [Google Scholar] [CrossRef]
- Jeon, J.R.; Lee, J.S.; Lee, C.H.; Kim, J.Y.; Kim, S.D.; Nam, D.H. Effect of ethanol extract of dried Chinese yam (Dioscorea batatas) flour containing dioscin on gastrointestinal function in rat model. Arch. Pharmacal Res. 2006, 29, 348–353. [Google Scholar] [CrossRef]
- Nishimura, N.; Tanabe, H.; Yamamoto, T.; Fukushima, M. Raw Chinese Yam (Dioscorea opposita) Promotes Cecal Fermentation and Reduces Plasma Non-HDL Cholesterol Concentration in Rats. J. Nutr. Sci. Vitaminol. 2011, 57, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Jie-Lun, H.; Shao-Ping, N.; Ming-Yong, X. Antidiabetic mechanism of dietary polysaccharides based on their gastrointestinal functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Chen, Z.; Gao, X.; Yuan, G.; Pan, Y.; Chen, H.J.F.R.I. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res. Int. 2018, 103, 280. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Kasaoka, S.; Kiriyama, S. Physiological functions of resistant proteins: Proteins and peptides regulating large bowel fermentation of indigestible polysaccharide. J. AOAC Int. 2004, 87, 792–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; San Cheang, W.; Liu, B.; Zhao, K.; Miao, S.; Riviere, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Shiga, T.M.; Cordenunsi, B.R.; Lajolo, F.M. Effect of cooking on non-starch polysaccharides of hard-to-cook beans. Carbohydr. Polym. 2009, 76, 100–109. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Pastoriza, S.; Jimenez-Hernandez, N.; D’Auria, G.; Francino, M.P.; Rufian-Henares, J.A. Effect of Food Thermal Processing on the Composition of the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Hu, L.; Feng, D.; Chi, J.; Wang, B.; Wang, J. Effects of different household cooking methods on the biological properties of Chinese yam. Food Chem. 2021, 363, 130246. [Google Scholar] [CrossRef]
- Carmody, R.N.; Bisanz, J.E.; Bowen, B.P.; Maurice, C.F.; Lyalina, S.; Louie, K.B.; Treen, D.; Chadaideh, K.S.; Rekdal, V.M.; Bess, E.N.; et al. Cooking shapes the structure and function of the gut microbiome. Nat. Microbiol. 2019, 4, 2052–2063. [Google Scholar] [CrossRef]
- Weiwei, L.; Jingya, W.; Zhongqin, C.; Xudong, G.; Yue, C.; Zihan, X.; Qingwen, G.; Qiqi, M.; Haixia, C. Physicochemical properties of polysaccharides from Lentinus edodes under high pressure cooking treatment and its enhanced anticancer effects. Int. J. Biol. Macromol. 2018, 115, 994–1001. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Zhang, C.; Tian, Y.; Zhang, F.; Li, X. Structural and functional analyses of three purified polysaccharides isolated from Chinese Huaishan-yams. Int. J. Biol. Macromol. 2018, 120, 693–701. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Peng, Y.; Rui, Y.; Zeng, X.; Ye, H. Simulated digestion and fermentation in vitro with human gut microbiota of polysaccharides from Coralline pilulifera. LWT 2019, 100, 167–174. [Google Scholar] [CrossRef]
- Gong, L.; Chi, H.; Wang, J.; Zhang, H.; Sun, B. In vitro fermentabilities of whole wheat as compared with refined wheat in different cultivars. J. Funct. Foods 2019, 52, 505–515. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Dong, F.; Liu, X.; Lv, Q.; Yang, Y.; Liu, F.; Chen, L.; Wang, T.; Wang, Z.; Zhang, Y. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr. Polym. 2016, 140, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Lam, S.C.; Cheong, K.L.; Wei, F.; Lin, P.C.; Long, Z.R.; Lv, X.J.; Zhao, J.; Ma, S.C.; Li, S.P. Simultaneous determination of molecular weights and contents of water-soluble polysaccharides and their fractions from Lycium barbarum collected in China. J. Pharm. Biomed. Anal. 2016, 129, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Li, X.; Yu, P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015, 117, 1021–1027. [Google Scholar] [CrossRef]
- Xing-en, G.; Yong-kang, J.; Hong-xin, W. Effect of cooking methods on the functional compounds of yam. Food Res. Dev. 2015, 36, 9–12. [Google Scholar] [CrossRef]
- Gu, R.; Chang, X.; Bai, G.; Li, X.; Di, Y.; Liu, X.; Sun, L.; Wang, Y. Effects of household cooking methods on changes of tissue structure, phenolic antioxidant capacity and active component bioaccessibility of quinoa. Food Chem. 2021, 350, 129138. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.L.; Nie, S.P.; Min, F.F.; Xie, M.Y. Artificial simulated saliva, gastric and intestinal digestion of polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. 2013, 92, 1143–1150. [Google Scholar] [CrossRef]
