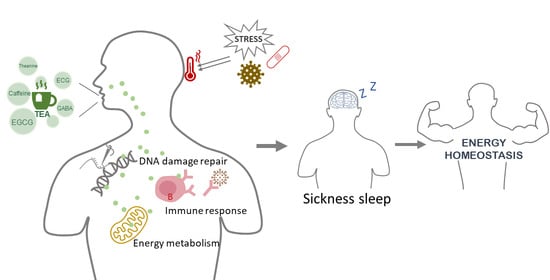
New Perspectives on Sleep Regulation by Tea: Harmonizing Pathological Sleep and Energy Balance under Stress
Abstract
:1. Introduction
2. Tea and Sleep
2.1. Tea
2.2. Sleep
2.3. Sickness Sleep
3. Tea Effects on Sleep
4. Does Tea Affect Sickness Sleep and Maintain Energy Homeostasis?
4.1. Tea Acts on the Brain–Gut Axis to Regulate Sickness Sleep
4.1.1. Tea Acts on the Nervous System to Regulate Sickness Sleep
4.1.2. Tea Regulates Intestinal Flora to Mediate Sickness Sleep
4.2. Tea Acts on Damage Repair to Mediate Sickness Sleep
4.2.1. DNA Damage Repair
4.2.2. Immune Response
4.3. Tea Acts on Energy Metabolism to Mediate Sickness Sleep
4.3.1. Lipid Metabolism
4.3.2. AMPK
4.3.3. IIS Signaling Pathway
4.3.4. mTOR Pathway
5. Conclusions and Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym | Full name |
| UV | Ultraviolet |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin gallate |
| AAI | Acute alcohol intoxication |
| EEG | Electroencephalogram |
| NREMS | Non-rapid eye movement sleep |
| REMS | Rapid eye movement sleep |
| DTS | Developmentally timed sleep |
| SIS | Stress-induced sleep |
| GABA | γ-aminobutyric acid |
| PSQI | Pittsburgh sleep quality index |
| ROS | Reactive oxygen species |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| FP | Flavonifractor plautii |
| SCFA | Short-chain fatty acid |
| AMP | Antimicrobial peptide |
| NLP | Neuropeptide-like protein |
| DDR | DNA damage response |
| MAPK | Mitogen-activated protein kinase |
| TNFα | Tumor necrosis factor-alpha |
| IL-1 | Interleukin-1 |
| AMPK | Adenosine monophosphate-activated protein kinase |
| IIS | Insulin/IGF-1 signaling |
| mTOR | Mammalian/mechanistic target of rapamycin |
| UCP1 | Uncoupling protein 1 |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| SREBP-1c | Sterol regulatory element binding protein-1c |
| FFA | Free fatty acid |
| Lk | Leucokinin |
| SIKs | Salt-inducible kinases |
| PKA | Protein kinase A |
| ACC | Acetyl-CoA carboxylase |
| G6Pase | Glucose 6-phosphatase |
| IGF | Insulin-like growth factor |
| FOXO | Forkhead box O |
| AKH | Adipokinetic hormone |
| CREB | cAMP-response element binding protein |
| TFEB | Transcription factor EB |
| mtROS | Mitochondrial reactive oxygen species |
References
- Bringmann, H. Genetic sleep deprivation: Using sleep mutants to study sleep functions. EMBO Rep. 2019, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, C.; Tononi, G. Is sleep essential? PLoS Biol. 2008, 6, e216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignot, E. Why we sleep: The temporal organization of recovery. PLoS Biol. 2008, 6, e106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.J.; Liu, Q.Q.; Li, Y.J.; Zhao, F.F.; Chang, H.; Lyu, J. Longitudinal study of the relationship between sleep duration and hypertension in Chinese adult residents (CHNS 2004–2011). Sleep Med. 2019, 58, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, N.S.; Rossi, N.; El-Sayed Moustafa, J.; Laverty, A.A.; Quint, J.K.; Freidin, M.; Visconti, A.; Murray, B.; Modat, M.; Ourselin, S.; et al. Multiple, objectively measured sleep dimensions including hypoxic burden and chronic kidney disease: Findings from the Multi-Ethnic Study of Atherosclerosis. Thorax 2021, 76, 714–722. [Google Scholar] [CrossRef]
