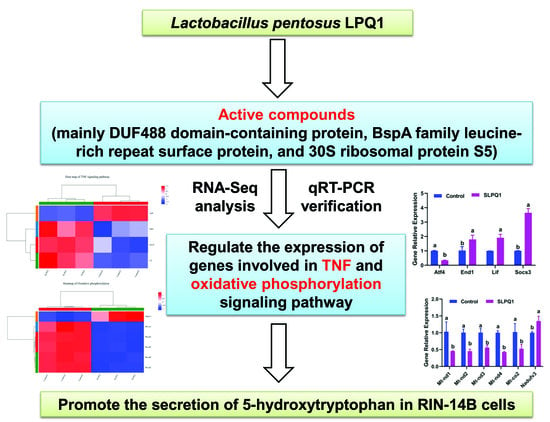
Promoting Effect and Potential Mechanism of Lactobacillus pentosus LPQ1-Produced Active Compounds on the Secretion of 5-Hydroxytryptophan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. RIN-14B Cell Culture
2.3. The Preparation of LAB Strains
2.4. Preparation of the Mixture of Compounds Secreted by L. pentosus LPQ1 (SLPQ1)
2.5. Evaluation of the Promoting Effect of LAB and Its Secreted Compounds on the Secretion of 5-HTP by RIN-14B Cells
2.6. Determination of 5-hydroxytryptophan Content
2.7. Methyl Thiazolyl Tetrazolium (MTT) Assay
2.8. RNA-Seq Analysis
2.9. qRT-PCR Analysis
2.10. Analysis of Active Compounds
2.11. Statistical Analysis
3. Results
3.1. Effect of LAB Strains on 5-HTP Secretion
3.2. Effect of SLPQ1 on 5-HTP Secretion by RIN-14B Cells
3.3. Analysis of the Transcriptional Changes
3.4. Differentially Expressed Genes Analysis by Gene Ontology Term and Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment Analyses
3.5. Effect of SLPQ1 on Oxidative Phosphorylation- and TNF Signaling Pathway-Related Gene Expression
3.6. Effects of the Active Compounds Present in SLPQ1 on 5-HTP Secretion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Wei, J.; Call, T.; Quintus, N.T.; Summers, A.J.; Carotenuto, S.; Johnson, R.; Ma, X.; Xu, C.; Park, J.G.; et al. Shisa6 mediates cell-type specific regulation of depression in the nucleus accumbens. Mol. Psychiatry 2021, 26, 7316–7327. [Google Scholar] [CrossRef] [PubMed]
- Steel, Z.; Marnane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Semahegn, A.; Torpey, K.; Manu, A.; Assefa, N.; Tesfaye, G.; Ankomah, A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. Pharm. 2020, 9, 17. [Google Scholar] [CrossRef]
- Pereira, C.A.; Rodrigues, F.L.; Ruginsk, S.G.; Zanotto, C.Z.; Rodrigues, J.A.; Duarte, D.A.; Costa-Neto, C.M.; Resstel, L.B.; Carneiro, F.S.; Tostes, R.C. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eur. J. Pharmacol. 2017, 800, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, B.F.; Zlotnik, A.; Frenkel, A.; Fleidervish, I.; Boyko, M. Glutamate Efflux across the Blood-Brain Barrier: New Perspectives on the relationship between depression and the glutamatergic system. Metabolites 2022, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Rayff da Silva, P.; Diniz Nunes Pazos, N.; Karla Silva do Nascimento Gonzaga, T.; Cabral de Andrade, J.; Brito Monteiro, Á.; Caroline Ribeiro Portela, A.; Fernandes Oliveira Pires, H.; Dos Santos Maia, M.; Vilar da Fonsêca, D.; T Scotti, M.; et al. Anxiolytic and antidepressant-like effects of monoterpene tetrahydrolinalool and in silico approach of new potential targets. Curr. Top. Med. Chem. 2022, 22, 1530–1552. [Google Scholar]
- Xu, J.; Tang, M.; Wu, X.; Kong, X.; Liu, Y.; Xu, X. Lactobacillus rhamnosus zz-1 exerts preventive effects on chronic unpredictable mild stress-induced depression in mice via regulating the intestinal microenvironment. Food Funct. 2022, 13, 4331–4343. [Google Scholar] [CrossRef]
- Hao, W.; Ma, Q.; Tao, G.; Huang, J.; Chen, J. Oral coniferyl ferulate attenuated depression symptoms in mice via reshaping gut microbiota and microbial metabolism. Food Funct. 2021, 12, 12550–12564. [Google Scholar] [CrossRef]
- Tian, P.; Zhu, H.; Zou, R.; Kong, Q.; Xu, M.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. An in vitro screening method for probiotics with antidepressant-like effect using the enterochromaffin cell model. Food Funct. 2021, 12, 646–655. [Google Scholar] [CrossRef]
- Harmer, C.J. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology 2008, 55, 1023–1028. [Google Scholar] [CrossRef]
