Influence of Gelatin-Chitosan-Glycerol Edible Coating Incorporated with Chlorogenic Acid, Gallic Acid, and Resveratrol on the Preservation of Fresh Beef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Edible Coating
2.3. Beef Treatment
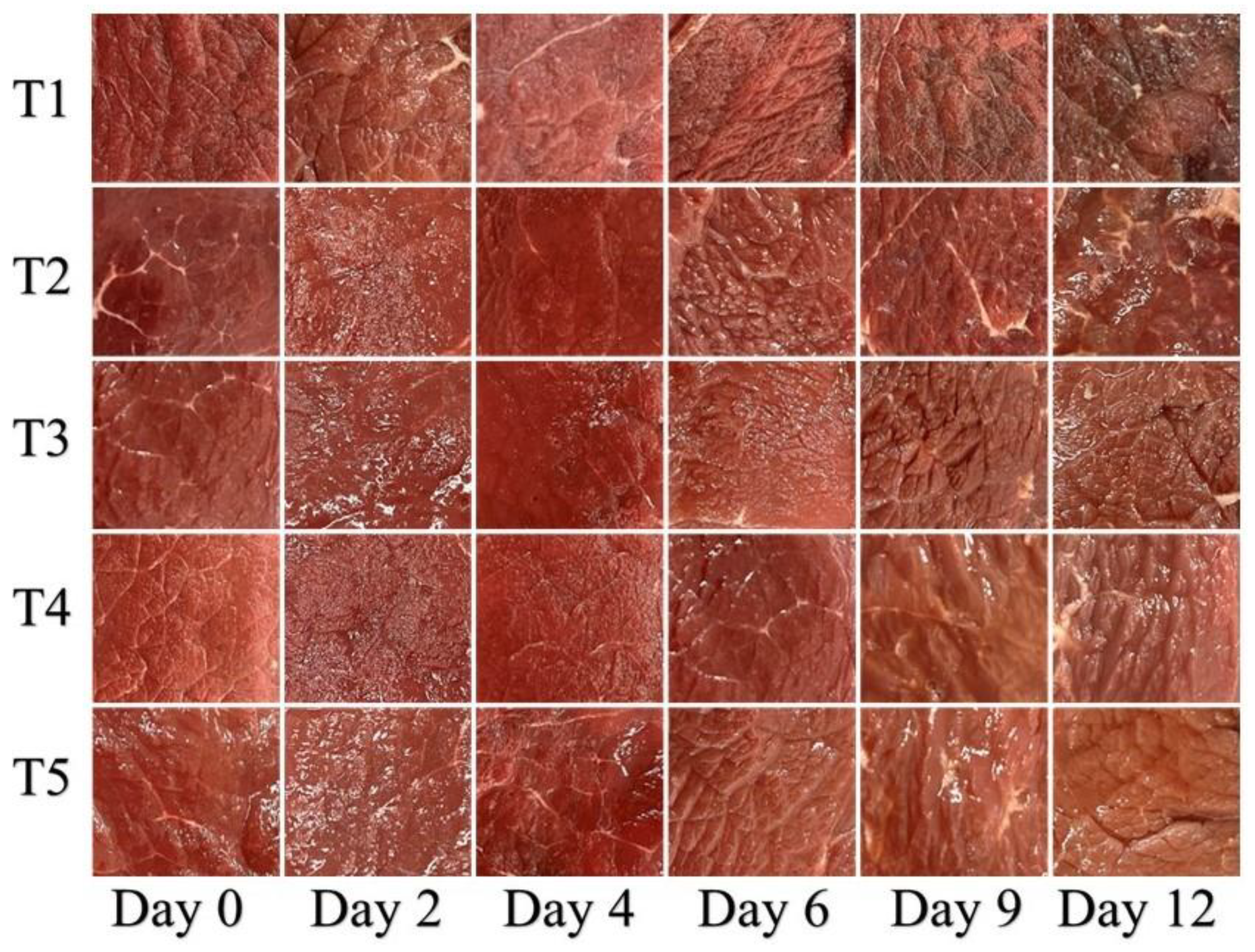
2.4. Determination of Color Values and Acquisition of Beef Images
2.5. Determination of pH Values
2.6. Determination of Total Viable Count
2.7. Determination of Total Phenolic Content
2.8. Determination of Total Volatile Base Nitrogen
2.9. Determination of Thiobarbituric Acid Reactive Substances
2.10. Determination of Texture
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Color Evaluation
3.2. pH Values and Microbiological Analysis
3.3. Total Phenolic Content
3.4. Total Volatile Base Nitrogen
3.5. Thiobarbituric Acid Reactive Substances
3.6. Texture Profile Analysis
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alirezalu, K.; Movlan, H.S.; Yaghoubi, M.; Pateiro, M.; Lorenzo, J.M. ε-polylysine coating with stinging nettle extract for fresh beef preservation. Meat Sci. 2021, 176, 108474. [Google Scholar] [CrossRef]
- Duran, A.; Kahve, H.I. The effect of chitosan coating and vacuum packaging on the microbiological and chemical properties of beef. Meat Sci. 2020, 162, 107961. [Google Scholar] [CrossRef] [PubMed]
- You, Y.S.; Her, J.Y.; Shafel, T.; Kang, T.Y.; Jun, S.J. Supercooling preservation on quality of beef steak. J. Food Eng. 2020, 274, 109840. [Google Scholar] [CrossRef]
- Kassem, A.; Meade, J.; Gibbons, J.; McGill, K.; Walsh, C.; Lyng, J.; Whyte, P. Evaluation of chemical immersion treatments to reduce microbial populations in fresh beef. Int. J. Food Microbiol. 2017, 261, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ünalan, İ.U.; Korel, F.; Yemenicioğlu, A. Active packaging of ground beef patties by edible zein films incorporated with partially purified lysozyme and Na2EDTA. Int. J. Food Sci. Technol. 2011, 46, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. 2017, 221, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J. Development of advanced edible coatings for fruits. Trends Food Sci. Technol. 1999, 10, 254–260. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Han, J.H. Edible films and coatings. Innov. Food Packag. 2014, 9, 213–255. [Google Scholar]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.L.S.; Gomide, L.A.M.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Andrade, M.P.D.; Rodrigues, L.M.; Massingue, A.A.; Fontes, P.R.; Ramos, A.L.S.; Ramos, E.M. Retail display of beef steaks coated with monolayer and bilayer chitosan-gelatin composites. Meat Sci. 2019, 152, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Heu, M.S.; Park, C.H.; Kim, H.J.; Lee, D.H.; Kim, J.S. Effects of a gelatin coating on the shelf life of salmon. Fish. Aquat. Sci. 2010, 13, 89–95. [Google Scholar] [CrossRef]
