Effect of Encapsulation Processes by Freeze and Spray Drying on the Antioxidant Properties of Red Wine from cv. Listan Prieto and Syrah
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Physicochemical, Color and Total Phenolic Content Analysis
2.4. Phenolic Profiling
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Physicochemical, Color and Total Phenolic Content Analysis
3.2. Phenolic Profiling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Higgins, S.; Duthie, G.G.; Duthie, S.J.; Howie, M.; Mullen, W.; Lean, M.E.J.; Crozier, A. The Influence of Moderate Red Wine Consumption on Antioxidant Status and Indices of Oxidative Stress Associated with CHD in Healthy Volunteers. Br. J. Nutr. 2005, 93, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]
- Landrault, N.; Poucheret, P.; Ravel, P.; Gasc, F.; Cros, G.; Teissedre, P.L. Antioxidant Capacities and Phenolics Levels of French Wines from Different Varieties and Vintages. J. Agric. Food Chem. 2001, 49, 3341–3348. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. Extraction of Phenolics and Changes in Antioxidant Activity of Red Wines during Vinification. J. Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Karumanchiri, A.; Tsang, E.; Soleas, G.J. Catechin and Epicatechin Concentrations of Red Wines: Regional and Cultivar-Related Differences. Am. J. Enol. Vitic. 1998, 49, 23–34. [Google Scholar]
- Oliva Oller, P.R. Characterization of the Winegrowers of the Major Wine Regions in Bolivia. RIVAR 2021, 8, 51–70. [Google Scholar] [CrossRef]
- Lacoste, P. Rise, Fall and Rebirth of Listán Prieto in the Southern Cone of America. Idesia 2021, 39, 75–84. [Google Scholar] [CrossRef]
- Aguilera, I.; Alvear, A. Pipeño and Terremoto Like National Drinks: Reflexions about Heritage and National Representation. RIVAR 2017, 12, 5–21. [Google Scholar]
- Bravo-Ávila, D.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Viticultural Characterization of Carignan (Vitis vinifera L.) Grapevine Variety Located in the Rainfed Area of the Maule Valley, Chile. RIVAR 2021, 8, 18–35. