Development and Application of Edible Coatings with Malva sylvestris L. Extract to Extend Shelf-Life of Small Loaf
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
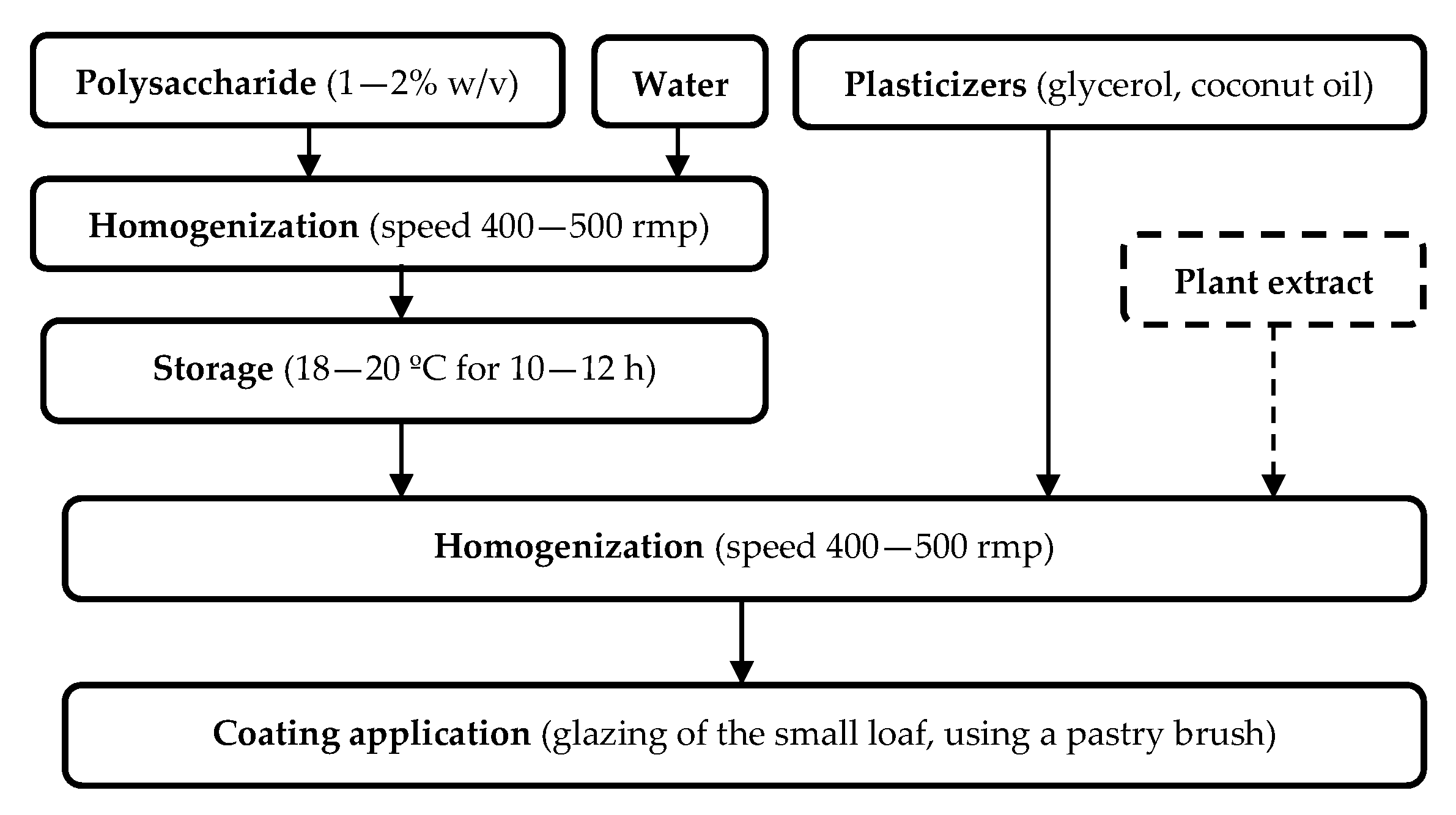
2.2. Preparation of Coatings
2.3. Preparation of Small Loaf
2.4. Antimicrobial Activity of Edible Coatings
2.4.1. Test Microorganisms
2.4.2. Determination of the Antimicrobial Activity of Edible Coatings
2.5. Storage of Small Loaves and Shelf-Life Analysis
2.5.1. Crumb Moisture Content and Moisture Loss
2.5.2. Texture Analysis of Loaf Crumb
2.5.3. Firming Kinetics of Crumb Loaf Using the Avrami Model
2.5.4. Color Analysis
2.5.5. Microbiological Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Effect of Edible Coatings
3.2. Effect of Edible Coatings on Food Properties (i.e., Texture, Crumb Moisture, Moisture Loss, Colour and Microbial Growth)
3.2.1. Crumb Moisture and Crumb Firmness
3.2.2. Color
3.2.3. Sanitary Hygienic Status
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fadda, C.; Sanguinetti, A.M.; Del Caro, A.; Collar, C.; Piga, A. Bread Staling: Updating the View. Compr. Rev. Food Sci. Food Saf. 2014, 13, 473–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.V.; Copetti, M.V. Alternative Methods for Mould Spoilage Control in Bread and Bakery Products. Int. Food Res. J. 2019, 26, 737–749. [Google Scholar]
- Le-Bail, A.; Boumali, K.; Jury, V.; Ben-Aissa, F.; Zuniga, R. Impact of the baking kinetics on staling rate and mechanical properties of bread crumb and degassed bread crumb. J. Cereal Sci. 2009, 50, 235–240. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Shulga, O.; Chorna, A.; Arsenieva, L. Edible coating as factor of preserving freshness and increasing biological value of gingerbread cakes. Food Sci. Technol. 2016, 10, 247. [Google Scholar] [CrossRef]
- Galvão, A.M.; Zambelli, R.A.; Araújo, A.W.; Bastos, M.S. Edible coating based on modified corn starch/tomato powder: Effect on the quality of dough bread. LWT 2018, 89, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A review of active packaging in bakery products: Applications and future trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Dos Santos Caetano, K.; Almeida Lopes, N.; Haas Costa, T.M.; Brandelli, A.; Rodrigues, E.; Hickmann Flôres, S.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Zahedi, Y. Edible/Biodegradable Films and Coatings from Natural Hydrocolloids. In Emerging Natural Hydrocolloids: Rheology and Functions; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 571–599. [Google Scholar] [CrossRef]
- Suput, D.Z.; Lazic, V.L.; Popovic, S.Z.; Hromis, N.M. Edible films and coatings: Sources, properties and application. Food Feed Res. 2015, 42, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Bartolozzo, J.; Borneo, R.; Aguirre, A. Effect of triticale-based edible coating on muffin quality maintenance during storage. J. Food Meas. Charact. 2015, 10, 88–95. [Google Scholar] [CrossRef]