- Suriagandhi, V.; Nachiappan, V. Protective Effects of Melatonin against Obesity-Induced by Leptin Resistance. Behav. Brain Res. 2022, 417, 9. [Google Scholar] [CrossRef]
- Carpena, M.X.; Matijasevich, A.; Loret de Mola, C.; Santos, I.S.; Munhoz, T.N.; Tovo-Rodrigues, L. The effects of persistent sleep disturbances during early childhood over adolescent ADHD, and the mediating effect of attention-related executive functions: Data from the 2004 Pelotas Birth Cohort. J. Affect. Disord. 2022, 296, 175–182. [Google Scholar] [CrossRef]
- Kang, X.; Jiang, L.; Lan, F.; Tang, Y.-Y.; Zhang, P.; Zou, W.; Chen, Y.-J.; Tang, X.-Q. Hydrogen sulfide antagonizes sleep deprivation-induced depression- and anxiety-like behaviors by inhibiting neuroinflammation in a hippocampal Sirt1-dependent manner. Brain Res. Bull. 2021, 177, 194–202. [Google Scholar] [CrossRef]
- Lau, E.Y.Y.; Wong, M.L.; Lam, Y.C.; Lau, K.N.T.; Chung, K.F.; Rusak, B. Sleep and Inhibitory Control Over Mood-Congruent Information in Emerging Adults With Depressive Disorder. Psychosom. Med. 2021, 83, 1004–1012. [Google Scholar] [CrossRef]
- Ma, T.; Wang, Y.-Y.; Lu, Y.; Feng, L.; Yang, Y.-T.; Li, G.-H.; Li, C.; Chu, Y.; Wang, W.; Zhang, H. Inhibition of Piezo1/Ca2+/calpain signaling in the rat basal forebrain reverses sleep deprivation-induced fear memory impairments. Behav. Brain Res. 2022, 417. [Google Scholar] [CrossRef]
- Davis, K.C.; Raizen, D.M. A mechanism for sickness sleep: Lessons from invertebrates. J. Physiol. 2017, 595, 5415–5424. [Google Scholar] [CrossRef] [Green Version]
- Konietzka, J.; Fritz, M.; Spiri, S.; McWhirter, R.; Leha, A.; Palumbos, S.; Costa, W.S.; Oranth, A.; Gottschalk, A.; Miller, D.M., III; et al. Epidermal Growth Factor Signaling Promotes Sleep through a Combined Series and Parallel Neural Circuit. Curr. Biol. 2020, 30, 1–16.e13. [Google Scholar] [CrossRef] [Green Version]
- Hill, V.M.; O’Connor, R.M.; Sissoko, G.B.; Irobunda, I.S.; Leong, S.; Canman, J.C.; Stavropoulos, N.; Shirasu-Hiza, M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [Google Scholar] [CrossRef]
- Tanaka, S.; Toyoda, H.; Honda, Y.; Seki, Y.; Sakurai, T.; Honda, K.; Kodama, T. Hypocretin/orexin prevents recovery from sickness. Biomed. Rep. 2015, 3, 648–650. [Google Scholar] [CrossRef] [Green Version]
- Sonnenschein, M.; Sorbi, M.J.; Verbraak, M.; Schaufeli, W.B.; Maas, C.J.M.; van Doomen, L.J.P. Influence of sleep on symptom improvement and return to work in clinical burnout. Scand. J. Work Environ. Health 2008, 34, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.H. The energy allocation function of sleep: A unifying theory of sleep, torpor, and continuous wakefulness. Neurosci. Biobehav. Rev. 2014, 47, 122–153. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.-J.; Tsai, S.-J.; Kuo, P.-H.; Lin, W.-Y.; Liu, Y.-L.; Yang, A.C.; Lin, E.; Lan, T.-H. An association study in the Taiwan Biobank elicits the GABAA receptor genes GABRB3, GABRA5, and GABRG3 as candidate loci for sleep duration in the Taiwanese population. BMC Med. Genom. 2021, 14, 223. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Jachimowicz, K. Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea-The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes. Nutrients 2021, 13, 3972. [Google Scholar] [CrossRef]