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Carrasco-Quiroz, M.; Martínez-Gil, A.M.; Pérez-Álvarez, E.P.; Garde-Cerdán, T.; Moreno-Simunovic, Y. Grape and Wine Amino Acid Composition from Carignan Noir Grapevines Growing under Rainfed Conditions in the Maule Valley, Chile: Effects of Location and Rootstock. Food Res. Int. 2018, 105, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Liu, S.Y.; Pszczólkowski, P. Resurgence of Minority and Autochthonous Grapevine Varieties in South America: A Review of Their Oenological Potential. J. Sci. Food Agric. 2020, 100, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I. Producción Vitivinícola En El Secano de Chile Central; Instituto Nacional de Investigaciones Agropecuarias, Ed.; Boletín INIA.: Villa Alegre, Chile, 2020. [Google Scholar]
- Cavallini, G.; Straniero, S.; Donati, A.; Bergamini, E. Resveratrol Requires Red Wine Polyphenols for Optimum Antioxidant Activity. J. Nutr. Health Aging 2016, 20, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Parra, D.F.; Lanari, M.C.; Zamora, M.C.; Chirife, J. Influence of Storage Conditions on Phenolic Compounds Stability, Antioxidant Capacity and Colour of Freeze-Dried Encapsulated Red Wine. LWT 2016, 70, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 829. [Google Scholar] [CrossRef] [Green Version]
- Alvarez Gaona, I.J.; Bater, C.; Zamora, M.C.; Chirife, J. Spray Drying Encapsulation of Red Wine: Stability of Total Monomeric Anthocyanins and Structural Alterations upon Storage. J. Food Process. Preserv. 2018, 42, e13457. [Google Scholar] [CrossRef]
- Gurak, P.D.; Cabral, L.M.C.; Rocha-Leão, M.H. Production of Grape Juice Powder Obtained by Freeze-Drying after Concentration by Reverse Osmosis. Braz. Arch. Biol. Technol. 2013, 56, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, V.; Baeza, R.; Galmarini, M.v.; Zamora, M.C.; Chirife, J. Freeze-Drying Encapsulation of Red Wine Polyphenols in an Amorphous Matrix of Maltodextrin. Food Bioprocess Technol. 2013, 6, 1350–1354. [Google Scholar] [CrossRef]
- Alvarez Gaona, I.J.; Fanzone, M.; Sari, S.; Assof, M.; Pérez, D.; Chirife, J.; Zamora, M.C. Spray-Dried Ancellotta Red Wine: Natural Colorant with Potential for Food Applications. Eur. Food Res. Technol. 2019, 245, 2621–2630. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Concha-Meyer, A.A.; Sepúlveda, G.; Pérez-Díaz, R.; Torres, C.A. Effect of Preservation Processing on Quality Attributes and Phenolic Profile of Maqui (Aristotelia chilensis Mol. Stuntz) Fruit. LWT 2021, 149, 111920. [Google Scholar] [CrossRef]