- Saraiva, L.E.F.; Naponucena, L.D.O.M.; Santos, V.D.S.; Silva, R.P.D.; de Souza, C.O.; Souza, I.E.G.L.; Mamede, M.E.D.O.; Druzian, J.I. Development and application of edible film of active potato starch to extend mini panettone shelf life. LWT 2016, 73, 311–319. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Cevoli, C.; Balestra, F.; Fabbri, A.; Rosa, M.D. Evaluation of drying of edible coating on bread using NIR spectroscopy. J. Food Eng. 2018, 240, 29–37. [Google Scholar] [CrossRef]
- Mouzakitis, C.-K.; Sereti, V.; Matsakidou, A.; Kotsiou, K.; Biliaderis, C.G.; Lazaridou, A. Physicochemical properties of zein-based edible films and coatings for extending wheat bread shelf life. Food Hydrocoll. 2022, 132, 107856. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Cevoli, C.; Balestra, F.; Fabbri, A.; Rosa, M.D. Evaluation of the effect of edible coating on mini-buns during storage by using NIR spectroscopy. J. Food Eng. 2019, 263, 46–52. [Google Scholar] [CrossRef]
- Marquez, G.R.; Di Pierro, P.; Esposito, M.; Mariniello, L.; Porta, R. Application of Transglutaminase-Crosslinked Whey Protein/Pectin Films as Water Barrier Coatings in Fried and Baked Foods. Food Bioprocess Technol. 2013, 7, 447–455. [Google Scholar] [CrossRef]
- Bravin, B.; Peressini, D.; Sensidoni, A. Development and application of polysaccharide–lipid edible coating to extend shelf-life of dry bakery products. J. Food Eng. 2006, 76, 280–290. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Shanmugam, S.; Bedi, J.S.; Selvamuthukumaran, S.; Yadav, D.N.; Anurag, R. Integrative Approach of MAP and Active Antimicrobial Packaging for Prolonged Shelf-Life of Composite Bottle Gourd Milk Cake. Coatings 2022, 12, 1204. [Google Scholar] [CrossRef]
- Solís-Contreras, G.; Rodríguez-Guillermo, M.; Reyes-Vega, M.D.L.L.; Aguilar, C.; Rebolloso-Padilla, O.; Corona-Flores, J.; Soriano-Melgar, L.D.A.A.; Ruelas-Chacon, X. Extending Shelf-Life and Quality of Minimally Processed Golden Delicious Apples with Three Bioactive Coatings Combined with Cinnamon Essential Oil. Foods 2021, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Jideani, V.A.; Vogt, K. Antimicrobial Packaging for Extending the Shelf Life of Bread—A Review. Crit. Rev. Food Sci. Nutr. 2013, 56, 1313–1324. [Google Scholar] [CrossRef]
- Luana, D.O.M.N.; Bruna, A.S.M.; Luciana, E.F.S.; Samantha, S.C.; Rejane, P.D.S.; Emanuelle, A.D.; Roseane, S.O.; Janice, I.D. Physicochemical and microbiological stability of muffins packed in actives edible coatings from cassava starch: Inverted sugar/sucrose and natural additives. Afr. J. Biotechnol. 2019, 18, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.-S.; Song, H.-G.; Choi, I.; Lee, J.-S.; Han, J. Effects of mung bean starch/guar gum-based edible emulsion coatings on the staling and safety of rice cakes. Carbohydr. Polym. 2020, 247, 116696. [Google Scholar] [CrossRef] [PubMed]
- De Pilli, T. Development of a vegetable oil and egg proteins edible film to replace preservatives and primary packaging of sweet baked goods. Food Control 2020, 114, 107273. [Google Scholar] [CrossRef]
