Effect of Probiotic Lactic Acid Bacteria (LAB) on the Quality and Safety of Greek Yogurt
Abstract
:1. Introduction
2. Materials and Methods
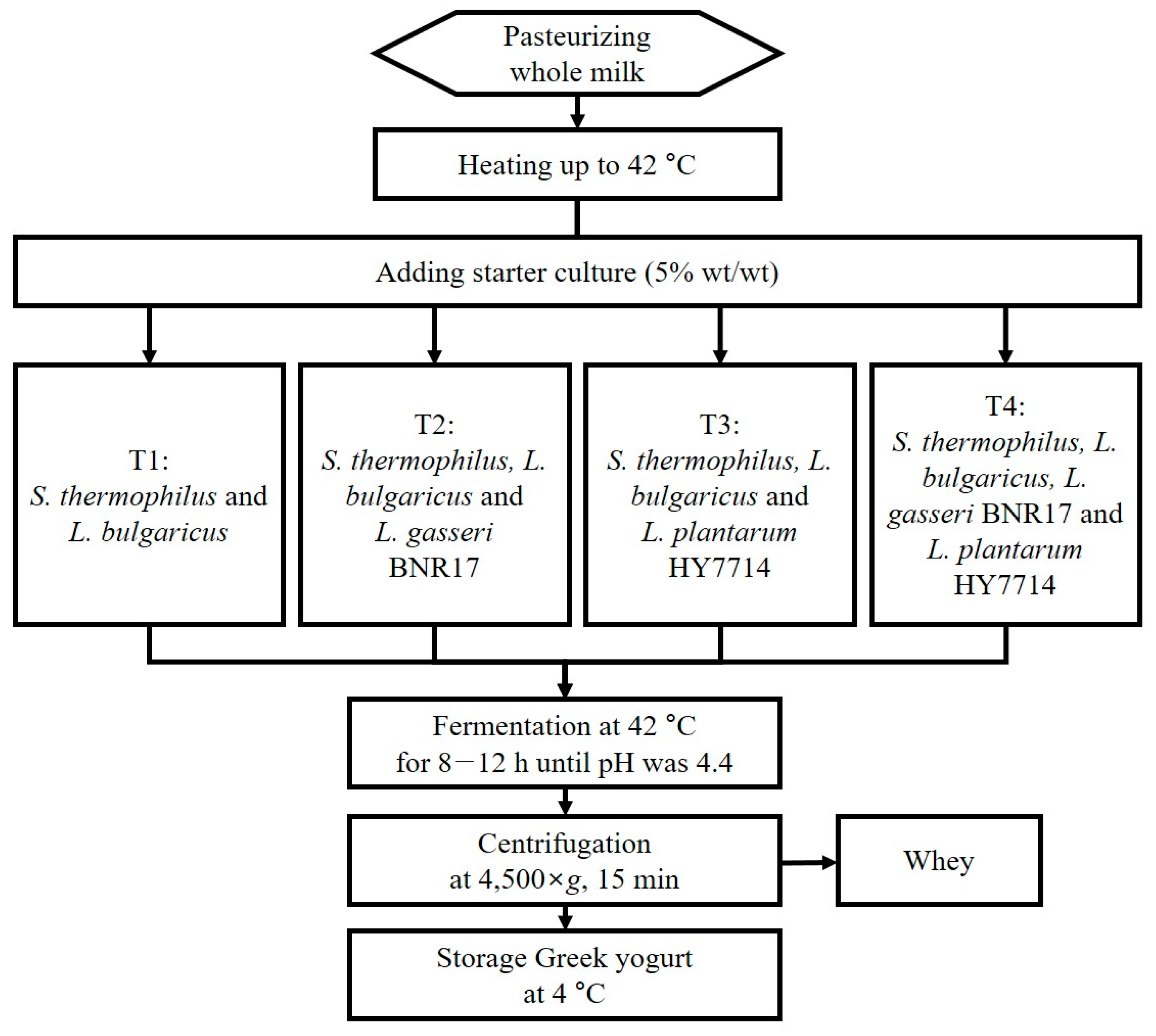
2.1. Starter Culture for Preparation of Greek Yogurt
2.2. Preparation of Enterohaemorrhagic E. coli for the Safety Study
2.3. Manufacturing of Greek Yogurt
2.4. pH, Titratable Acidity, and Viscosity
2.5. Consumer Prefernce Test
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Probiotic LAB on pH and Titratable Acidity
3.2. Effect of Probiotic LAB on Viscosity and LAB Population
3.3. Antimicrobial Effect of Probiotic LAB on EHEC
3.4. Consumer Preference Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, W.J.; Lucey, J.A. Formation and physical properties of yogurt. Asian Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Li, S.; Ye, A.; Singh, H. Effects of seasonal variations on the quality of set yogurt, stirred yogurt, and Greek-style yogurt. J. Dairy Sci. 2021, 104, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Moineau-Jean, A.; Champagne, C.P.; Roy, D.; Raymond, Y.; LaPointe, G. Effect of Greek-style yoghurt manufacturing processes on starter and probiotic bacteria populations during storage. Int. Dairy J. 2019, 93, 35–44. [Google Scholar] [CrossRef]
- Douglas, S.M.; Ortinau, L.C.; Hoertel, H.A.; Leidy, H.J. Low, moderate, or high protein yogurt snacks on appetite control and subsequent eating in healthy women. Appetite 2013, 60, 117–122. [Google Scholar] [CrossRef]