- Torres, C.A.; Sepúlveda, G.; Concha-Meyer, A.A. Effect of Processing on Quality Attributes and Phenolic Profile of Quince Dried Bar Snack. J. Sci. Food Agric. 2019, 99, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cánovas, G.v.; Fontana, A.J.; Schmidt, S.J.; Labuza, T.P. Water Activity in Foods: Fundamentals and Applications; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; ISBN 0813824087. [Google Scholar]
- Allan, M.C.; Grush, E.N.; Rajwa, B.P.; Butzke, C.E.; Mauer, L.J. Determination of the Water Activities of Wines and Spirits. Food Anal. Methods 2019, 12, 2753–2763. [Google Scholar] [CrossRef]
- Sadras, V.O.; Collins, M.; Soar, C.J. Modelling Variety-Dependent Dynamics of Soluble Solids and Water in Berries of Vitis Vinifera. Aust. J. Grape Wine Res. 2008, 14, 250–259. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Semenov, G.V.; Kasyanov, G.I.; Petkov, I.I.; Krasnova, I.S. Freeze Drying of Grape Raw Materials in Red Winemaking. Online J. Biol. Sci. 2017, 17, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Xia, N.; Cheng, H.; Yao, X.; Pan, Q.; Meng, N.; Yu, Q. Effect of Cold Stabilization Duration on Organic Acids and Aroma Compounds during Vitis vinifera L. Cv. Riesling Wine Bottle Storage. Foods 2022, 11, 1179. [Google Scholar] [CrossRef]
- Heredia, F.J.; Francia-Aricha, E.M.; Rivas-Gonzalo, J.C.; Vicario, I.M.; Santos-Buelga, C. Chromatic Characterization of Anthocyanins from Red Grapes—I. PH Effect. Food Chem. 1998, 63, 491–498. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Extraction of Anthocyanins from Red Cabbage and Purification Using Adsorption. Food Bioprod. Process. 2012, 90, 615–623. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional Properties of Anthocyanins and Betalains in Plants, Food, and in Human Nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. A Comprehensive Study of Red Wine Properties According to Variety. Food Chem. 2016, 196, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Davila-Trujillo, R.; Santos Fernandes, S.; Lima Dora, C.; Maria Monserrat, J.; Prentice, C.; de las Mercedes Salas-Mellado, M. Physical, Chemical, and Biological Evaluation of Nanoparticles Containing Phenolic Compounds from Wine Production Residues. J. Food Process. Preserv. 2021, 45, e15629. [Google Scholar] [CrossRef]