- Fu, S.L.; Wu, C.H.; Wu, T.T.; Yu, H.X.; Yang, S.B.; Hu, Y.Q. Preparation and characterisation of Chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Wang, Z.; Kadouh, H.; Zhou, K.Q. The antimicrobial, mechanical, physical and structural properties of chitosan–gallic acid films. LWT-Food Sci. Technol. 2014, 57, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Li, S.M.; Warner, R.D.; Fang, Z.X. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.F.; Gao, Y.P.; Ye, C.; Wang, H.Y. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT-Food Sci. Technol. 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Montano-Sanchez, E.; Torres-Martinez, B.D.M.; Vargas-Sanchez, R.D.; Huerta-Leidenz, N.; Sanchez-Escalante, A.; Beriain, M.J.; Torrescano-Urrutia, G.R. Effects of chitosan coating with green tea aqueous extract on lipid oxidation and microbial growth in pork chops during chilled storage. Foods 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Mhalla, D.; Bouaziz, A.; Ennouri, K.; Chawech, R.; Smaoui, S.; Jarraya, R.; Tounsi, S.; Trigui, M. Antimicrobial activity and bioguided fractionation of Rumex tingitanus extracts for meat preservation. Meat Sci. 2017, 125, 22–29. [Google Scholar] [CrossRef]
- Radha krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.R.; Xiang, R.; Tang, D.B.; Zhu, M.J.; Liu, X.M. Regulation of protein oxidation in Cantonese sausages by rutin, quercetin and caffeic acid. Meat Sci. 2021, 175, 108422. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, H.L.; Chen, M.M.; Jiang, S.W.; Cheng, J.F.; Li, X.J.; Zhang, M.Z.; Jiang, S.T. Gelatin/zein fiber mats encapsulated with resveratrol: Kinetics, antibacterial activity and application for pork preservation. Food Hydrocoll. 2020, 101, 105577. [Google Scholar] [CrossRef]
- Fang, Z.X.; Lin, D.; Warner, R.D.; Ha, M. Effect of gallic acid/chitosan coating on fresh pork quality in modified atmosphere packaging. Food Chem. 2018, 260, 90–96. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Wang, H.H.; Liu, W.H.; Cheong, K.L.; Teng, B. Effect of sodium alginate-agar coating containing ginger essential oil on the shelf life and quality of beef. Food Control 2021, 130, 108216. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Holman, B.W.B.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding beef flavour and overall liking traits using two different methods for determination of thiobarbituric acid reactive substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Tsau, R.T.; Lin, Y.C.; Jan, S.S.; Tan, F.J. Effect of various levels of rosemary or Chinese mahogany on the quality of fresh chicken sausage during refrigerated storage. Food Chem. 2009, 117, 106–113. [Google Scholar] [CrossRef]