- Calva-Estrada, S.D.J.; Jimenez-Fernandez, M.; Vallejo-Cardona, A.A.; Castillo-Herrera, G.A.; Lugo-Cervantes, E.D.C. Cocoa Nanoparticles to Improve the Physicochemical and Functional Properties of Whey Protein-Based Films to Extend the Shelf Life of Muffins. Foods 2021, 10, 2672. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.; Chang, Y.; Lee, E.-S.; Choi, H.-D.; Han, J. Development of a starch/gum-based edible coating for rice cakes to retard retrogradation during storage. LWT 2018, 97, 516–522. [Google Scholar] [CrossRef]
- Ghafoor, K.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ahmed, I.A.M.; Shahzad, S.A.; Alsawmahi, O.N. Quality Attributes of Refrigerated Barhi Dates Coated with Edible Chitosan Containing Natural Functional Ingredients. Foods 2022, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.A.; Soares, N.D.F.F.; Lopes, C.D.C.P.; da Silva, W.A.; Júnior, J.C.B.; Medeiros, E.A.A. Conservation of Bakery Products Through Cinnamaldehyde Antimicrobial Films. Packag. Technol. Sci. 2013, 27, 293–302. [Google Scholar] [CrossRef]
- Sachdeva, A.; Vashist, S.; Chopra, R.; Puri, D. Antimicrobial Activity of Active Packaging Film to Prevent Bread Spoilage. Int. J. Food Sci. Nutr. 2017, 2, 29–37. [Google Scholar]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef]
- Hajyani, S.; Modaresi, M.; Madani, M. Effect of Malva sylvestris L. Extract on Blood Cell Parameters in Mice with Candida Albicans Infection. Sch. Res. Libr. Der Pharma Chem. 2015, 7, 302–305. [Google Scholar]
- Mihaylova, D.; Popova, A.; Denkova, R.; Alexieva, I.; Krastanov, A. In Vitro Antioxidant and Antimicrobial Activity of Extracts of Bulgarian Malva sylvestris L. In Proceedings of the Annual of Sofia University, Faculty of Biology; Sofia University: Sofia, Bulgaria, 2014; pp. 41–48. [Google Scholar]
- Sharifi-Rad, J.; Melgar-Lalanne, G.; Álvarez, A.J.H.; Taheri, Y.; Shaheen, S.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Brdar-Jokanovic, M.; Rajkovic, J.; et al. Malva species: Insights on its chemical composition towards pharmacological applications. Phytother. Res. 2019, 34, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, I.N.; Baeva, M.R.; Popova, A.T.; Petrova, I.V.; Fidan, H.N.; Milkova-Tomova, I.V.; Mihov, R.B. Edible coatings enriched with Malva sylvestris L. extract. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 012113. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; Radwan, H.A.; Mohammed, N.S.; Aloraini, M.A.; Albezrah, N.K.A.; Alharbi, M.A.; Sayed, H.H.; Daoud, M.A.; Elmahallawy, E.K. Antimicrobial Activity of Some Plant Extracts and Their Applications in Homemade Tomato Paste and Pasteurized Cow Milk as Natural Preservatives. Fermentation 2022, 8, 428. [Google Scholar] [CrossRef]
- ISO—BDS—Bulgarian Institute for Standardization. Available online: https://www.iso.org/member/1597.html (accessed on 16 October 2022).