- Pardridge, W.M. The role of blood-brain barrier transport of tryptophan and other neutral amino acids in the regulation of substrate-limited pathways of brain amino acid metabolism. J. Neural Transm. Suppl. 1979, 15, 43–54. [Google Scholar]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: A complex interconnected loop influencing human health status. Int. J. Tryptophan Res. 2019, 12, 1178646919852996. [Google Scholar] [CrossRef] [PubMed]
- Teunis, C.; Nieuwdorp, M.; Hanssen, N. Interactions between tryptophan metabolism, the gut microbiome and the immune system as potential drivers of non-alcoholic fatty liver disease (NAFLD) and metabolic diseases. Metabolites 2022, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Sharma, A.; Castellani, R.J.; Smith, M.A.; Muresanu, D.F.; Dey, P.K.; Sharma, H.S. 5-Hydroxytryptophan: A precursor of serotonin influences regional blood-brain barrier breakdown, cerebral blood flow, brain edema formation, and neuropathology. Int. Rev. Neurobiol. 2019, 146, 1–44. [Google Scholar]
- Fukuda, K. 5-HTP hypothesis of schizophrenia. Med. Hypotheses 2014, 82, 20–23. [Google Scholar] [CrossRef]
- Jangid, P.; Malik, P.; Singh, P.; Sharma, M.; Gulia, A.K.D. Comparative study of efficacy of l-5-hydroxytryptophan and fluoxetine in patients presenting with first depressive episode. Asian J. Psychiatr. 2013, 6, 29–34. [Google Scholar] [CrossRef]
- Nakajima, T.; Kudo, Y.; Kaneko, Z. Clinical evaluation of 5-hydroxy-L-tryptophan as an antidepressant drug. Folia Psychiatr. Neurol. Jpn. 1978, 32, 223–230. [Google Scholar] [CrossRef]
- Sha, J.; Song, J.; Huang, Y.; Zhang, Y.; Wang, H.; Zhang, Y.; Suo, H. Inhibitory effect and potential mechanism of Lactobacillus plantarum YE4 against dipeptidyl peptidase-4. Foods 2021, 11, 80. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Zhou, X.; Zhang, L.; Gu, L.; Liu, Z.; Ma, J.; Hou, J.; Jiang, Z. Lactobacillus plantarum combined with Galactooligosaccharides supplement: A neuroprotective regimen against neurodegeneration and memory impairment by regulating short-chain fatty acids and the c-Jun N-Terminal kinase signaling pathway in mice. J. Agric. Food Chem. 2022, 70, 8619–8630. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, N.; Zou, L.; Shi, Z.; Dun, B.; Ren, G.; Yao, Y. Soy protein alleviates malnutrition in weaning rats by regulating gut microbiota composition and serum metabolites. Front. Nutr. 2021, 8, 774203. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yu, T.; Wang, H.; Li, Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; O’Riordan, K.J.; Lee, Y.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Chen, H.; Wang, R.; Ma, Y. The structure and properties of MFG-E8 and the In vitro assessment of its toxic effects on myoblast cells. Protein Expr. Purif. 2021, 178, 105720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, L.; Zhang, P.; Dong, T.; Fang, J. Transcriptome and metabolite profiling reveal that spraying calcium fertilizer reduces grape berry cracking by modulating the flavonoid biosynthetic metabolic pathway. Food Chem. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Chen, J.; Ren, Y.; Zhang, K.; Xiong, Y.L.; Wang, Q.; Shang, K.; Zhang, D. Site-specific incorporation of sodium tripolyphosphate into myofibrillar protein from mantis shrimp (Oratosquilla oratoria) promotes protein crosslinking and gel network formation. Food Chem. 2020, 312, 126113. [Google Scholar] [CrossRef]
- Zettlitz, K.A.; Lorenz, V.; Landauer, K.; Munkel, S.; Herrmann, A.; Scheurich, P.; Pfizenmaier, K.; Kontermann, R. ATROSAB, a humanized antagonistic anti-tumor necrosis factor receptor one-specific antibody. mAbs 2010, 2, 639–647. [Google Scholar] [CrossRef]
- Liu, S.; Guo, R.; Liu, F.; Yuan, Q.; Yu, Y.; Ren, F. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr. Dis. Treat. 2020, 16, 859–869. [Google Scholar] [CrossRef]