- Moore, M.E.; Han, I.Y.; Acton, J.C.; Ogale, A.A.; Barmore, C.R.; Dawson, P.L. Effects of antioxidants in polyethylene film on fresh beef color. Food Chem. Toxicol. 2003, 68, 99–104. [Google Scholar] [CrossRef]
- Cheng, Y.X.; Hu, J.F.; Wu, S.J. Chitosan based coatings extend the shelf-life of beef slices during refrigerated storage. LWT-Food Sci. Technol. 2021, 138, 110694. [Google Scholar] [CrossRef]
- Cao, Y.; Warner, R.D.; Fang, Z.X. Effect of chitosan/nisin/gallic acid coating on preservation of pork loin in high oxygen modified atmosphere packaging. Food Control 2019, 101, 9–16. [Google Scholar] [CrossRef]
- Zarandona, I.; López-Caballero, M.E.; Montero, M.P.; Guerrero, P.; de la Caba, K.; Gómez-Guillén, M.C. Horse mackerel (Trachurus trachurus) fillets biopreservation by using gallic acid and chitosan coatings. Food Control 2021, 120, 107511. [Google Scholar] [CrossRef]
- Wong, L.W.; Loke, X.J.; Chang, C.K.; Ko, W.C.; Hou, C.Y.; Hsieh, C.W. Use of the plasma-treated and chitosan/gallic acid-coated polyethylene film for the preservation of tilapia (Orechromis niloticus) fillets. Food Chem. 2020, 329, 126989. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional betanin nanoliposomes-incorporated gelatin/chitosan nanofiber/ZnO nanoparticles nanocomposite film for fresh beef preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. Int. J. Biol. Macromol. 2017, 94, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Makwana, S.; Choudhary, R.; Haddock, J.; Kohli, P. In-vitro antibacterial activity of plant based phenolic compounds for food safety and preservation. LWT-Food Sci. Technol. 2015, 62, 935–939. [Google Scholar] [CrossRef]
- Silva, C.S.; Figueiredo, H.M.; Stamford, T.L.M.; Silva, L. Inhibition of Listeria monocytogenes by Melaleuca alternifolia (tea tree) essential oil in ground beef. Int. J. Food Microbiol. 2019, 293, 79–86. [Google Scholar] [CrossRef]
- Nie, X.; Wang, L.; Wang, Q.; Lei, J.; Hong, W.; Huang, B.; Zhang, C. Effect of a sodium alginate coating infused with tea polyphenols on the quality of fresh Japanese sea bass (Lateolabrax japonicas) fillets. J. Food Sci. 2018, 83, 1695–1700. [Google Scholar] [CrossRef]
- GB 2707-2016; Chinese Food Safety Standard—Fresh (Frozen) Livestock and Poultry Products. China Food and Drug Administration: Beijing, China, 2016.