- BDS 3412:1979 Bread and Bread Products. Regulation for Taking Samples and Testing Methods. Available online: https://bds-bg.org/en/project/show/bds:proj:23125 (accessed on 20 October 2022).
- AACC Approved Methods of Analysis, 11th Edition—AACC Method 44-15.02. Moisture—Air-Oven Methods. Available online: http://methods.aaccnet.org/summaries/44-15-02.aspx (accessed on 15 August 2021).
- Armero, E.; Collar, C. Crumb Firming Kinetics of Wheat Breads with Anti-staling Additives. J. Cereal Sci. 1998, 28, 165–174. [Google Scholar] [CrossRef]
- Khanna, Y.P.; Taylor, T.J. Comments and recommendations on the use of the Avrami equation for physico-chemical kinetics. Polym. Eng. Sci. 1988, 28, 1042–1045. [Google Scholar] [CrossRef]
- Soukoulis, C.; Yonekura, L.; Gan, H.-H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.S.; Tatol, M. Color Difference ΔE: A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- ISO/TC 34/SC 9; Microbiology ISO 4833-1:2013-Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. Association française de normalisation: Saint-Denis, France, 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 12 November 2020).
- SIST ISO 21527-2:2011; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds. Agricultural food products: Saint-Denis, France, 2011. Available online: https://standards.iteh.ai/catalog/standards/sist/97646659-6073-41ee-905e-c1c523b9859f/sist-iso-21527-2-2011 (accessed on 16 October 2022).
- ISO 4831:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Coliforms—Most Probable Number Technique. Association française de normalisation: Saint-Denis, France, 1982. Available online: https://www.iso.org/standard/38280.html (accessed on 16 October 2022).
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. [WWW Document], n.d. Association française de normalisation: Saint-Denis, France, 1982. Available online: https://www.iso.org/standard/56712.html (accessed on 15 November 2022).
- UNE EN ISO 6888-1:2000; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar Medium (ISO 6888-1:1999). European Standards: Brussel, Belgium, 1999. Available online: https://www.en-standard.eu/une-en-iso-6888-1-2000-microbiology-of-food-and-animal-feeding-stuffs-horizontal-method-for-the-enumera-tion-of-coagulase-positive-staphylococci-staphylococcus-aureus-and-other-species-part-1-technique-using-baird-parker-agar-medium-iso-6888-1-1999/ (accessed on 16 October 2022).
- Assaad, H.I.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables for one-way ANOVA. SpringerPlus 2014, 3, 474. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yu, H.; Tian, B.; Jiang, B.; Xu, J.; Li, D.; Feng, Z.; Liu, C. Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese. Coatings 2019, 9, 583. [Google Scholar] [CrossRef] [Green Version]
- Sarengaowa; Wang, L.; Liu, Y.; Yang, C.; Feng, K.; Hu, W. Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes. Coatings 2022, 12, 1492. [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Cerqueira, M.A. Active Carboxymethyl Cellulose-Based Edible Coatings for the Extension of Fresh Goldenberries Shelf-Life. Horticulturae 2022, 8, 936. [Google Scholar] [CrossRef]
- Pahwa, A.; Kaur, A.; Puri, R. Influence of Hydrocolloids on the Quality of Major Flat Breads: A Review. J. Food Process. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Kim, S.K.; D’Apolonia, B.L. Bread Staling Studies. I. Effect of Protein Content on Staling Rate and Bread Crumb Pasting Properties. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201302501603 (accessed on 17 October 2022).