- Kotni, M.K.; Zhao, M.; Wei, D.Q. Gene expression profiles and protein-protein interaction networks in amyotrophic lateral sclerosis patients with C9orf72 mutation. Orphanet J. Rare Dis. 2016, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhou, W. Amarogentin has protective effects against sepsis-induced brain injury via modulating the AMPK/SIRT1/NF-κB pathway. Brain Res. Bull. 2022, 189, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, B.; Ai, L. Advances in the microbial synthesis of 5-Hydroxytryptophan. Front. Bioeng. Biotechnol. 2021, 9, 624503. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.B.; Brinckmann, J.A.; Harter, D.E.V. From forest to pharmacy: Should we be depressed about a sustainable Griffonia simplicifolia (Fabaceae) seed supply chain? J. Ethnopharmacol. 2021, 278, 114202. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ran, L.; Wu, Y.; Liang, M.; Zeng, J.; Ke, F.; Wang, F.; Yang, J.; Lao, X.; Liu, L.; et al. Oral administration of 5-hydroxytryptophan restores gut microbiota dysbiosis in a mouse model of depression. Front. Microbiol. 2022, 13, 864571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, X.; Zheng, J. Transcriptome profiling using Illumina- and SMRT-based RNA-seq of hot pepper for in-depth understanding of genes involved in CMV infection. Gene 2018, 666, 123–133. [Google Scholar] [CrossRef]
- Leonard, B.E. Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Das, R.; Emon, M.P.Z.; Shahriar, M.; Nahar, Z.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, S.N.; Islam, M.R. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. 2021, 295, 113568. [Google Scholar] [CrossRef]
- Bavaresco, D.V.; Uggioni, M.L.R.; Ferraz, S.D.; Marques, R.M.M.; Simon, C.S.; Dagostin, V.S.; Grande, A.J.; da Rosa, M.I. Efficacy of infliximab in treatment-resistant depression: A systematic review and meta-analysis. Pharmacol. Biochem. Behav. 2020, 188, 172838. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Subramaniapillai, M.; Lee, Y.; Pan, Z.; Carmona, N.E.; Shekotikhina, M.; Rosenblat, J.D.; Brietzke, E.; Soczynska, J.K.; Cosgrove, V.E.; et al. Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar I/II depression: A randomized clinical trial. JAMA Psychiatry 2019, 76, 783–790. [Google Scholar] [CrossRef]
- Uzzan, S.; Azab, A.N. Anti-TNF-α compounds as a treatment for depression. Molecules 2021, 26, 2368. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Rozpedek, W.; Markiewicz, L.; Diehl, J.A.; Pytel, D.; Majsterek, I. Unfolded protein response and PERK Kinase as a new therapeutic target in the pathogenesis of Alzheimer’s disease. Curr. Med. Chem. 2015, 22, 3169–3184. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, W.; Yin, J.; Chen, Y.; Guo, S.; Fan, H.; Li, X.; Zhang, X.; He, X.; Duan, C. TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J. Neuroinflamm. 2018, 15, 231. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, Y.; Kurihara, H.; Morita, H.; Cao, W.H.; Ling, G.Y.; Kumada, M.; Kimura, S.; Nagai, R.; Yazaki, Y.; Kuwaki, T. Role of endothelin-1 in stress response in the central nervous system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R515–R521. [Google Scholar] [CrossRef] [PubMed]
- Pantazatos, S.P.; Huang, Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Mann, J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: Evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatry 2017, 22, 760–773. [Google Scholar] [CrossRef]
- Lari, A.; Gholami Pourbadie, H.; Sharifi-Zarchi, A.; Aslani, S.; Nejatbakhsh Samimi, L.; Jamshidi, A.; Mahmoudi, M. Evaluation of the ankylosing spondylitis transcriptome for oxidative phosphorylation pathway: The shared pathway with neurodegenerative diseases. Iran. J. Allergy Asthma Immunol. 2021, 20, 563–573. [Google Scholar] [CrossRef]