- Daniali, M.; Nikfar, S.; Abdollahi, M. A brief overview on the use of probiotics to treat overweight and obese patients. Expert Rev. Endocrinol. Metab. 2020, 15, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Yun, S.I.; Park, H.O. Composition for Prevention and Treatment of Diabetes Mellitus with Lactobacillus gasseri BNR17. Korean Patent KR20080048976A, 29 November 2007. [Google Scholar]
- Yun, S.I.; Park, H.O.; Kang, J.H. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J. Appl. Microbiol. 2009, 107, 1681–1686. [Google Scholar] [CrossRef]
- Kang, J.H.; Yun, S.I.; Park, M.H.; Park, J.H.; Jeong, S.Y.; Park, H.O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yun, J.M.; Kim, M.K.; Kwon, O.; Cho, B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: A randomized, double-blind, placebo-controlled trial. J. Med. Food. 2018, 21, 454–461. [Google Scholar] [CrossRef]
- Lee, D.E.; Kim, H.M.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Lee, J.H.; Myoung, K.S.; Jang, S.S.; Huh, C.S. Probiotics of Lactobacillus plantarum HY7714 for skin wrinkle inhibitory and moisurizing effects and use of thereof as skin anti-wrinkle or moisturizing products. Korean Patent KR101492003B1, 29 April 2013. [Google Scholar]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Health Functional Food Code. Available online: https://www.khsa.or.kr/assets/extra/hfood/01.pdf (accessed on 2 July 2021).