- Barzegar, H.; Alizadeh Behbahani, B.; Mehrnia, M.A. Quality retention and shelf life extension of fresh beef using Lepidium sativum seed mucilage-based edible coating containing Heracleum lasiopetalum essential oil: An experimental and modeling study. Food Sci. Biotechnol. 2020, 29, 717–728. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z.X. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.R.; Mies, P.D.; Baird, B.E.; Pfeiffer, K.D.; Ellebracht, J.W.; Savell, J.W. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 2005, 70, 665–682. [Google Scholar] [CrossRef]
- Ripoll, G.; Joy, M.; Munoz, F. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Sci. 2011, 87, 88–93. [Google Scholar] [CrossRef]
- Bermudez-Oria, A.; Rodriguez-Gutierrez, G.; Rubio-Senent, F.; Fernandez-Prior, A.; Fernandez-Bolanos, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Descalzo, A.M.; Sancho, A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Basurto, B.E.; Ramírez-Gilly, M.; Tecante, A.; Severiano-Pérez, P.; Wacher, C.; Valdivia-López, M.A. Effect of high hydrostatic pressure treatment on the preservation of beef meat. Ind. Eng. Chem. Res. 2011, 51, 5932–5938. [Google Scholar] [CrossRef]
- Romero de Avila, M.D.; Isabel Cambero, M.; Ordonez, J.A.; de la Hoz, L.; Herrero, A.M. Rheological behaviour of commercial cooked meat products evaluated by tensile test and texture profile analysis (TPA). Meat Sci. 2014, 98, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.R.; Xu, L.; Xiang, R.; Liu, X.M.; Zhu, M.J. Effects of mulberry polyphenols on oxidation stability of sarcoplasmic and myofibrillar proteins in dried minced pork slices during processing and storage. Meat Sci. 2020, 160, 107973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lv, W.J.; Wang, J.L.; Li, J.R.; Liu, X.X.; Zhu, J.L. Effects of tea polyphenols on the post-mortem integrity of large yellow croaker (Pseudosciaena crocea) fillet proteins. Food Chem. 2013, 141, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Sun, J.J.; Thavaraj, P.; Yang, X.B.; Guo, Y.R. Effects of thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) surimi during cold storage. Food Chem. 2017, 224, 372–381. [Google Scholar] [CrossRef] [PubMed]




| Beef | Storage Time (Day) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 9 | 12 | ||
| L* | T1 | 41.7 ± 0.5 Ba | 40.6 ± 0.5 Bab | 41.8 ± 1.0 ABa | 39.0 ± 0.5 Bbc | 37.0 ± 0.6 Bc | 37.6 ± 0.5 CDc |