- Moussou, N.; Corzo-Martínez, M.; Sanz, M.L.; Zaidi, F.; Montilla, A.; Villamiel, M. Assessment of Maillard reaction evolution, prebiotic carbohydrates, antioxidant activity and α-amylase inhibition in pulse flours. J. Food Sci. Technol. 2016, 54, 890–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sopiwnyk, E.; Young, G.; Frohlich, P.; Borsuk, Y.; Lagassé, S.; Boyd, L.; Bourré, L.; Sarkar, A.; Dyck, A.; Malcolmson, L. Effect of pulse flour storage on flour and bread baking properties. LWT 2019, 121, 108971. [Google Scholar] [CrossRef]
- Shadid, K.A.; Shakya, A.K.; Naik, R.R.; Jaradat, N.; Farah, H.S.; Shalan, N.; Khalaf, N.A.; Oriquat, G.A. Phenolic Content and Antioxidant and Antimicrobial Activities of Malva sylvestris L., Malva oxyloba Boiss., Malva parviflora L., and Malva aegyptia L. Leaves Extract. J. Chem. 2021, 2021, 8867400. [Google Scholar] [CrossRef]
- Bashir, S.; Arshad, M.S.; Khalid, W.; Nayik, G.A.; Al Obaid, S.; Ansari, M.J.; Moreno, A.; Karabagias, I.K. Effect of Antimicrobial and Antioxidant Rich Pomegranate Peel Based Edible Coatings on Quality and Functional Properties of Chicken Nuggets. Molecules 2022, 27, 4500. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, Y. Antimicrobial and Antioxidant Effects of Kappa-Carrageenan Coatings Enriched with Cinnamon Essential Oil in Pork Meat. Foods 2022, 11, 2885. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Allam, A.Y.; Oz, E.; Khan, M.R.; Proestos, C.; Oz, F. Edible Xanthan/Propolis Coating and Its Effect on Physicochemical, Microbial, and Sensory Quality Indices in Mackerel Tuna (Euthynnus affinis) Fillets during Chilled Storage. Gels 2022, 8, 405. [Google Scholar] [CrossRef] [PubMed]




| Microorganism | Type of Edible Coating | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | P1 | C | C1 | X | X1 | ||||||||
| Concentration of Edible Coating, mL | |||||||||||||
| 0.15 | 0.10 | 0.15 | 0.10 | 0.15 | 0.10 | 0.15 | 0.10 | 0.15 | 0.10 | 0.15 | 0.10 | ||
| Listeria monocytogenes NCTC 11994 | Diameter * amimiof the zones, mm | 19 | * | * | * | * | * | * | * | 12 | 10 | * | * |
| Staphylococcus aureus ATCC 25093 | * | * | * | * | * | * | * | * | * | * | * | * | |
| Escherichia coli ATCC 8739 | * | * | * | * | 17 | 15 | 15 | * | 14 | * | 16 | 11 | |
| Salmonella NCTC 6017 | * | * | 36 | 10 | 12 | * | * | * | 14 | 11 | * | * | |
| Candida albicans ATCC 10231 | * | * | * | * | * | * | 13 | * | * | * | 17 | 14 | |
| Aspergillus brasiliensis ATCC 16404 | * | * | * | * | * | * | * | * | * | * | 15 | 12 | |
| Parameters | Crumb Moisture Content | ||