- Zhu, K.X.; Yao, S.W.; Zhang, Y.J.; Liu, Q.B.; Xu, F.; Wu, G.; Dong, W.J.; Tan, L.H. Effects of in vitro saliva, gastric and intestinal digestion on the chemical properties, antioxidant activity of polysaccharide from Artocarpus heterophyllus Lam. (Jackfruit) Pulp. Food Hydrocoll. 2019, 87, 952–959. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, R.; You, L.; Ma, Y.; Liao, L.; Pedisic, S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. Food Chem. Toxicol. 2021, 151, 112145. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Liu, F.; Chen, W. Effects of Chinese yam (Dioscorea oppositifolia L.) dietary supplementation on intestinal microflora, digestive enzyme activity and immunity in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2020, 51, 4698–4712. [Google Scholar] [CrossRef]
- Meng, X.; Hu, W.; Wu, S.; Zhu, Z.; Lu, R.; Yang, G.; Qin, C.; Yang, L.; Nie, G. Chinese yam peel enhances the immunity of the common carp (Cyprinus carpio L.) by improving the gut defence barrier and modulating the intestinal microflora. Fish Shellfish Immunol. 2019, 95, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Nirmalkar, K.; Murugesan, S.; Pizano-Zarate, M.L.; Villalobos-Flores, L.E.; Garcia-Gonzalez, C.; Morales-Hernandez, R.M.; Nunez-Hernandez, J.A.; Hernandez-Quiroz, F.; Romero-Figueroa, M.D.S.; Hernandez-Guerrero, C.; et al. Gut Microbiota and Endothelial Dysfunction Markers in Obese Mexican Children and Adolescents. Nutrients 2018, 10, 2009. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Huang, F.; Zhao, A.; Lei, S.; Zhang, Y.; Xie, G.; Chen, T.; Qu, C.; Rajani, C.; Dong, B.; et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017, 15, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Hong, R.; Yi, Y.; Bai, Y.; Dong, L.; Jia, X.; Zhang, R.; Wang, G.; Zhang, M.; Wu, J. In vitro digestion and human gut microbiota fermentation of longan pulp polysaccharides as affected by Lactobacillus fermentum fermentation. Int. J. Biol. Macromol. 2020, 147, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food Sci. Technol. 2019, 92, 65–70. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Wu, D.T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.R.; Wang, S.P.; Gan, R.Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Huang, Y.-C.; Yin, M.-C.; Lin, S.-J. Effect of Yam (Dioscorea alata Compared to Dioscorea japonica) on Gastrointestinal Function and Antioxidant Activity in Mice. J. Food Sci. 2006, 71, S513–S516. [Google Scholar] [CrossRef]
- Han, R.; Pang, D.; Wen, L.; You, L.; Huang, R.; Kulikouskaya, V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. J. Funct. Foods 2020, 64, 103652. [Google Scholar] [CrossRef]
- Wu, D.T.; Feng, K.L.; Li, F.; Hu, Y.C.; Wang, S.P.; Gan, R.Y.; Zou, L. In vitro digestive characteristics and microbial degradation of polysaccharides from lotus leaves and related effects on the modulation of intestinal microbiota. Curr. Res. Food Sci. 2022, 5, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Chen, L.; Zhang, P.F.; Zhou, J.Q.; Lu, X.F.; Tian, W. Carbohydrate polymers exhibit great potential as effective elicitors in organic agriculture: A review. Carbohydr. Polym. 2020, 230, 15. [Google Scholar] [CrossRef] [PubMed]
- Paesani, C.; Salvucci, E.; Moiraghi, M.; Canigia, L.F.; Perez, G.T. Arabinoxylan from Argentinian whole wheat flour promote the growth of Lactobacillus reuteri and Bifidobacterium breve. Lett. Appl. Microbiol. 2019, 68, 142–148. [Google Scholar] [CrossRef]

| NPSY | NPBY | HPSY | HPBY | MY | FY | Raw | |||
|---|---|---|---|---|---|---|---|---|---|
| Content (mg/g) * | Undigested | 161.4 ± 0.6 e | 80.2 ± 0.9 b | 100.9 ± 6.5 d | 83.9 ± 1.8 b | 93.9 ± 2.1 c | 68.1 ± 5.5 a | 77.4 ± 4.7 b | |
| Digested | 188.6 ± 2.1 e | 175.8 ± 7.7 d | 172.1 ± 1.9 cd | 162.1 ± 7.9 bc | 162.2 ± 4.8 bc | 149.8 ± 0.9 a | 154.9 ± 8.7 ab | ||
| Molecular weight ** | Undigested | 200 Da–130 kDa | 53.23% | 65.82% | 49.11% | 62.37% | 67.88% | 26.98% | 44.66% |
| >130 kDa | 46.66% | 34.18% | 50.89% | 37.63% | 32.12% | 73.02% | 55.34% | ||
| Digested | 200 Da–10 kDa | 18.03% | 17.48% | 18.13% | 17.59% | 17.86% | 17.85% | 20.52% | |
| 10 kDa~130 kDa | 81.97% | 82.52% | 81.87% | 82.41% | 82.14% | 82.15% | 79.48% | ||
| PI | |||
|---|---|---|---|
| NPSYP | 121.13 | NPSY | 30.47 |
| NPBYP | 55.67 | NPBY | 8.37 |
| HPSYP | 86.08 | HPSY | −8.07 |
| HPBYP | 107.84 | HPBY | −2.78 |
| FYP | 60.40 | FY | −11.84 |
| MYP | 75.40 | MY | 5.11 |
| RYP | 100.81 | RY | 16.77 |
| Control | |||
| NC | −118.07 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Hu, L.; Liu, F.; Chi, J.; Chen, R.; Wang, J. Domestic Cooking Affects the Prebiotic Performances of Chinese Yam. Foods 2022, 11, 3794. https://doi.org/10.3390/foods11233794
Gong L, Hu L, Liu F, Chi J, Chen R, Wang J. Domestic Cooking Affects the Prebiotic Performances of Chinese Yam. Foods. 2022; 11(23):3794. https://doi.org/10.3390/foods11233794
Chicago/Turabian StyleGong, Lingxiao, Linlin Hu, Feiyue Liu, Jingwen Chi, Rui Chen, and Jing Wang. 2022. "Domestic Cooking Affects the Prebiotic Performances of Chinese Yam" Foods 11, no. 23: 3794. https://doi.org/10.3390/foods11233794