| T2 | 39.7 ± 0.5 Cbc | 43.5 ± 0.4 Aa | 40.2 ± 0.8 Bb | 42.0 ± 0.4 Aa | 38.0 ± 0.3 Bc | 36.6 ± 0.4 Dd | |
| T3 | 43.3 ± 0.8 Aba | 38.1 ± 0.3 Cc | 39.9 ± 0.2 Bbc | 41.7 ± 0.6 Aab | 42.5 ± 0.8 Aa | 42.1 ± 0.7 ABa | |
| T4 | 44.6 ± 0.4 Aa | 38.6 ± 0.0 Cc | 40.6 ± 0.1 ABbc | 41.5 ± 0.2 Ab | 43.9 ± 0.4 Aa | 39.5 ± 1.6 BCbc | |
| T5 | 44.3 ± 0.7 Aa | 38.6 ± 0.1 Cb | 42.6 ± 0.5 Aa | 43.1 ± 0.7 Aa | 43.4 ± 0.5 Aa | 42.7 ± 0.4 Aa | |
| a* | T1 | 14.8 ± 0.2 Aa | 13.3 ± 0.0 Ab | 12.0 ± 0.3 Cc | 11.4 ± 0.2 Cc | 9.2 ± 0.3 Cd | 7.4 ± 0.1 Ce |
| T2 | 12.9 ± 0.1 Bb | 12.7 ± 0.2 Bb | 14.2 ± 0.3 Aa | 13.2 ± 0.2 ABb | 11.1 ± 0.1 Bc | 9.5 ± 0.3 Bd | |
| T3 | 12.8 ± 0.2 Bb | 11.8 ± 0.1 Ccd | 13.8 ± 0.2 Ba | 12.8 ± 0.1 Bb | 12.3 ± 0.2 Abc | 10.5 ± 0.2 Ae | |
| T4 | 12.6 ± 0.1 Bb | 12.5 ± 0.1 Bb | 12.4 ± 0.1 Cb | 13.5 ± 0.1 Aa | 12.8 ± 0.4 Ab | 10.1 ± 0.1 ABc | |
| T5 | 12.8 ± 0.3 Bb | 12.4 ± 0.2 Bb | 14.1 ± 0.2 Aa | 11.4 ± 0.0 Cc | 10.5 ± 0.1 Bd | 9.8 ± 0.4 ABe | |
| b* | T1 | 7.2 ± 0.2 Aa | 6.5 ± 0.0 Ab | 6.2 ± 0.2 Ab | 6.1 ± 0.1 Cb | 4.4 ± 0.1 Cc | 3.7 ± 0.1 Dd |
| T2 | 6.2 ± 0.3 Bbc | 5.7 ± 0.1 Bcd | 6.7 ± 0.2 Aab | 6.8 ± 0.1 ABa | 6.1 ± 0.1 Bc | 5.3 ± 0.2 Cd | |
| T3 | 5.6 ± 0.2 Ccd | 5.4 ± 0.1 Bd | 6.5 ± 0.1 Ab | 7.2 ± 0.2 Aa | 6.1 ± 0.1 Bbc | 7.7 ± 0.3 Aa | |
| T4 | 5.3 ± 0.0 Cd | 5.7 ± 0.0 Bcd | 6.4 ± 0.1 Aab | 6.0 ± 0.0 Cbc | 6.2 ± 0.3 Bab | 6.7 ± 0.2 Ba | |
| T5 | 5.2 ± 0.2 Cd | 5.6 ± 0.2 Bcd | 5.5 ± 0.2 Bd | 6.3 ± 0.1 BCbc | 7.2 ± 0.4 Aa | 6.4 ± 0.2 Bb | |
| Beef | Storage Time (Day) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 9 | 12 | ||
| Hardness/(g) | T1 | 871.4 ± 27.7 Ca | 731.5 ± 38.4 Db | 537.2 ± 35.0 Dc | 530.0 ± 5.1 Cc | 316.8 ± 34.3 Dd | 282.0 ± 10.9 Ee |
| T2 | 954.2 ± 44.3 Aa | 784.1 ± 43.6 Cb | 573.6 ± 28.2 Cc | 501.8 ± 56.5 CDd | 416.1 ± 40.8 Ce | 370.5 ± 10.0 Cf | |
| T3 | 699.9 ± 23.8 Da | 512.9 ± 16.9 Eb | 481.3 ± 62.3 Ec | 438.7 ± 21.4 Dd | 409.0 ± 19.0 Ce | 349.2 ± 8.0 Df | |
| T4 | 949.0 ± 44.1 Aa | 836.8 ± 71.5 Bb | 749.2 ± 26.7 Ac | 668.6 ± 117.2 Ad | 488.9 ± 60.3 Be | 418.7 ± 34.3 Bf | |
| T5 | 912.6 ± 89.2 Ba | 899.5 ± 31.1 Aa | 624.0 ± 19.3 Bb | 586.6 ± 21.6 Bc | 585.5 ± 43.1 Ac | 454.1 ± 70.6 Ad | |
| Springiness | T1 | 0.78 ± 0.08 Ba | 0.65 ± 0.07 Db | 0.61 ± 0.04 Dc | 0.56 ± 0.02 Ed | 0.53 ± 0.03 Dde | 0.47 ± 0.01 De |
| T2 | 0.85 ± 0.08 Aa | 0.80 ± 0.09 Bab | 0.75 ± 0.05 ABb | 0.62 ± 0.04 Dc | 0.58 ± 0.11 Ccd | 0.55 ± 0.04 Cd | |