|---|---|---|---|
| 1 Day | 2 Days | 3 Days | |
| Control | |||
| Firmness | −0.961 | −0.999 | −0.756 |
| P | |||
| Firmness | −0.8663 | −0.870 | −1 |
| P1 | |||
| Firmness | −0.866 | −0.925 | −0.935 |
| C | |||
| Firmness | −1 | −0.866 | −0.859 |
| C1 | |||
| Firmness | −0.993 | −0.997 | −0.658 |
| X | |||
| Firmness | −0.786 | −0.866 | −0.914 |
| X1 | |||
| Firmness | −0.999 | −0.999 | −0.756 |
| Type of Loaf * | Fo, g/cm2 | Fmax (t = 3), g/cm2 | Avrami Equation | Rate Constant (k), Day−1 | R2 | Time Constant (1/k), Days |
|---|---|---|---|---|---|---|
| Control | 1.10 ± 0.10 a | 1.87 ± 0.55 a | y = −0.5280x − 0.1905 | 0.5280 | 0.9426 | 1.89 |
| P | 0.45 ± 0.10 e | 1.00 ± 0.10 c | y = −0.3031x − 0.5516 | 0.3031 | 0.9347 | 3.30 |
| P1 | 0.50 ± 0.10 d | 0.83 ± 0.12 d | y = −0.3466x − 1.0575 | 0.3466 | 0.9594 | 2.89 |
| C | 0.58 ± 0.06 bc | 0.67 ± 0.21 f | y = −0.4777x − 2.3739 | 0.4777 | 0.9365 | 2.09 |
| C1 | 0.53 ± 0.12 c | 0.77 ± 0.06 e | y = −0.4237x − 1.5006 | 0.4237 | 0.9668 | 2.36 |
| X | 0.60 ± 0.10 b | 1.37 ± 0.15 b | y = −0.1815x − 0.2355 | 0.1815 | 0.9235 | 5.51 |
| X1 | 0.50 ± 0.10 d | 1.07 ± 0.23 c | y = −0.2177x − 0.5372 | 0.2177 | 0.9432 | 4.59 |
| Parameter | Storage Time, Days | Type of Loaf * | ||||||
|---|---|---|---|---|---|---|---|---|
| C | C1 | X | X1 | P | P1 | Control | ||
| L* | 1 | 54.41 ± 3.03 b | 52.14 ± 0.17 c | 50.22 ± 3.09 e | 51.83 ± 1.75 d | 56.62 ± 2.84 a | 55.32 ± 1.84 a | 55.19 ± 4.42 a |
| 2 | 55.81 ± 3.05 a | 51.71 ± 1.71 b | 49.75 ± 3.67 c | 50.61 ± 0.48 b | 54.26 ± 0.76 a | 55.42 ± 2.30 a | 50.00 ± 2.94 b | |
| 3 | 54.98 ± 4.28 b | 51.43 ± 0.66 d | 50.83 ± 5.47 de | 53.24 ± 2.05 c | 53.30 ± 0.53 c | 56.56 ± 1.61 a | 49.22 ± 1.67 e | |
| a* | 1 | 18.06 ± 0.93 b | 19.60 ± 0.22 a | 19.97 ± 0.61 a | 18.10 ± 0.32 b | 18.33 ± 1.27 b | 18.25 ± 1.34 b | 18.66 ± 1.52 ab |
| 2 | 16.63 ± 1.10 c | 19.68 ± 0.11 a | 19.00 ± 0.29 a | 18.94 ± 0.40 a | 19.03 ± 0.45 a | 16.82 ± 1.34 c | 18.31 ± 3.20 ab | |
| 3 | 17.49 ± 1.57 c | 18.99 ± 0.67 b | 17.98 ± 1.09 c | 18.16 ± 0.69 b | 18.68 ± 0.55 b | 17.52 ± 1.97 c | 20.70 ± 0.28 a | |
| b* | 1 | 32.84 ± 0.76 a | 32.96 ± 0.47 a | 32.63 ± 0.93 a | 31.13 ± 0.86 a | 33.22 ± 2.13 b | 32.64 ± 1.17 a | 32.69 ± 0.62 a |
| 2 | 30.91 ± 3.43 b | 32.93 ± 0.71 a | 31.77 ± 1.66 a | 30.90 ± 0.85 b | 32.72 ± 0.22 a | 30.69 ± 1.47 b | 31.40 ± 2.07 a | |
| 3 | 32.30 ± 1.57 a | 31.37 ± 1.02 b | 30.78 ± 0.94 b | 30.46 ± 0.58 b | 31.66 ± 0.85 ab | 32.95 ± 1.72 a | 33.34 ± 1.00 a | |
| C* | 1 | 37.48 ± 0.95 a | 38.35 ± 0.51 a | 38.26 ± 0.53 a | 36.01 ± 0.78 b | 37.95 ± 2.14 a | 37.40 ± 1.67 a | 37.66 ± 0.26 a |