- Gomes, W.F.; França, F.R.M.; Denadai, M.; Andrade, J.K.S.; da Silva Oliveira, E.M.; de Brito, E.S.; Rodrigues, S.; Narain, N. Effect of Freeze- and Spray-Drying on Physico-Chemical Characteristics, Phenolic Compounds and Antioxidant Activity of Papaya Pulp. J. Food Sci. Technol. 2018, 55, 2102. [Google Scholar] [CrossRef] [PubMed]
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia Powders Obtained by Different Drying Processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Hammami, C.; René, F. Determination of Freeze-Drying Process Variables for Strawberries. J. Food Eng. 1997, 32, 133–154. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Alonso-Alonso, O.; Gallo-Montesdeoca, A.; Díaz-Romero, C.; Darias-Martín, J. Influence of Copigmentation and Phenolic Composition on Wine Color. J. Food Sci. Technol. 2016, 53, 2540. [Google Scholar] [CrossRef] [Green Version]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanin-Derived Pigments and Colour of Red Wines. Anal. Chim. Acta 2002, 458, 147–155. [Google Scholar] [CrossRef]
- Gris, E.F.; Ferreira, E.A.; Falcão, L.D.; Bordignon-Luiz, M.T. Caffeic Acid Copigmentation of Anthocyanins from Cabernet Sauvignon Grape Extracts in Model Systems. Food Chem. 2007, 100, 1289–1296. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D. Effects of Spray Drying Conditions on the Physicochemical and Antioxidant Properties of the Gac (Momordica Cochinchinensis) Fruit Aril Powder. J. Food Eng. 2010, 98, 385–392. [Google Scholar] [CrossRef]
- Favre, G.; Piccardo, D.; Gómez-Alonso, S.; Pérez-Navarro, J.; García-Romero, E.; Mena-Morales, A.; González-Neves, G. Stilbenes in Grapes and Wines of Tannat, Marselan and Syrah from Uruguay. OENO ONE 2020, 54, 27–36. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. Effects of Sunlight Exposure on Flavonol Content and Wine Sensory of the White Winegrape Grechetto Gentile. Am. J. Enol. Vitic. 2019, 70, 277–285. [Google Scholar] [CrossRef]
- Gawel, R.; Day, M.; van Sluyter, S.C.; Holt, H.; Waters, E.J.; Smith, P.A. White Wine Taste and Mouthfeel as Affected by Juice Extraction and Processing. J. Agric. Food Chem. 2014, 62, 10008–10014. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Waterhouse, A.L.; de La Torre-Boronat, M.C. Levels of Cis- and Trans-Resveratrol and Their Glucosides in White and Rosé Vitis Vinifera Wines from Spain. J. Agric. Food Chem. 1996, 44, 2124–2128. [Google Scholar] [CrossRef]