- Kehong, L.; Enshuo, L.; Ling, L.; Yuan, H.; Yong, Y.; Wenjun, X. L-Theanine mediates the p38MAPK signaling pathway to alleviate heat-induced oxidative stress and inflammation in mice. Food Funct. 2022, 13, 2120–2130. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.; Chen, R.; Wen, S.; Li, Q.; Lai, X.; Zhang, Z.; Cao, F.; Sun, S. Chinese Tea Alleviates CCl4-Induced Liver Injury through the NF-kappaBorNrf2Signaling Pathway in C57BL-6J Mice. Nutrients 2022, 14, 972. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, H.; Ling, C.; Bi-Feng, Z.; Zhao-Dong, H.; Shibata, H.; Kiso, Y.; Tanaka, T.; Xin-Sheng, Y. Anti-stress effect of oolong tea in women loaded with vigil. J. Health Sci. 2003, 49, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Mahdavi, S.; Mansourian, M.; Shams, E.; Qorbani, M.; Heshmat, R.; Motlagh, M.E.; Ziaodini, H.; Dashti, R.; Taheri, M.; Kelishadi, R. Association of Sunlight Exposure with Sleep Hours in Iranian Children and Adolescents: The CASPIAN-V Study. J. Trop. Pediatr. 2020, 66, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.C.; Corcoran, K.; Perng, W.; Dunietz, G.L.; Cantoral, A.; Zhou, L.; Tellez-Rojo, M.M.; Peterson, K.E. Relationships of beverage consumption and actigraphy-assessed sleep parameters among urban-dwelling youth from Mexico. Public Health Nutr. 2021, 25, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Manzar, M.D.; Alghadir, A.H.; Khan, M.; Salahuddin, M.; Albougami, A.; Maniago, J.D.; Vasquez, B.A.; Pandi-Perumal, S.R.; Bahammam, A.S. Anxiety Symptoms Are Associated With Higher Psychological Stress, Poor Sleep, and Inadequate Sleep Hygiene in Collegiate Young Adults-A Cross-Sectional Study. Front. Psychiatry 2021, 12, 677136. [Google Scholar] [CrossRef]
- Althakafi, K.A.; Alrashed, A.A.; Aljammaz, K.I.; Abdulwahab, I.J.; Hamza, R.; Hamad, A.F.; Alhejaili, K.S. Prevalence of short sleep duration and effect of co-morbid medical conditions—A cross-sectional study in Saudi Arabia. J. Fam. Med. Prim. Care 2019, 8, 3334–3339. [Google Scholar] [CrossRef]
- Hou, S.J.; Tsai, S.J.; Kuo, P.H.; Liu, Y.L.; Yang, A.C.; Lin, E.; Lan, T.H. An association study in the Taiwan Biobank reveals RORA as a novel locus for sleep duration in the Taiwanese Population. Sleep Med. 2020, 73, 70–75. [Google Scholar] [CrossRef]
- Lai, X.F.; Wang, X.R.; Wen, S.; Sun, L.L.; Chen, R.H.; Zhang, Z.B.; Li, Q.H.; Cao, J.X.; Lai, Z.X.; Li, Z.G.; et al. Six Types of Tea Reduce Acute Alcoholism in Mice by Enhancing Ethanol Metabolism, Suppressing Oxidative Stress and Inflammation. Front. Nutr. 2022, 9, 848918. [Google Scholar] [CrossRef]
- Tung, Y.C.; Liang, Z.R.; Yang, M.J.; Ho, C.T.; Pan, M.H. Oolong tea extract alleviates weight gain in high-fat diet-induced obese rats by regulating lipid metabolism and modulating gut microbiota. Food Funct. 2022, 13, 2846–2856. [Google Scholar] [CrossRef]
- Ladeira, L.C.M.; dos Santos, E.C.; Santos, T.A.; da Silva, J.; Lima, G.D.D.; Machado-Neves, M.; da Silva, R.C.; Freitas, M.B.; Maldonado, I. Green tea infusion prevents diabetic nephropathy aggravation in recent-onset type 1 diabetes regardless of glycemic control. J. Ethnopharmacol. 2021, 274, 114032. [Google Scholar] [CrossRef]
- Mo, T.; Zhang, W.; Li, P. The change and regulation mechanism of key components during tea processing. J. Chin. Inst. Food Sci. Technol. 2011, 11, 176–180. [Google Scholar]
