Characterisation of a High Fibre Flour Prepared from Soy Milk By-Product and Its Potential Use in White Wheat Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. SMB Sample Preparation
2.2.2. Proximate Composition Analysis of SMB
2.2.3. Determination of Phytochemicals
Extraction of Phytochemicals from SMB100
Total Phenolic Content (TPC)
Total Saponins (TS)
HPLC for Genistin, Diadzin, Daidzein and Genistein
2.2.4. Physical Properties of SMB100 Flour
2.2.5. Bread Dough Formulation and Baking
2.2.6. Volume, Relative Density, and Texture Profile Analysis (TPA) of SMB Bread
2.2.7. Colour of the Crust and Crumb
2.3. Statistical Analysis
3. Results and Discussion
3.1. Quality of SMB100 Flour
3.1.1. Proximate Composition of SMB100 Flour
3.1.2. Physical Properties of SMB100 Flour
3.2. Substitution of Wheat Flour with SMB100 in Production of White Wheat Bread
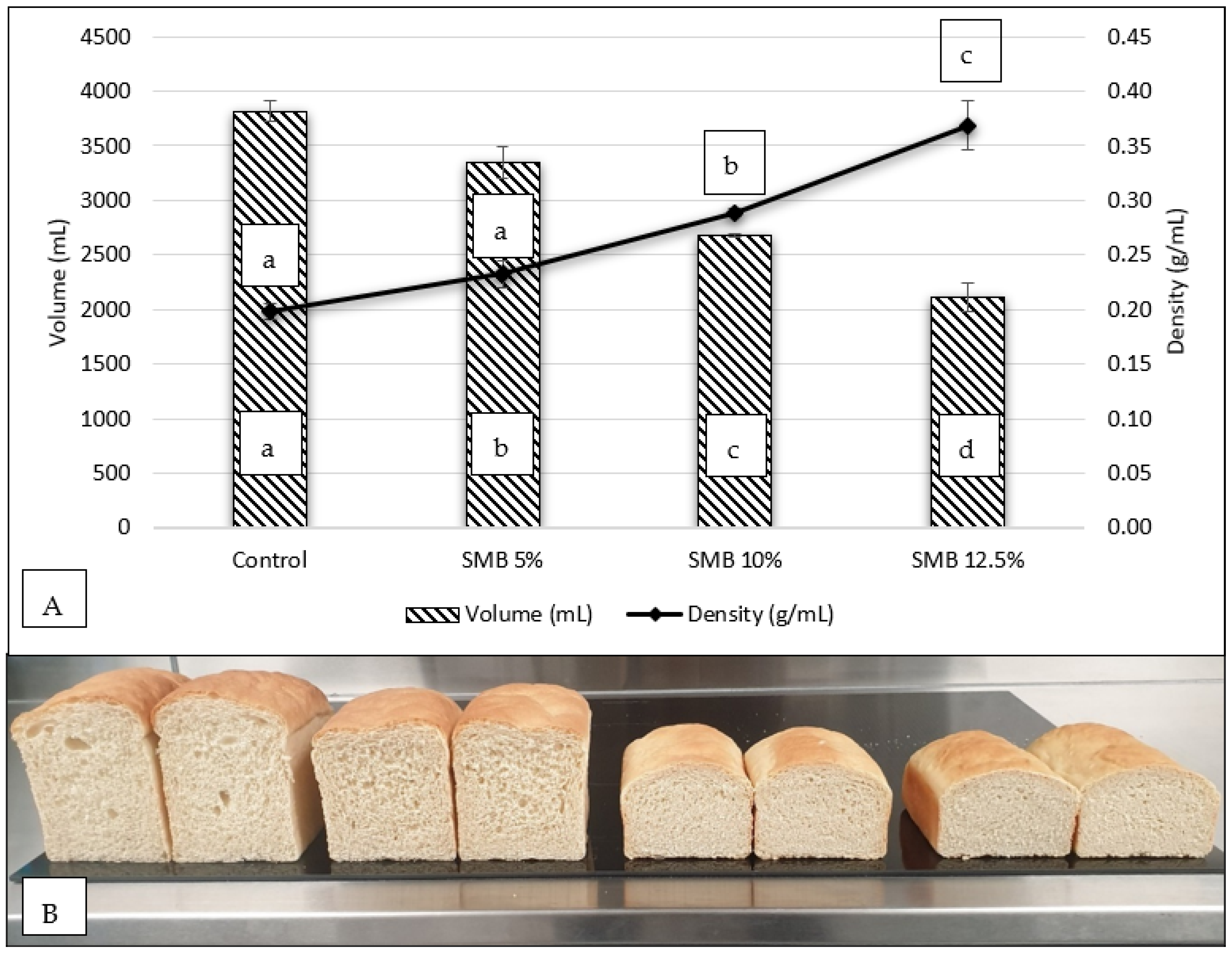
3.2.1. Impact of SMB100 Flour Substitution on Loaf Mass, Volume, Relative Density and of White Bread
3.2.2. Impact of SMB100 Flour Substitution on Colour of Crust and Crumb of White Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, N.; Hussain, S.; Raza, M.A.; Yang, C.; Safdar, M.E.; Brestic, M.; Aziz, A.; Hayyat, M.S.; Asghar, M.A.; Yang, W. Drought tolerance of soybean (Glycine max L. Merr.) by improved photosynthetic characteristics and an efficient antioxidant enzyme system under a split-root system. Front. Physiol. 2019, 10, 786. [Google Scholar] [CrossRef] [PubMed]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- O’Toole, D.K. Characteristics and use of okara, the soybean residue from soy milk production a review. J. Agric. Food Chem. 1999, 47, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Radočaj, O.; Dimić, E. Valorization of Wet Okara, a Value-Added Functional Ingredient, in a Coconut-Based Baked Snack. Cereal Chem. 2013, 90, 256–262. [Google Scholar] [CrossRef]
- Van der Riet, W.; Wight, A.; Cilliers, J.; Datel, J. Food chemical investigation of tofu and its byproduct okara. Food Chem. 1989, 34, 193–202. [Google Scholar] [CrossRef]
- Voss, G.B.; Rodríguez-Alcalá, L.M.; Valente, L.M.P.; Pintado, M.M. Impact of different thermal treatments and storage conditions on the stability of soybean byproduct (okara). J. Food Meas. Charact. 2018, 12, 1981–1996. [Google Scholar] [CrossRef]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Liu, Y.; Li, B. Okara dietary fiber and hypoglycemic effect of okara foods. Bioact. Carbohydr. Diet. Fibre 2013, 2, 126–132. [Google Scholar] [CrossRef]
- Davy, P.; Vuong, Q.V. The fate of phenolics, soysaponins, major isoflavones and antioxidant activity in soy milk by-product during conventional drying process. Future Foods 2021, 4, 100084. [Google Scholar] [CrossRef]
- Evžen, Š.; Miroslava, K.; Iva, W.; Pavel, H.; Petra, S.; Václav, D.; David, C. Two resistant starches applied in bread. Czech J. Food Sci. 2017, 35, 67–72. [Google Scholar] [CrossRef]