| 2 | 35.10 ± 3.51 b | 38.37 ± 0.57 a | 37.03 ± 1.45 a | 36.25 ± 0.88 b | 37.85 ± 0.32 a | 35.01 ± 1.67 b | 36.38 ± 3.32 b | |
| 3 | 36.75 ± 1.61 b | 36.67 ± 1.20 b | 35.67 ± 0.28 b | 35.47 ± 0.16 c | 36.76 ± 1.01 b | 37.32 ± 2.42 b | 39.25 ± 0.99 a | |
| h* | 1 | 61.20 ± 1.10 a | 59.26 ± 0.11 b | 58.52 ± 1.45 b | 59.81 ± 0.75 b | 61.10 ± 1.86 a | 60.81 ± 0.97 a | 60.29 ± 2.47 a |
| 2 | 61.64 ± 1.35 a | 59.14 ± 0.66 a | 59.09 ± 1.32 a | 58.49 ± 0.49 b | 59.34 ± 0.64 a | 61.28 ± 1.77 a | 59.93 ± 3.03 a | |
| 3 | 61.57 ± 2.40 a | 58.81 ± 0.35 b | 59.70 ± 2.28 a | 59.19 ± 1.44 a | 59.47 ± 0.07 a | 62.06 ± 1.53 a | 58.16 ± 0.45 b | |
| ∆E | 1 | 1.00 | 3.20 | 5.14 | 3.75 | 1.56 | 0.43 | - |
| 2 | 6.07 | 2.67 | 0.82 | 1.01 | 4.52 | 5.67 | - | |
| 3 | 6.68 | 3.42 | 4.07 | 5.56 | 4.85 | 8.01 | - | |
| Parameter | Storage Time, Days | Type of Loaf * | ||||||
|---|---|---|---|---|---|---|---|---|
| C | C1 | X | X1 | P | P1 | Control | ||
| L* | 1 | 63.04 ± 1.23 c | 66.05 ± 2.11 b | 70.62 ± 3.60 a | 63.01 ± 1.66 c | 66.25 ± 5.57 b | 63.10 ± 0.38 c | 66.13 ± 0.27 b |
| 2 | 66.81 ± 1.28 ab | 63.59 ± 0.36 c | 68.25 ± 3.10 a | 64.88 ± 1.35 b | 63.44 ± 1.44 c | 66.99 ± 4.80 ab | 68.17 ± 2.51 a | |
| 3 | 66.16 ± 3.26 a | 64.77 ± 1.37 a | 65.24 ± 3.92 a | 66.05 ± 2.85 a | 66.97 ± 1.40 a | 65.41 ± 1.52 a | 67.36 ± 2.14 a | |
| a* | 1 | 1.71 ± 0.04 b | 2.12 ± 0.50 a | 2.24 ± 0.17 a | 2.03 ± 0.49 a | 1.86 ± 0.07 b | 1.70 ± 0.13 b | 2.07 ± 0.26 a |
| 2 | 2.10 ± 0.61 a | 1.70 ± 0.24 b | 2.27 ± 0.37 a | 2.30 ± 0.20 a | 1.98 ± 0.10 a | 1.71 ± 0.05 b | 1.91 ± 0.38 a | |
| 3 | 1.96 ± 0.17 a | 1.89 ± 0.12 a | 2.31 ± 0.30 a | 2.15 ± 0.15 a | 1.97 ± 0.08 a | 1.78 ± 0.11 b | 2.20 ± 0.20 a | |
| b* | 1 | 12.99 ± 0.46 b | 13.93 ± 1.10 b | 15.22 ± 1.92 a | 14.45 ± 1.82 a | 13.75 ± 0.16 b | 13.87 ± 0.23 b | 13.83 ± 1.25 b |
| 2 | 13.90 ± 0.67 a | 13.59 ± 0.33 a | 14.73 ± 0.29 a | 14.94 ± 0.56 a | 14.07 ± 0.60 a | 13.64 ± 0.74 a | 14.09 ± 0.49 a | |
| 3 | 13.38 ± 1.12 b | 13.97 ± 0.44 a | 15.37 ± 0.42 a | 14.58 ± 0.56 a | 13.78 ± 0.36 a | 13.14 ± 0.68 a | 14.79 ± 0.92 a | |
| C* | 1 | 13.09 ± 0.47 b | 14.10 ± 1.16 a | 15.38 ± 1.92 a | 14.60 ± 1.85 a | 13.88 ± 0.16 a | 13.98 ± 0.23 a | 13.99 ± 1.28 a |
| 2 | 14.06 ± 0.76 a | 13.70 ± 0.31 b | 14.91 ± 0.32 a | 15.12 ± 0.56 a | 14.21 ± 0.60 a | 13.75 ± 0.74 b | 14.22 ± 0.52 a | |
| 3 | 13.52 ± 1.27 b | 14.10 ± 0.44 a | 15.54 ± 0.37 a | 14.74 ± 0.57 a | 13.92 ± 0.35 a | 13.29 ± 0.68 b | 14.95 ± 0.90 a | |
| h* | 1 | 82.93 ± 0.55 a | 81.40 ± 1.36 a | 81.57 ± 0.81 a | 82.03 ± 1.34 a | 82.32 ± 0.29 a | 82.99 ± 0.55 a | 81.51 ± 0.34 a |