- Sanlier, N.; Gokcen, B.B.; Altug, M. Tea consumption and disease correlations. Trends Food Sci. Technol. 2018, 78, 95–106. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods 2022, 11, 620. [Google Scholar] [CrossRef]
- Kan, Z.P.; Wang, Y.J.; Chen, Q.; Tang, X.Y.; Thompson, H.J.; Huang, J.B.; Zhang, J.S.; Gao, F.; Shen, Y.; Wan, X.C. Green Tea Suppresses Amyloid beta Levels and Alleviates Cognitive Impairment by Inhibiting APP Cleavage and Preventing Neurotoxicity in 5XFAD Mice. Mol. Nutr. Food Res. 2021, 65, 2100626. [Google Scholar] [CrossRef]
- Perdices, L.; Fuentes-Broto, L.; Segura, F.; Cavero, A.; Orduna-Hospital, E.; Insa-Sanchez, G.; Sanchez-Cano, A.I.; Fernandez-Sanchez, L.; Cuenca, N.; Pinilla, I. Systemic epigallocatechin gallate protects against retinal degeneration and hepatic oxidative stress in the P23H-1 rat. Neural Regen. Res. 2022, 17, 625–631. [Google Scholar] [CrossRef]
- Li, M.Z.; Duan, Y.J.; Wang, Y.; Chen, L.; Abdelrahim, M.E.A.; Yan, J. The effect of Green green tea consumption on body mass index, lipoprotein, liver enzymes, and liver cancer: An updated systemic review incorporating a meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 1–9. [Google Scholar] [CrossRef]
- Colonetti, L.; Grande, A.J.; Toreti, I.R.; Ceretta, L.B.; da Rosa, M.I.; Colonetti, T. Green tea promotes weight loss in women with polycystic ovary syndrome: Systematic review and meta-analysis. Nutr. Res. 2022, 104, 1–9. [Google Scholar] [CrossRef]
- Kwak, J.; Shin, D. Association between Green Tea Consumption and Abdominal Obesity Risk in Middle-Aged Korean Population: Findings from the Korean Genome and Epidemiology Study. Int. J. Environ. Res. Public Health 2022, 19, 2735. [Google Scholar] [CrossRef]
- Bedran, T.B.L.; Morin, M.P.; Spolidorio, D.P.; Grenier, D. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and beta-defensin secretion in oral epithelial cells. PLoS ONE 2015, 10, e0143158. [Google Scholar] [CrossRef]
- Korystova, A.F.; Kublik, L.N.; Samokhvalova, T.V.; Shaposhnikova, V.V.; Korystov, Y.N. Black tea is more effective than green tea in prevention of radiation-induced oxidative stress in the aorta of rats. Biomed. Pharmacother. 2021, 142, 112064. [Google Scholar] [CrossRef] [PubMed]
- Yusufoglu, B.; Karakus, E.; Yaman, M. Determining the amount and bioaccessibility of methylglyoxal and glyoxal in functional snack foods with herbal teas: Effect of different herbal teas on alpha-Dicarbonyls. Food Sci. Technol. 2022, 42, e82621. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Qian, Y.; Wang, R. In vitro antioxidative activity of yellow tea and its in vivo preventive effect on gastric injury. Exp. Ther. Med. 2013, 6, 423–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, T.; Daxiang, L.; Ponmari, G.; Na, X.; Zhongwen, X. Dietary supplement of large yellow tea ameliorates metabolic syndrome and attenuates hepatic steatosis in db/db Mice. Nutrients 2018, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Shaoxiong, Z.; Chatrawee, D.; Tewin, T.; Jianghong, L.; Jinke, L.; Wink, M. Neuroprotective effects of oolong tea extracts against glutamate-induced toxicity in cultured neuronal cells and beta-amyloid-induced toxicity in Caenorhabditis elegans. Food Funct. 2020, 11, 8179–8192. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, X.F.; Lai, C.C.; Gao, H.Y.; Zheng, Y.F.; Huang, J.Q.; Lin, B. Selenium enrichment improves anti-proliferative effect of oolong tea extract on human hepatoma HuH-7 cells. Food Chem. Toxicol. 2021, 147, 111873. [Google Scholar] [CrossRef]