- Davy, P.; Vuong, Q.V. Soy Milk By-product: Its Composition and Utilisation. Food Rev. Int. 2020, 1–23. [Google Scholar] [CrossRef]
- Lu, F.; Li, B.; Zhang, Y.; Zhang, Z. Application of bean curd residue in bread. Sci. Technol. Food Ind. 2011, 4. [Google Scholar]
- Yang, L.; Bo, L.; Fei, L.; Mingshan, S. Effect of okara powder on textural properties of bread dough. J. Henan Inst. Sci. Technol. 2012, 3, 015. [Google Scholar]
- Rinaldi, V.; Ng, P.; Bennink, M. Effects of extrusion on dietary fiber and isoflavone contents of wheat extrudates enriched with wet okara. Cereal Chem. 2000, 77, 237–240. [Google Scholar] [CrossRef]
- Wickramarathna, G.; Arampath, P. Utilization of okara in bread making. J. Biosci. 2003, 31, 29–33. [Google Scholar]
- Silva, L.H.D.; Paucar-Menacho, L.M.; Vicente, C.A.; Salles, A.S.; Steel, C.J.; Chang, Y. Development of loaf bread with the addition of “okara” flour. Braz. J. Food Technol. 2009, 12, 315–322. [Google Scholar] [CrossRef]
- Guimarães, R.M.; Silva, T.E.; Lemes, A.C.; Boldrin, M.C.F.; da Silva, M.A.P.; Silva, F.G.; Egea, M.B. Okara: A soybean by-product as an alternative to enrich vegetable paste. LWT 2018, 92, 593–599. [Google Scholar] [CrossRef]
- Ikanone, C.E.O.; Oyekan, P.O. Effect of Boiling and Frying on the Total Carbohydrate, Vitamin C and Mineral Contents of Irish (Solanun tuberosum) and Sweet (Ipomea batatas) Potato Tubers. Niger. Food J. 2014, 32, 33–39. [Google Scholar] [CrossRef]
- Randall, E. Improved method for fat and oil analysis by a new process of extraction. J. Assoc. Off. Anal. Chem. 1974, 57, 1165–1168. [Google Scholar] [CrossRef]
- Thiex, N.J.; Anderson, S.; Gildemeister, B. Crude fat, hexanes extraction, in feed, cereal grain, and forage (Randall/soxtec/submersion method): Collaborative study. J. AOAC Int. 2003, 86, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Methods of Analysis; AOAC: Arlington, VA, USA, 1989; Volume 14. [Google Scholar]
- Jung, S.; Rickert, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of kjeldahl and dumas methods for determining protein contents of soybean products. J. Am. Oil Chem. Soc. 2003, 80, 1169. [Google Scholar] [CrossRef]
- Mosse, J. Nitrogen-to-protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed protein content. J. Agric. Food Chem. 1990, 38, 18–24. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; Villanueva-Suárez, M.J.; Mateos-Aparicio, I. Soybean seeds and its by-product okara as sources of dietary fibre. Measurement by AOAC and Englyst methods. Food Chem. 2008, 108, 1099–1105. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Analysis. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 147–177. [Google Scholar]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfur1c acid. Planta Med. 1976, 29, 116–122. [Google Scholar] [CrossRef]