- Galmarini, M.v.; Maury, C.; Mehinagic, E.; Sanchez, V.; Baeza, R.I.; Mignot, S.; Zamora, M.C.; Chirife, J. Stability of Individual Phenolic Compounds and Antioxidant Activity During Storage of a Red Wine Powder. Food Bioprocess Technol. 2013, 6, 3585–3595. [Google Scholar] [CrossRef] [Green Version]
- Kontaxakis, E.; Trantas, E.; Ververidis, F. Resveratrol: A Fair Race Towards Replacing Sulfites in Wines. Molecules 2020, 25, 2378. [Google Scholar] [CrossRef]
- Manfredi, C.; Trifuoggi, M.; Amoresano, A.; Vasca, E.; Pepe, C.; Volino, S.; Annetta, M. On Trans-Resveratrol in Aqueous Solutions. J. Solut. Chem. 2017, 46, 2214–2230. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Pharmaceutics 2008, 349, 83–93. [Google Scholar] [CrossRef]
- Ma, Q.; Bi, J.; Yi, J.; Wu, X.; Li, X.; Zhao, Y. Stability of Phenolic Compounds and Drying Characteristics of Apple Peel as Affected by Three Drying Treatments. Food Sci. Hum. Wellness 2021, 10, 174–182. [Google Scholar] [CrossRef]
- Elhamirad, A.H.; Zamanipoor, M.H. Thermal Stability of Some Flavonoids and Phenolic Acids in Sheep Tallow Olein. Eur. J. Lipid Sci. Technol. 2012, 114, 602–606. [Google Scholar] [CrossRef]
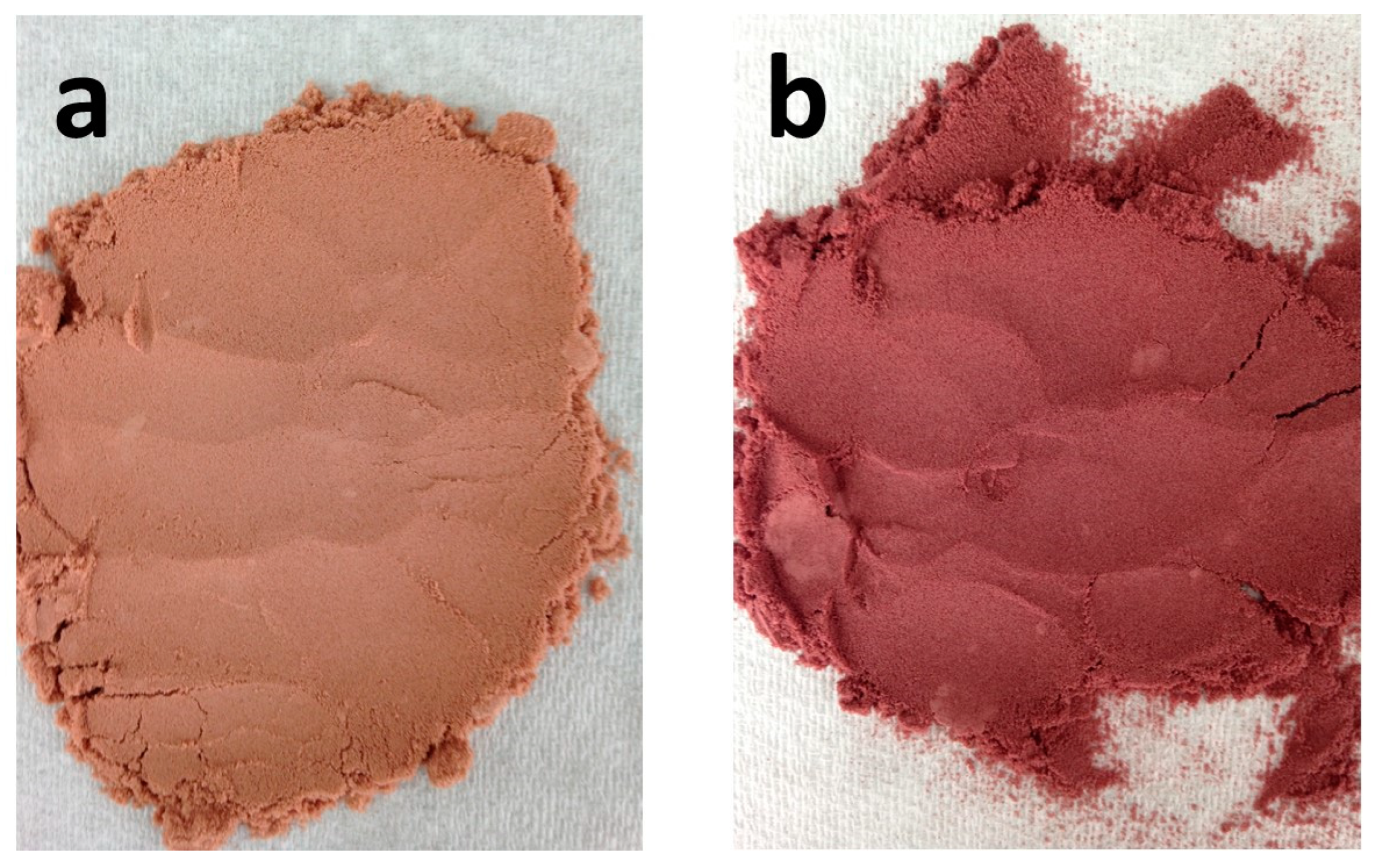
| Wine Variety | Treatment | Water Activity (aw) | pH | Soluble Solids (°Brix) | Total Phenolic Content (mg GAE g−1) |
|---|---|---|---|---|---|
| Listan Prieto | Non-dealcoholized | 0.93 ± 0.01 a | 3.94 ± 0.07 a | 4.20 ± 0.04 a | 1.45 ± 0.07 a |
| Dealcoholized | 0.92 ± 0.01 a | 3.48 ± 0.11 abc | 5.20 ± 0.61 a | 4.05 ± 0.07 a | |
| Spray Dry | 0.17 ± 0.01 b | 3.57 ± 0.16 ab | 9.30 ± 0.18 c | 41.70 ± 0.70 c | |
| Freeze Dry | 0.24 ± 0.05 b | 2.91 ± 0.27 c | 9.00 ± 0.21 c | 33.50 ± 0.57 b | |
| Syrah | Non-dealcoholized | 0.95 ± 0.01 a | 3.66 ± 0.16 a | 7.10 ± 0.12 b | 1.85 ± 0.07 a |
| Dealcoholized | 0.92 ± 0.00 a | 3.55 ± 0.14 ab | 5.10 ± 0.19 a | 5.45 ± 0.07 a | |
| Spray Dry | 0.14 ± 0.03 b | 3.62 ± 0.13 a | 9.23 ± 0.11 c | 51.40 ± 1.98 d | |
| Freeze Dry | 0.24 ± 0.07 b | 2.98 ± 0.14 bc | 8.70 ± 0.21 c | 40.35 ± 2.90 c |
| Wine Variety | Treatment | L* | a | b | HEX Code | Color |