- Asokan Shibu, M.; Chia-Hua, K.; Bih-Cheng, C.; Da-Tong, J.; Ray-Jade, C.; Chao-Hung, L.; Pei-Jane, H.; Padma Viswanadha, V.; Wei-Wen, K.; Chih-Yang, H. Oolong tea prevents cardiomyocyte loss against hypoxia by attenuating p-JNK mediated hypertrophy and enhancing P-IGF1R, p-akt, and p-Badser136 activity and by fortifying NRF2 antioxidation system. Environ. Toxicol. 2018, 33, 220–233. [Google Scholar] [CrossRef]
- Duangjan, C.; Curran, S.P. Oolonghomobisflavans from Camellia sinensis increase Caenorhabditis elegans lifespan and healthspan. Geroscience 2022, 44, 533–545. [Google Scholar] [CrossRef]
- Santana-Rios, G.; Orner, G.A.; Xu, M.; Izquierdo-Pulido, M.; Dashwood, R.H. Inhibition by white tea of 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine-induced colonic aberrant crypts in the F344 rat. Nutr. Cancer 2001, 41, 98–103. [Google Scholar] [CrossRef] [Green Version]
- He, M.R.; Lyu, X.H. Application of BRAFO-tiered approach for health benefit-risk assessment of dark tea consumption in China. Food Chem. Toxicol. 2021, 158, 112615. [Google Scholar] [CrossRef]
- Siqiang, W.; Zhuoting, G.; Beichao, W.; Na, Z.; Kun, L.; Ting, Y. Effect of brewing conditions on polyphenols in the dark tea (Camellia sinensis L.) infusions: Content, composition and antioxidant activities. Food Sci. Technol. Cienc. Tecnol. Aliment. 2022, 42, e36322. [Google Scholar] [CrossRef]
- Wang, C.Q.; Hu, M.H.; Yi, Y.H.; Wen, X.N.; Lv, C.H.; Shi, M.; Zeng, C.X. Multiomic analysis of dark tea extract on glycolipid metabolic disorders in db/db mice. Front. Nutr. 2022, 9, 1006517. [Google Scholar] [CrossRef]
- Liming, W.; Ting, L. Effect of Eurotium cristatum fermented dark tea extract on body weight and blood lipid in rats. Curr. Dev. Nutr. 2018, 2, A77. [Google Scholar] [CrossRef] [Green Version]
- Campbell, S.S.; Tobler, I. Animal sleep: A review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 1984, 8, 269–300. [Google Scholar] [CrossRef]
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef] [Green Version]
- Bringmann, H. Sleep-Active Neurons: Conserved Motors of Sleep. Genetics 2018, 208, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Vorster, A.P.; Born, J. Sleep and memory in mammals, birds and invertebrates. Neurosci. Biobehav. Rev. 2015, 50, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Orem, J.; Lovering, A.T.; Dunin-Barkowski, W.; Vidruk, E.H. Endogenous excitatory drive to the respiratory system in rapid eye movement sleep in cats. J. Physiol. 2000, 527 Pt 2, 365–376. [Google Scholar] [CrossRef]
- Peever, J.; Fuller, P.M. The Biology of REM Sleep. Curr. Biol. 2017, 27, R1237–R1248. [Google Scholar] [CrossRef]
- Trojanowski, N.F.; Nelson, M.D.; Flavell, S.W.; Fang-Yen, C.; Raizen, D.M. Distinct Mechanisms Underlie Quiescence during Two Caenorhabditis elegans Sleep-Like States. J. Neurosci. 2015, 35, 14571–14584. [Google Scholar] [CrossRef] [Green Version]