- Beroun, A.; Mitra, S.; Michaluk, P.; Pijet, B.; Stefaniuk, M.; Kaczmarek, L. MMPs in learning and memory and neuropsychiatric disorders. Cell. Mol. Life Sci. 2019, 76, 3207–3228. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef]
- Coba de la Peña, T.; Redondo, F.J.; Fillat, M.F.; Lucas, M.M.; Pueyo, J.J. Flavodoxin overexpression confers tolerance to oxidative stress in beneficial soil bacteria and improves survival in the presence of the herbicides paraquat and atrazine. J. Appl. Microbiol. 2013, 115, 236–246. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Sergi, D.; Lane, M.M.; Naumovski, N.; Gamage, E.; Rajendran, A.; Kouvari, M.; Gauci, S.; Dissanayka, T.; Marx, W.; et al. The effects of dietary advanced glycation End-products on neurocognitive and mental disorders. Nutrients 2022, 14, 2421. [Google Scholar] [CrossRef] [PubMed]






| Accession of Uniprot | Compounds | Relative Content (%) |
|---|---|---|
| A0A241RQC3 | DUF488 domain-containing protein | 27.441 |
| A0A3M6KN02 | BspA family leucine-rich repeat surface protein | 25.096 |
| A0A241RM09 | 30S ribosomal protein S5 | 9.968 |
| A0A3M6LAF7 | Polysaccharide biosynthesis protein | 7.482 |
| A0A3M6LTI6 | Phage terminase small subunit P27 family | 6.731 |
| A0A3M6KR91 | UDP-N-acetylmuramoylalanine-d-glutamate ligase | 5.230 |
| A0A241RT34 | Zinc metalloprotease | 4.151 |
| A0A3M6L0D5 | CDP-glycerol-glycerophosphate glycerophosphotransferase | 2.603 |
| A0A3M6LA56 | Glycosyltransferase family 1 protein | 2.580 |
| A0A2K9I2H2 | Metal ABC transporter substrate-binding protein | 2.087 |
| A0A2S9W1Z2 | Lipid II isoglutaminyl synthase (glutamine-hydrolyzing) subunit MurT | 1.848 |
| A0A3M6KKY7 | Cell surface protein | 1.281 |
| A0A3M6L8T2 | ABC transporter ATP-binding protein | 0.626 |
| A0A2S9VI33 | Fumarate hydratase class II | 0.575 |
| A0A3M6L2B3 | Uncharacterized protein | 0.558 |
| A0A3M6L2S8 | Uncharacterized protein | 0.556 |
| A0A3M6LRB4 | Non-canonical purine NTP pyrophosphatase | 0.227 |
| A0A3M6LSL4 | UvrABC system protein A | 0.217 |
| A0A3M6KNV5 | Orotate phosphoribosyltransferase | 0.150 |
| A0A3M6LLL3 | Transporter | 0.131 |
| A0A3M6LM94 | ABC transporter ATP-binding protein | 0.126 |
| A0A3M6KWU4 | Phage tail tape measure protein | 0.078 |
| A0A2K9HYL8 | DNA polymerase | 0.077 |
| A0A3M6LFN8 | Glycosyl hydrolase family protein | 0.058 |
| A0A3M6L3I3 | DUF2201 domain-containing protein | 0.045 |
| A0A3M6LN80 | Phosphoenolpyruvate carboxykinase (ATP) | 0.039 |
| A0A387EW54 | DUF2075 domain-containing protein | 0.024 |
| A0A3M6LKY6 | Uncharacterized protein | 0.009 |
| A0A2S9VNG1 | Flavodoxin | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Song, J.; Zhang, Y.; Huang, Y.; Zhang, F.; Suo, H. Promoting Effect and Potential Mechanism of Lactobacillus pentosus LPQ1-Produced Active Compounds on the Secretion of 5-Hydroxytryptophan. Foods 2022, 11, 3895. https://doi.org/10.3390/foods11233895
Zeng Y, Song J, Zhang Y, Huang Y, Zhang F, Suo H. Promoting Effect and Potential Mechanism of Lactobacillus pentosus LPQ1-Produced Active Compounds on the Secretion of 5-Hydroxytryptophan. Foods. 2022; 11(23):3895. https://doi.org/10.3390/foods11233895
Chicago/Turabian StyleZeng, Yixiu, Jiajia Song, Yuhong Zhang, Yechuan Huang, Feng Zhang, and Huayi Suo. 2022. "Promoting Effect and Potential Mechanism of Lactobacillus pentosus LPQ1-Produced Active Compounds on the Secretion of 5-Hydroxytryptophan" Foods 11, no. 23: 3895. https://doi.org/10.3390/foods11233895
APA StyleZeng, Y., Song, J., Zhang, Y., Huang, Y., Zhang, F., & Suo, H. (2022). Promoting Effect and Potential Mechanism of Lactobacillus pentosus LPQ1-Produced Active Compounds on the Secretion of 5-Hydroxytryptophan. Foods, 11(23), 3895. https://doi.org/10.3390/foods11233895