- Lee, K.; Kim, H.J.; Kim, S.A.; Park, S.D.; Shim, J.J.; Lee, J.L. Exopolysaccharide from Lactobacillus plantarum HY7714 protects against skin aging through skin–gut axis communication. Molecules 2021, 26, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Breidt, F.; Fratamico, P.; Oh, D.H. Acid resistance and molecular characterization of Escherichia coli O157: H7 and different non-O157 Shiga toxin-producing E. coli serogroups. J. Food Sci. 2015, 80, M2257–M2264. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Newman, C.P.; Hutchinson, D.N.; Walker, A.M.; Rowe, B.; Majid, F. Verotoxin producing Escherichia coli O 157 infections associated with the consumption of yoghurt. Epidemiol. Infect. 1993, 111, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Yoon, K.S. Quantitative Microbial Risk Assessment of Listeria monocytogenes and Enterohemorrhagic Escherichia coli in Yogurt. Foods 2022, 11, 971. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, A.R.U.; Jahid, I.K. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef]
- Choi, S.J.; Yang, S.Y.; Yoon, K.S. Lactic acid bacteria starter in combination with sodium chloride controls pathogenic Escherichia coli (EPEC, ETEC, and EHEC) in kimchi. Food Microbiol. 2021, 100, 103868. [Google Scholar] [CrossRef]
- AOAC International. Method Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Ghasempour, Z.; Javanmard, N.; Langroodi, A.M.; Alizadeh-Sani, M.; Ehsani, A.; Kia, E.M. Development of probiotic yogurt containing red beet extract and basil seed gum; techno-functional, microbial and sensorial characterization. Biocatal. Agric. Biotechnol. 2020, 29, 101785. [Google Scholar] [CrossRef]
- Karagul-Yuceer, Y.; Drake, M. Sensory analysis of yogurt. In Manufacturing Yogurt and Fermented Milk, 2nd ed.; Chandan, R.C., Kilara, A., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Desai, N.T.; Shepard, L.; Drake, M.A. Sensory properties and drivers of liking for Greek yogurts. J. Dairy Sci. 2013, 96, 7454–7466. [Google Scholar] [CrossRef] [Green Version]
- Cayot, P.; Schenker, F.; Houze, G.; Sulmont-Rosse, C.; Colas, B. Creaminess in relation to consistency and particle size in stirred fat-free yogurt. Int. Dairy J. 2008, 18, 303–311. [Google Scholar] [CrossRef]
- Greis, M.; Sainio, T.; Katina, K.; Kinchla, A.K.; Nolden, A.; Partanen, R.; Seppa, L. Dynamic texture perception in plant-based yogurt alternatives: Identifying temperal drivers of liking by TDS. Food Qual. Prefer. 2020, 86, 104019. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguérinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Serafeimidou, A.; Zlatanos, S.; Laskaridis, K.; Sagredos, A. Chemical characteristics, fatty acid composition, and conjugated linoleic acid (CLA) content of traditional Greek yogurts. Food Chem. 2012, 134, 1839–1846. [Google Scholar] [CrossRef]
- Sodini, I.; Mattas, J.; Tong, P.S. Influence of pH and heat treatment of whey on the functional properties of whey protein concentrates in yoghurt. Int. Dairy, J. 2006, 16, 1464–1469. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Huo, R.; Kwok, L.Y.; Li, C.; Ma, Y.; Mi, Z.; Chen, Y. Effects of applying Lactobacillus helveticus H9 as adjunct starter culture in yogurt fermentation and storage. J. Dairy Sci. 2019, 102, 223–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, R.; Jain, N.K.; Shah, V.; Soni, J.; Suthar, D.; Gohel, P. Development of probiotic yogurt: Effect of strain combination on nutritional, rheological, organoleptic and probiotic properties. J. Food Sci. Technol. 2020, 57, 2038–2050. [Google Scholar] [CrossRef]
- Jia, R.; Chen, H.; Chen, H.; Ding, W. Effects of fermentation with Lactobacillus rhamnosus GG on product quality and fatty acids of goat milk yogurt. J. Dairy Sci. 2016, 99, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, L.; Zhang, S.; Uluko, H.; Cui, W.; Lv, J. Potential use of Lactobacillus casei AST18 as a bioprotective culture in yogurt. Food Control 2013, 34, 675–680. [Google Scholar] [CrossRef]
- Duboc, P.; Mollet, B. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- İspirli, H.; Dertli, E. Isolation and identification of exopolysaccharide producer lactic acid bacteria from Turkish yogurt. J. Food Process. Preserv. 2018, 42, e13351. [Google Scholar] [CrossRef]
- Nambiar, R.B.; Sellamuthu, P.S.; Perumal, A.B.; Sadiku, E.R.; Phiri, G.; Jayaramudu, J. Characterization of an exopolysaccharide produced by Lactobacillus plantarum HM47 isolated from human breast milk. Process. Biochem. 2018, 73, 15–22. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Milk and Cream Products and Yogurt Products; Final Rule to Revoke the Standards for Lowfat Yogurt and Nonfat Yogurt and to Amend the Standard for Yogurt. Available online: https://www.federalregister.gov/documents/2021/06/11/2021-12220/milk-and-cream-products-and-yogurt-products-final-rule-to-revoke-the-standards-for-lowfat-yogurt-and (accessed on 16 November 2021).