- Arnason, B.B.; Thornorsteinsson, H.; Karlsson, K.A.E. Absence of rapid eye movements during sleep in adult zebrafish. Behav. Brain Res. 2015, 291, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, T.; Marin, W.; Faraco, J.; Pezeron, G.; Appelbaum, L.; Zhang, J.; Rosa, F.; Mourrain, P.; Mignot, E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007, 5, e277. [Google Scholar] [CrossRef] [PubMed]
- Rihel, J.; Prober, D.A.; Schier, A.F. Monitoring sleep and arousal in zebrafish. Methods Cell Biol. 2010, 100, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Nitz, D.A.; van Swinderen, B.; Tononi, G.; Greenspan, R.J. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr. Biol. 2002, 12, 1934–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, P.J.; Cirelli, C.; Greenspan, R.J.; Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 2000, 287, 1834–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, N.F.; Dikmen, S.; Machamer, J.; Doherty, M.; Temkin, N. Hypersomnia Following Traumatic Brain Injury. J. Clin. Sleep Med. 2007, 3, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Karshikoff, B.; Sundelin, T.; Lasselin, J. Role of inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front. Immunol. 2017, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Nagai, M.; Nagai, H.; Numa, C.; Furuyashiki, T. Stress-induced sleep-like inactivity modulates stress susceptibility in mice. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Olivadoti, M.D.; Weinberg, J.B.; Toth, L.A.; Opp, M.R. Sleep and fatigue in mice infected with murine gammaherpesvirus 68. Brain Behav. Immun. 2011, 25, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Lenz, O.; Xiong, J.; Nelson, M.D.; Raizen, D.M.; Williams, J.A. FMRFamide signaling promotes stress-induced sleep in Drosophila. Brain Behav. Immun. 2015, 47, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Zhang, H.Y.; Zhang, Z.Y.; Sun, B.Q.; Tang, C.; Zhang, L.; Jiang, Z.H.; Ding, B.; Liao, Y.Y.; Cai, P. Simulated mobile communication frequencies (3.5 GHz) emitted by a signal generator affects the sleep of Drosophila melanogaster. Environ. Pollut. 2021, 283, 117087. [Google Scholar] [CrossRef]
- Goetting, D.L.; Mansfield, R.; Soto, R.; Van Buskirk, C. Cellular damage, including wounding, drives C. elegans stress-induced sleep. J. Neurogenet. 2020, 34, 430–439. [Google Scholar] [CrossRef]
- Hill, A.J.; Mansfield, R.; Lopez, J.M.N.G.; Raizen, D.M.; Van Buskirk, C. Cellular Stress Induces a Protective Sleep-like State in C. elegans. Curr. Biol. 2014, 24, 2399–2405. [Google Scholar] [CrossRef] [Green Version]
- Freiberg, A.S. Why We Sleep: A Hypothesis for an Ultimate or Evolutionary Origin for Sleep and Other Physiological Rhythms. J. Circadian Rhythm. 2020, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 2021, CD005004. [Google Scholar] [CrossRef] [Green Version]
- Kleiser, C.; Wawro, N.; Stelmach-Mardas, M.; Boeing, H.; Gedrich, K.; Himmerich, H.; Linseisen, J. Are sleep duration, midpoint of sleep and sleep quality associated with dietary intake among Bavarian adults? Eur. J. Clin. Nutr. 2017, 71, 631–637. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, J.; Shen, H.; Ai, L.; Wu, R. A randomized controlled pilot study of the effectiveness of magnolia tea on alleviating depression in postnatal women. Food Sci. Nutr. 2020, 8, 1554–1561. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.F.; Li, Y.; Ma, W.; Ge, Y.Z.; Huang, Y.H. A study on quality components and sleep-promoting effects of GABA black tea. Food Funct. 2015, 6, 3393–3398. [Google Scholar] [CrossRef]