- Wang, H.; Murphy, P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Montero, G.; Günther, G.; Valdés, K.; Arriagada, F.; Morales, J. An HPLC Method for the Determination of Isoflavones and the Evaluation of Their Antioxidant Capacity in Both Homogeneous and Microheterogeneous Systems. J. AOAC Int. 2018, 101, 235–241. [Google Scholar] [CrossRef]
- Barretto, R.; Buenavista, R.M.; Pandiselvam, R.; Siliveru, K. Influence of milling methods on the flow properties of ivory teff flour. J. Texture Stud. 2021. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Encapsulation of phenolic-rich extract from banana (Musa cavendish) peel. J. Food Sci. Technol. 2020, 57, 2089–2098. [Google Scholar] [CrossRef]
- Ostermann-Porcel, M.V.; Rinaldoni, A.N.; Rodriguez-Furlán, L.T.; Campderrós, M.E. Quality assessment of dried okara as a source of production of gluten-free flour. J. Sci. Food Agric. 2017, 97, 2934–2941. [Google Scholar] [CrossRef]
- Markovic, I.; Ilic, J.; Markovic, D.; Simonovic, V.; Kosanic, N. Color measurement of food products using CIE L * a * b * and RGB color space. J. Hyg. Eng. Des 2013, 4, 50–53. [Google Scholar]
- Allen, H.; Pleming, D.; Pumpa, J. The development of a rapid dough bread baking method using a doughlab. In Proceedings of the 55th Australian Cereal Chemistry Conference and AACC International Pacific Rim Symposium, Sydney, NSW, Australia, 3–7 July 2005; p. 293. [Google Scholar]
- AACC. Method 10-05.01 Guidelines for Measurement of Volume by Rapeseed Displacement; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Zambelli, R.A.; Galvao, A.M.T.; Pinto, L.I.F.; Santos, G.B.M.; da Silva, A.C.; Melo, C.A.L.; Farias, M.M.; de Mendonca, L.G. Effect of Vegetable Powders on the Bread Quality Made from Frozen Dough. Int. J. Nutr. Food Sci. 2017, 6, 1–8. [Google Scholar]
- Zielinska, M.; Markowski, M. Color characteristics of carrots: Effect of drying and rehydration. Int. J. Food Prop. 2012, 15, 450–466. [Google Scholar] [CrossRef]
- Farzana, T.; Mohajan, S. Effect of incorporation of soy flour to wheat flour on nutritional and sensory quality of biscuits fortified with mushroom. Food Sci. Nutr. 2015, 3, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ocheme, O.B.; Adedeji, O.E.; Chinma, C.E.; Yakubu, C.M.; Ajibo, U.H. Proximate composition, functional, and pasting properties of wheat and groundnut protein concentrate flour blends. Food Sci. Nutr. 2018, 6, 1173–1178. [Google Scholar] [CrossRef]
- Muliterno, M.M.; Rodrigues, D.; de Lima, F.S.; Ida, E.I.; Kurozawa, L.E. Conversion/degradation of isoflavones and color alterations during the drying of okara. LWT-Food Sci. Technol. 2017, 75, 512–519. [Google Scholar] [CrossRef]