| T3 | 0.74 ± 0.05 Ca | 0.70 ± 0.02 Cab | 0.67 ± 0.01 Cb | 0.65 ± 0.07 Cbc | 0.63 ± 0.05 Bcd | 0.58 ± 0.05 Bd | |
| T4 | 0.85 ± 0.09 Aa | 0.84 ± 0.06 Aa | 0.73 ± 0.02 Bb | 0.72 ± 0.05 Bb | 0.72 ± 0.05 Ab | 0.70 ± 0.05 Ab | |
| T5 | 0.86 ± 0.04 Aa | 0.85 ± 0.05 Aa | 0.79 ± 0.04 Ab | 0.78 ± 0.08 Ab | 0.71 ± 0.03 Ac | 0.58 ± 0.07 Bd | |
| Chewiness/(g) | T1 | 232.2 ± 17.0 Ba | 227.8 ± 5.3 Bab | 171.2 ± 32.0 Db | 167.5 ± 21.3 CDbc | 107.9 ± 21.3 Ec | 65.9 ± 9.6 Cd |
| T2 | 364.9 ± 29.6 Aa | 215.1 ± 28.6 BCb | 209.8 ± 32.8 Cbc | 199.7 ± 29.4 Ccd | 197.1 ± 28.9 Ccd | 116.6 ± 27.9 Bd | |
| T3 | 233.3 ± 43.6 Ba | 198.0 ± 8.4 Cb | 194.5 ± 4.5 CDb | 154.5 ± 17.6 Dc | 138.7 ± 25.1 Dd | 115.7 ± 8.0 Be | |
| T4 | 378.2 ± 59.0 Aa | 338.7 ± 20.8 ABb | 310.5 ± 26.9 Ac | 294.7 ± 31.1 Ad | 213.6 ± 40.1 Be | 178.0 ± 3.9 Af | |
| T5 | 375.1 ± 56.6 Aa | 345.4 ± 35.6 Ab | 260.2 ± 31.7 Bc | 256.7 ± 50.9 Bc | 243.7 ± 8.0 Ad | 183.3 ± 21.2 Ae | |
| Sensory | Color | Odor | Texture | |||
|---|---|---|---|---|---|---|
| 0 | 12 | 0 | 12 | 0 | 12 | |
| T1 | 8.3 ± 0.1 Aa | 2.2 ± 0.2 Cb | 8.3 ± 0.2 Aa | 1.9 ± 0.2 Cb | 8.3 ± 0.1 Aa | 1.5 ± 0.1 Cb |
| T2 | 8.0 ± 0.1 Aa | 4.4 ± 0.2 Bb | 8.5 ± 0.1 Aa | 3.5 ± 0.2 Bb | 7.9 ± 0.1 Aa | 3.7 ± 0.1 Bb |
| T3 | 7.8 ± 0.1 Aa | 6.2 ± 0.2 Ab | 8.4 ± 0.1 Aa | 6.2 ± 0.1 Ab | 8.03 ± 0.1 Aa | 5.7 ± 0.2 Ab |
| T4 | 8.0 ± 0.2 Aa | 6.2 ± 0.1 Ab | 8.4 ± 0.0 Aa | 6.4 ± 0.1 Ab | 8.2 ± 0.2 Aa | 5.8 ± 0.2 Ab |
| T5 | 8.3 ± 0.2 Aa | 6.3 ± 0.1 Ab | 8.4 ± 0.2 Aa | 6.3 ± 0.1 Ab | 8.3 ± 0.1 Aa | 6.1 ± 0.1 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Liu, X.; Wang, X.; Yang, H.; Cheng, J.; Lin, Y.; Tang, D. Influence of Gelatin-Chitosan-Glycerol Edible Coating Incorporated with Chlorogenic Acid, Gallic Acid, and Resveratrol on the Preservation of Fresh Beef. Foods 2022, 11, 3813. https://doi.org/10.3390/foods11233813
Zou J, Liu X, Wang X, Yang H, Cheng J, Lin Y, Tang D. Influence of Gelatin-Chitosan-Glycerol Edible Coating Incorporated with Chlorogenic Acid, Gallic Acid, and Resveratrol on the Preservation of Fresh Beef. Foods. 2022; 11(23):3813. https://doi.org/10.3390/foods11233813
Chicago/Turabian StyleZou, Jinhao, Xueming Liu, Xuping Wang, Huaigu Yang, Jingrong Cheng, Yaosheng Lin, and Daobang Tang. 2022. "Influence of Gelatin-Chitosan-Glycerol Edible Coating Incorporated with Chlorogenic Acid, Gallic Acid, and Resveratrol on the Preservation of Fresh Beef" Foods 11, no. 23: 3813. https://doi.org/10.3390/foods11233813