- Kolar, M.H.; Plajnsek, K.T.; Dimitrijevic, D.; Gay, T.; Cohen, O.O. Mixture for Reducing Body Mass. WO 2015/099616 A1, 21 August 2015. [Google Scholar]
- Sun, J. Morning/evening menopausal formula relieves menopausal symptoms: A pilot study. J. Altern. Complement. Med. 2003, 9, 403–409. [Google Scholar] [CrossRef]
- Hossain, S.J.; Aoshima, H.; Koda, H.; Kiso, Y. Fragrances in oolong tea that enhance the response of GABAA receptors. Biosci. Biotechnol. Biochem. 2004, 68, 1842–1848. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, F.; Gholami, J.; Foroughnia, M.; Payvar, B.; Nemati, S.; Khodadadegan, M.A.; Saheb, M.; Hajali, V. The beneficial effects of green tea on sleep deprivation-induced cognitive deficits in rats: The involvement of hippocampal antioxidant defense. Heliyon 2021, 7, e08336. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Alcaraz, P.E.; Chung, L.H.; Cases, J. Regular consumption of HolisFiit, a polyphenol-rich extract-based food supplement, improves mind and body well-being of overweight and slightly obese volunteers: A randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2017, 68, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xie, J.; Mu, W.; Ruan, X.; Zhang, J.; Yao, L.; Diao, Z.; Wu, M.; Li, Y.; Ren, W.; et al. Tea polyphenols protect learning and memory in sleep-deprived mice by promoting AMPA receptor internalization. Neuroreport 2020, 31, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Byrne, G.J.; Cribb, L.; Oliver, G.; Murphy, J.; Macdonald, P.; Nazareth, S.; Karamacoska, D.; Galea, S.; Short, A.; et al. L-theanine in the adjunctive treatment of generalized anxiety disorder: A double-blind, randomised, placebo-controlled trial. J. Psychiatr. Res. 2019, 110, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients 2019, 11, 2362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jia, X.Z.; Chen, X.M.; Liu, Y.J.; Zhao, Z.F.; Hao, J.Y.; Wu, R.; Feng, H.T.; Ren, X.N. L-theanine and Neumentix mixture improves sleep quality and modulates brain neurotransmitter levels in mice. Ann. Palliat. Med. 2021, 10, 4572–4581. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.B.; Han, S.H.; Suh, H.J. GABA and L-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.S.A.; Weibel, J.; Landolt, H.P.; Santini, F.; Meyer, M.; Brunmair, J.; Meier-Menches, S.M.; Gerner, C.; Borgwardt, S.; Cajochen, C.; et al. Daily Caffeine Intake Induces Concentration-Dependent Medial Temporal Plasticity in Humans: A Multimodal Double-Blind Randomized Controlled Trial. Cerebral Cortex 2021, 31, 3096–3106. [Google Scholar] [CrossRef]
- Kwon, S.; Yoon, M.; Lee, J.; Moon, K.D.; Kim, D.; Kim, S.B.; Cho, S. A Standardized Phlorotannin Supplement Attenuates Caffeine-Induced Sleep Disruption in Mice. Nutrients 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Ohno, A. Sleep Aid. WO 2020/059808 A1, 28 May 2020. [Google Scholar]
- Zhong, Y.; Zheng, Q.; Hu, P.; Huang, X.; Yang, M.; Ren, G.; Du, Q.; Luo, J.; Zhang, K.; Li, J.; et al. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef]