- Ogwaro, B.A.; Gibson, H.; Whitehead, M.; Hill, D.J. Survival of Escherichia coli O157: H7 in traditional African yoghurt fermentation. Int. J. Food Microbiol. 2002, 79, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Moineau-Jean, A.; Guévremont, E.; Champagne, C.P.; Roy, D.; Raymond, Y.; LaPointe, G. Fate of Escherichia coli and Kluyveromyces marxianus contaminants during storage of Greek-style yogurt produced by centrifugation or ultrafiltration. Int. Dairy J. 2017, 72, 36–43. [Google Scholar] [CrossRef]
- Cutrim, C.S.; de Barros, R.F.; da Costa, M.P.; Franco, R.M.; Conte-Junior, C.A.; Cortez, M.A.S. Survival of Escherichia coli O157: H7 during manufacture and storage of traditional and low lactose yogurt. LWT 2016, 70, 178–184. [Google Scholar] [CrossRef]
- Hu, C.H.; Ren, L.Q.; Zhou, Y.; Ye, B.C. Characterization of antimicrobial activity of three Lactobacillus plantarum strains isolated from Chinese traditional dairy food. Food Sci. Nutr. 2019, 7, 1997–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, M.; Shimizu, K.; Nomoto, K.; Tanaka, R.; Hamabata, T.; Yamasaki, S.; Takeda, T.; Takeda, Y. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157: H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 2001, 68, 135–140. [Google Scholar] [CrossRef]
- Guraya, R.; Frank, J.F.; Hassan, A.N. Effectiveness of salt, pH, and diacetyl as inhibitors for Escherichia coli O157: H7 in dairy foods stored at refrigeration temperatures. J. Food Prot. 1998, 61, 1098–1102. [Google Scholar] [CrossRef]
- Coggins, P.C.; Schilling, M.W.; Kumari, S.; Gerrard, P.D. Development of a sensory lexicon for conventional milk yogurt in the United States. J. Sens. Stud. 2008, 23, 671–687. [Google Scholar] [CrossRef]
- Allgeyer, L.C.; Miller, M.J.; Lee, S.Y. Sensory and microbiological quality of yogurt drinks with prebiotics and probiotics. J. Dairy Sci. 2010, 93, 4471–4479. [Google Scholar] [CrossRef]
- Steele, J.; Broadbent, J.; Kok, J. Perspectives on the contribution of lactic acid bacteria to cheese flavor development. Curr. Opin. Biotechnol. 2013, 24, 135–141. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef] [Green Version]
- Boumerdassi, H.; Monnet, C.; Desmazeaud, M.; Corrieu, G. Isolation and properties of Lactococcus lactis subsp. lactis biovar diacetylactis CNRZ 483 mutants producing diacetyl and acetoin from glucose. Appl. Environ. Microbiol. 1997, 63, 2293–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zareba, D.; Ziarno, M.; Obiedzinski, M. Volatile profile of non-fermented milk and milk fermented by Bifidobacterium animalis subsp. lactis. Int. J. Food Prop. 2012, 15, 1010–1021. [Google Scholar] [CrossRef]
- Majchrzak, D.; Lahm, B.; Duerrschmid, K. Conventional and probiotic yogurts differ in sensory properties but not in consumers’ preferences. J. Sens. Stud. 2010, 25, 431–446. [Google Scholar] [CrossRef]