- Méndez Sevillano, D.; Jankowiak, L.; van Gaalen, T.L.T.; van der Wielen, L.A.M.; Hooshyar, N.; van der Goot, A.-J.; Ottens, M. Mechanism of Isoflavone Adsorption from Okara Extracts onto Food-Grade Resins. Ind. Eng. Chem. Res. 2014, 53, 15245–15252. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Khan, M.A.; El-Harty, E.H.; Ammar, M.H.; Farooq, M.; Migdadi, H.M. Comparative phytochemical profiling of different soybean (Glycine max (L.) Merr) genotypes using GC–MS. Saudi J. Biol. Sci 2018, 25, 15–21. [Google Scholar] [CrossRef]
- Kang, J.; Badger, T.M.; Ronis, M.J.J.; Wu, X. Non-isoflavone Phytochemicals in Soy and Their Health Effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef]
- Davy, P.; Vuong, Q.V. Soybean Processing By-Products and Potential Health Benefits. In Phytochemicals in Soybeans; CRC Press: Boca Raton, FL, USA, 2022; pp. 333–358. [Google Scholar]
- Jankowiak, L.; Kantzas, N.; Boom, R.; van der Goot, A.J. Isoflavone extraction from okara using water as extractant. Food Chem. 2014, 160, 371–378. [Google Scholar] [CrossRef]
- Rochfort, S.; Panozzo, J. Phytochemicals for Health, the Role of Pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Swallah, M.S.; Li, J.; Yu, H. Value-Added Processing and Function of Okara. In Phytochemicals in Soybeans; CRC Press: Boca Raton, FL, USA, 2022; pp. 419–436. [Google Scholar]
- Carter, B.; Galloway, M.; Morris, C.; Weaver, G.; Carter, A. The case for water activity as a specification for wheat tempering and flour production. Cereal Foods World 2015, 60, 166–170. [Google Scholar] [CrossRef]
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G.; Islam, M.S.; Goodwin, I. A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards. Horticulturae 2022, 8, 459. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint Who/Fao Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Standard 1.2.7; Nutrition, Health and Related Claims. Australian Government: Canberra, Australian, 2013.
- Feili, R.; Zzaman, W.; Abdullah, W.N.W.; Yang, T.A. Physical and sensory analysis of high fiber bread incorporated with jackfruit rind flour. Food Sci. Technol. 2013, 1, 30–36. [Google Scholar]
- Ibidapo, O.P.; Henshaw, F.O.; Shittu, T.A.; Afolabi, W.A. Quality evaluation of functional bread developed from wheat, malted millet (Pennisetum Glaucum) and ‘Okara’ flour blends. Sci. Afr. 2020, 10, e00622. [Google Scholar] [CrossRef]
- Hung, P.V.; Yamamori, M.; Morita, N. Formation of Enzyme-Resistant Starch in Bread as Affected by High-Amylose Wheat Flour Substitutions. Cereal Chem. J. 2005, 82, 690–694. [Google Scholar] [CrossRef]
- Ostermann-Porcel, M.V.; Campderrós, M.E.; Rinaldoni, A.N. Effect of Okara Flour Addition on the Physical and Sensory Quality of Wheat Bread. MOJ Food Process Technol. 2017, 4, 184–190. [Google Scholar]

| Ingredients (g) | Control | 5% SMB | 10% SMB | 12.5% SMB |
|---|---|---|---|---|
| Flour | 500 | 475 | 450 | 437.5 |
| SMB flour | 0 | 25 | 50 | 62.5 |
| Water | 320 | 320 | 320 | 320 |
| Yeast | 5 | 5 | 5 | 5 |
| Oil | 15 | 15 | 15 | 15 |
| Sugar | 10 | 10 | 10 | 10 |
| Salt | 7.5 | 7.5 | 7.5 | 7.5 |
| Nutritive Components | ||||||