| 2 | 81.49 ± 2.01 a | 82.88 ± 1.11 a | 81.24 ± 1.31 b | 81.26 ± 0.65 b | 81.98 ± 0.08 a | 82.83 ± 0.23 a | 82.29 ± 1.36 b | |
| 3 | 81.65 ± 0.07 a | 82.27 ± 0.50 a | 81.42 ± 1.31 c | 81.62 ± 0.49 a | 81.84 ± 0.54 a | 82.31 ± 0.35 a | 81.51 ± 1.08 a | |
| ∆E | 1 | 3.22 | 0.14 | 4.70 | 3.18 | 0.26 | 3.05 | - |
| 2 | 1.39 | 4.61 | 0.74 | 3.42 | 4.73 | 1.28 | - | |
| 3 | 1.87 | 2.73 | 2.20 | 1.33 | 1.11 | 2.59 | - | |
| Storage Time, [Day] | Type of Loaf 1 | Total Plate Count, [CFU/g] 2 | Coliforms, [CFU/g] | Coagulase-Positive Staphylococci, [CFU/g] | Salmonella spp. in 25 g | Molds and Yeasts, [CFU/g] |
|---|---|---|---|---|---|---|
| 1 | Control | 0 | ND 3 | ND | ND | 0 |
| P | 0 | ND | ND | ND | 0 | |
| P1 | 0 | ND | ND | ND | 0 | |
| C | 0 | ND | ND | ND | 0 | |
| C1 | 0 | ND | ND | ND | 0 | |
| X | 0 | ND | ND | ND | 0 | |
| X1 | 0 | ND | ND | ND | 0 | |
| 2 | Control | 3.1 × 102 | ND3 | ND | ND | 1.1 × 102 |
| P | 0 | ND | ND | ND | 0 | |
| P1 | 0 | ND | ND | ND | 0 | |
| C | 0 | ND | ND | ND | 0 | |
| C1 | 0 | ND | ND | ND | 0 | |
| X | 0 | ND | ND | ND | 0 | |
| X1 | 0 | ND | ND | ND | 0 | |
| 3 | Control | 5.8 × 104 | ND3 | ND | ND | 4.9 × 103 |
| P | 0 | ND | ND | ND | 0 | |
| P1 | 0 | ND | ND | ND | 0 | |
| C | 2.4 × 102 | ND | ND | ND | 0 | |
| C1 | 0 | ND | ND | ND | 0 | |
| X | 3.3 × 102 | ND | ND | ND | 0 | |
| X1 | 0 | ND | ND | ND | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexieva, I.; Baeva, M.; Popova, A.; Fidan, H.; Goranova, Z.; Milkova-Tomova, I. Development and Application of Edible Coatings with Malva sylvestris L. Extract to Extend Shelf-Life of Small Loaf. Foods 2022, 11, 3831. https://doi.org/10.3390/foods11233831
Alexieva I, Baeva M, Popova A, Fidan H, Goranova Z, Milkova-Tomova I. Development and Application of Edible Coatings with Malva sylvestris L. Extract to Extend Shelf-Life of Small Loaf. Foods. 2022; 11(23):3831. https://doi.org/10.3390/foods11233831
Chicago/Turabian StyleAlexieva, Iordanka, Marianna Baeva, Aneta Popova, Hafize Fidan, Zhivka Goranova, and Iliana Milkova-Tomova. 2022. "Development and Application of Edible Coatings with Malva sylvestris L. Extract to Extend Shelf-Life of Small Loaf" Foods 11, no. 23: 3831. https://doi.org/10.3390/foods11233831
APA StyleAlexieva, I., Baeva, M., Popova, A., Fidan, H., Goranova, Z., & Milkova-Tomova, I. (2022). Development and Application of Edible Coatings with Malva sylvestris L. Extract to Extend Shelf-Life of Small Loaf. Foods, 11(23), 3831. https://doi.org/10.3390/foods11233831