- Lim, S.M.; Lee, N.K.; Kim, K.T.; Paik, H.D. Probiotic Lactobacillus fermentum KU200060 isolated from watery kimchi and its application in probiotic yogurt for oral health. Microb. Pathog. 2020, 147, 104430. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.D.; Lee, H.S.; Kim, K.T.; Paik, H.D. Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Yogurt Supplemented with Lactiplantibacillus plantarum NK181 and Lactobacillus delbrueckii KU200171 and Sensory Evaluation. Foods 2021, 10, 2324. [Google Scholar] [CrossRef]



| Attribute | Definition 1 |
|---|---|
| Flavor | The tangy and dairy-sour flavor |
| Sweetness | The basic taste associated with sugar |
| Sourness | The basic taste associated with acid |
| Viscosity | The force required to move the spoon back and forth |
| Creaminess | Smooth texture and behave like a fluid product. |
| Mouthfeel | The physical sensation created by food in the mouth. |
| Sample 1 | Temperature | ||
|---|---|---|---|
| 4 | 10 | 25 | |
| T1 1 | 15.47 ± 0.11 d | 8.30 ± 0.23 e | 1.63 ± 0.24 d |
| T2 | 40.93 ± 0.91 bc | 24.90 ± 1.54 c | 1.98 ± 0.06 c |
| T3 | 37.30 ± 3.34 c | 19.69 ± 2.23 d | 1.79 ± 0.05 d |
| T4 | 42.04 ± 1.51 b | 31.06 ± 5.96 b | 2.65 ± 0.18 b |
| Commercial 2 | 47.76 ± 6.16 a | 36.77 ± 2.67 a | 5.00 ± 0.07 a |
| Sample 2 | Sensory Scores 1 | ||||||
|---|---|---|---|---|---|---|---|
| Flavor | Sweetness | Sourness | Viscosity | Creaminess | Mouthfeel | Overall Acceptability | |
| T1 | 5.03 ± 1.35 | 4.23 ± 1.47 b | 4.37 ± 1.58 b | 5.15 ± 1.30 | 5.18 ± 1.26 b | 5.30 ± 1.45 b | 4.78 ± 1.56 b |
| T2 | 4.98 ± 1.35 | 4.77 ± 1.21 a | 4.75 ± 1.27 b | 5.25 ± 1.04 | 5.40 ± 1.17 ab | 5.40 ± 1.18 b | 5.20 ± 1.33 b |
| T3 | 4.82 ± 1.41 | 4.77 ± 1.20 a | 4.42 ± 1.36 b | 5.28 ± 1.01 | 5.55 ± 1.11 ab | 5.57 ± 1.21 ab | 5.20 ± 1.27 b |
| T4 | 5.08 ± 1.33 | 5.05 ± 1.47 a | 5.30 ± 1.34 a | 5.42 ± 1.12 | 5.68 ± 1.11 a | 5.87 ± 0.96 a | 5.80 ± 1.13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-Y.; Yoon, K.-S. Effect of Probiotic Lactic Acid Bacteria (LAB) on the Quality and Safety of Greek Yogurt. Foods 2022, 11, 3799. https://doi.org/10.3390/foods11233799
Yang S-Y, Yoon K-S. Effect of Probiotic Lactic Acid Bacteria (LAB) on the Quality and Safety of Greek Yogurt. Foods. 2022; 11(23):3799. https://doi.org/10.3390/foods11233799
Chicago/Turabian StyleYang, So-Young, and Ki-Sun Yoon. 2022. "Effect of Probiotic Lactic Acid Bacteria (LAB) on the Quality and Safety of Greek Yogurt" Foods 11, no. 23: 3799. https://doi.org/10.3390/foods11233799
APA StyleYang, S.-Y., & Yoon, K.-S. (2022). Effect of Probiotic Lactic Acid Bacteria (LAB) on the Quality and Safety of Greek Yogurt. Foods, 11(23), 3799. https://doi.org/10.3390/foods11233799