|---|---|---|---|---|---|---|
| Proteins (g/100 g) | Fat (g/100 g) | Carbohydrates (g GE/100 g) | Total Fibre (g/100 g) | Insoluble Fibre (g/100 g) | Soluble Fibre (g/100 g) | Ash (g/100 g) |
| 26.5 ± 0.51 | 13.8 ± 0.93 | 23.7 ± 3.2 | 40.6 ± 0.41 | 38.4 ± 0.95 | 2.2 ± 0.95 | 4.1 ± 0.23 |
| Phytochemicals | ||||||
| TPC (GAE mg/g) | Saponins (ESE mg/100 g) | Daidzin (µg/g) | Genistin (µg/g) | Daidzein (µg/g) | Genistein (µg/g) | |
| 8.23 ± 3.66 | 31.4 ± 2.80 | 286.3 ± 62.36 | 339.0 ± 73.35 | 44.5 ± 11.24 | 28.6 ± 1.75 | |
| Physical properties | ||||||
| Moisture (%) | Water activity | Bulk density level (g/mL) | Tap bulk density (g/mL) | CI (%) | HR | Swelling Capacity (mL/g) |
| 8.67 ± 0.44 | 0.55 ± 0.01 | 0.24 ± 0.003 | 0.44 ± 0.01 | 44.65 ± 0.07 | 1.81 ± 0.002 | 6.11 ± 0.15 |
| Water Holding (mL/g) | Water absorbance (mL/g) | Water solubility (%) | Oil binding (mL/g) | Lightness (L*) | Hue (H°) | Chroma (C) |
| 9.92 ± 1.5 | 7.86 ± 0.36 | 12.7 ± 1.5 | 3.16 ± 0.17 | 89.3 ± 0.17 | 83.3 ± 0.08 | 16.9 ± 0.30 |
| Crust | L* | H° | C | ΔE | ΔC | ΔH° |
| Control | 55.5 ± 3.4 a | 64.9 ± 2.4 a | 29.6 ± 1.6 a | |||
| SMB 5% | 54.7 ± 0.38 a | 64.3 ± 0.88 a | 30.7 ± 1.3 a | 1.62 ± 0.46 a | 1.14 ± 1.3 a | 0.42 ± 0.14 a |
| SMB 10% | 59.0 ± 0.85 ab | 65.9 ± 1.3 a | 31.9 ± 3.1 a | 4.85 ± 1.2 ab | 2.33 ± 3.1 a | 0.70 ± 0.32 ab |
| SMB 12.5% | 63.1 ± 3.6 b | 65.2 ± 3.3 a | 27.3 ± 2.0 a | 8.19 ± 3.8 b | −2.34 ± 2.0 a | 1.34 ± 0.45 b |
| Crumb | L* | H° | C | ΔE | ΔC | ΔH° |
| Control | 65.2 ± 2.8 a | 87.2 ± 0.32 a | 14.5 ± 0.67 a | |||
| SMB 5% | 65.2 ± 0.78 a | 85.0 ± 0.21 b | 17.2 ± 0.41 b | 2.79 ± 0.46 a | 2.62 ± 0.41 a | 0.75 ± 0.07 a |
| SMB 10% | 68.9 ± 1.5 ab | 82.3 ± 0.23 c | 19.7 ± 0.58 c | 6.64 ± 1.2 b | 5.19 ± 0.58 b | 1.56 ± 0.04 b |
| SMB 12.5% | 70.8 ± 0.55 b | 81.3 ± 0.50 d | 18.4± 0.75 bc | 7.11 ± 0.47 b | 3.90 ± 0.75 ab | 1.78 ± 0.18 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davy, P.; Kirkman, T.; Scarlett, C.J.; Vuong, Q. Characterisation of a High Fibre Flour Prepared from Soy Milk By-Product and Its Potential Use in White Wheat Bread. Foods 2022, 11, 3921. https://doi.org/10.3390/foods11233921
Davy P, Kirkman T, Scarlett CJ, Vuong Q. Characterisation of a High Fibre Flour Prepared from Soy Milk By-Product and Its Potential Use in White Wheat Bread. Foods. 2022; 11(23):3921. https://doi.org/10.3390/foods11233921
Chicago/Turabian StyleDavy, Philip, Timothy Kirkman, Christopher J. Scarlett, and Quan Vuong. 2022. "Characterisation of a High Fibre Flour Prepared from Soy Milk By-Product and Its Potential Use in White Wheat Bread" Foods 11, no. 23: 3921. https://doi.org/10.3390/foods11233921
APA StyleDavy, P., Kirkman, T., Scarlett, C. J., & Vuong, Q. (2022). Characterisation of a High Fibre Flour Prepared from Soy Milk By-Product and Its Potential Use in White Wheat Bread. Foods, 11(23), 3921. https://doi.org/10.3390/foods11233921