|---|---|---|---|---|---|---|
| Listan Prieto | Spray Dry | 66.25 ± 0.05 d | 45.04 ± 0.04 d | 37.63 ± 0.04 d | #F97D60 | |
| Freeze Dry | 59.41 ± 0.07 c | 25.32 ± 0.06 c | 24.72 ± 0.05 c | #C57D65 | ||
| Syrah | Spray Dry | 37.10 ± 0.04 b | 21.72 ± 0.08 b | 13.89 ± 0.03 b | #7E4942 | |
| Freeze Dry | 29.59 ± 0.08 a | 8.58 ± 0.02 a | 1.92 ± 0.05 a | #544143 |
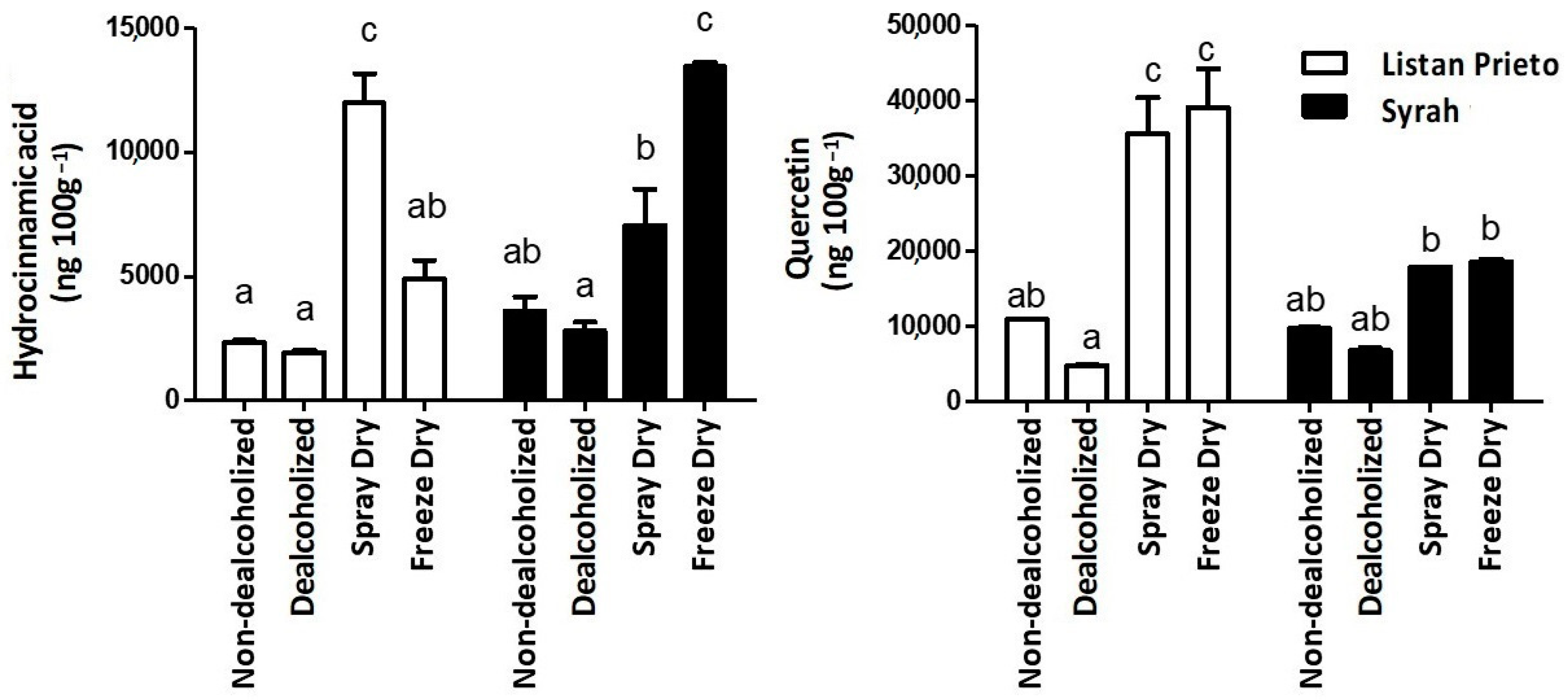
| Trans-Resveratrol | Hydrocinnamic Acid | Phloretin | Kaempferol | Quercetin | |
|---|---|---|---|---|---|
| Wine variety (A) | |||||
| Syrah | 87.8 ± 30.7 a | 6715.5 ± 2419.0 a | 297.5 ± 37.4 | 2654.6 ± 456.4 a | 13,238.3 ± 2946.0 a |
| Listan Prieto | 82.5 ± 25.5 a | 5273.5 ± 2324.2 a | 336.2 ± 21.2 | 2718.6 ± 548.9 a | 22,613.9 ± 8615.0 a |
| p-value | 0.80 | 0.62 | 0.09 | 0.58 | 0.60 |
| Treatment (B) | |||||
| Non-dealcoholized | 60.6 ± 12.1 a | 2961.2 ± 389.0 a | 333.7 ± 23.0 b | 2126.9 ± 151.0 b | 10,344.5 ± 273.1 a |
| Dealcoholized | 33.4 ± 5.3 a | 2366.9 ± 252.3 a | 244.1 ± 23.0 a | 1585.6 ± 22.5 a | 5806.1 ± 4499.2 a |
| Freeze Dry | 93.7 ± 25.4 ab | 9153.1 ± 1946.1 b | 331.4 ± 30.0 ab | 3700.8 ± 151.3 c | 28,804.2 ± 5134.1 b |
| Spray Dry | 152.8 ± 30.1 b | 9496.9 ± 1388.1 b | 358.3 ± 23.0 b | 3333.23 ± 115.0 c | 26,749.7 ± 467.0 b |
| p-value | 0.00 | 0.00 | 0.013 | 0.00 | 0.00 |
| Interaction (A × B) | |||||
| p-value | 0.35 | 0.00 | 0.09 | 0.13 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Gálvez, I.; Gutiérrez-Gamboa, G.; Plaza, A.; Concha-Meyer, A.A. Effect of Encapsulation Processes by Freeze and Spray Drying on the Antioxidant Properties of Red Wine from cv. Listan Prieto and Syrah. Foods 2022, 11, 3880. https://doi.org/10.3390/foods11233880
Díaz-Gálvez I, Gutiérrez-Gamboa G, Plaza A, Concha-Meyer AA. Effect of Encapsulation Processes by Freeze and Spray Drying on the Antioxidant Properties of Red Wine from cv. Listan Prieto and Syrah. Foods. 2022; 11(23):3880. https://doi.org/10.3390/foods11233880
Chicago/Turabian StyleDíaz-Gálvez, Irina, Gastón Gutiérrez-Gamboa, Andrea Plaza, and Anibal A. Concha-Meyer. 2022. "Effect of Encapsulation Processes by Freeze and Spray Drying on the Antioxidant Properties of Red Wine from cv. Listan Prieto and Syrah" Foods 11, no. 23: 3880. https://doi.org/10.3390/foods11233880