- Komagata, N.; Latifi, B.; Rusterholz, T.; Bassetti, C.L.A.; Adamantidis, A.; Schmidt, M.H. Dynamic REM Sleep Modulation by Ambient Temperature and the Critical Role of the Melanin-Concentrating Hormone System. Curr. Biol. 2019, 29, 1976–1987.e4. [Google Scholar] [CrossRef]
- Latifi, B.; Adamantidis, A.; Bassetti, C.; Schmidt, M.H. Sleep-Wake Cycling and Energy Conservation: Role of Hypocretin and the Lateral Hypothalamus in Dynamic State-Dependent Resource Optimization. Front. Neurol. 2018, 9, 16. [Google Scholar] [CrossRef]
- Bandaru, S.S.; Khanday, M.A.; Ibrahim, N.; Naganuma, F.; Vetrivelan, R. Sleep-Wake Control by Melanin-Concentrating Hormone (MCH) Neurons: A Review of Recent Findings. Curr. Neurol. Neurosci. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, J.; Miao, S.; Wei, K.; Peng, L.; Wang, Y.; Wei, X. Recent advances in the utilization of tea active ingredients to regulate sleep through neuroendocrine pathway, immune system and intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- Tsunematsu, T. Elucidation of Neural Circuits Involved in the Regulation of Sleep/Wakefulness Using Optogenetics. In Optogenetics: Light-Sensing Proteins and Their Applications in Neuroscience and Beyond, 2nd ed.; Springer: Singapore, 2021; Volume 1293, pp. 391–406. [Google Scholar] [CrossRef]
- Maluck, E.; Busack, I.; Besseling, J.; Masurat, F.; Turek, M.; Busch, K.E.; Bringmann, H. A wake-active locomotion circuit depolarizes a sleep-active neuron to switch on sleep. PLoS Biol. 2020, 18, e3000361. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cai, S.; Li, J.; Xiong, L.; Tian, L.; Liu, J.; Huang, J.; Liu, Z. Neuroprotective Effects of Theaflavins Against Oxidative Stress-Induced Apoptosis in PC12 Cells. Neurochem. Res. 2016, 41, 3364–3372. [Google Scholar] [CrossRef]
- Xu, Y.J.; Liu, S.J.; Zhu, L.Y.; Dai, L.G.; Qian, W.; Zhang, J.Z.; Li, X.; Pan, W. Green tea protects against hippocampal neuronal apoptosis in diabetic encephalopathy by inhibiting JNK/MLCK signaling. Mol. Med. Rep. 2021, 24, 1–13. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Hua, F.; Chen, W.; Wang, W.; Chu, G.X.; Bao, G.H. Interaction between Ester-Type Tea Catechins and Neutrophil Gelatinase-Associated Lipocalin: Inhibitory Mechanism. J. Agric. Food Chem. 2018, 66, 1147–1156. [Google Scholar] [CrossRef]
- Kim, J.; Funayama, S.; Izuo, N.; Shimizu, T. Dietary supplementation of a high-temperature-processed green tea extract attenuates cognitive impairment in PS2 and Tg2576 mice. Biosci. Biotechnol. Biochem. 2019, 83, 2364–2371. [Google Scholar] [CrossRef]
- Unno, K.; Nakamura, Y. Green Tea Suppresses Brain Aging. Molecules 2021, 26, 4897. [Google Scholar] [CrossRef]
- Bae, H.J.; Kim, J.; Jeon, S.J.; Kim, J.; Goo, N.; Jeong, Y.; Cho, K.; Cai, M.; Jung, S.Y.; Kwon, K.J.; et al. Green tea extract containing enhanced levels of epimerized catechins attenuates scopolamine-induced memory impairment in mice. J. Ethnopharmacol. 2020, 258, 112923. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.I.; Schafer, K.H.; Naseem, M.; Weyland, M.; Meiser, P. Troxerutin flavonoid has neuroprotective properties and increases neurite outgrowth and migration of neural stem cells from the subventricular zone. PLoS ONE 2020, 15, e0237025. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Yu, X.Z.; Papouin, T.; Cheong, E.J.; Freeman, M.R.; Monk, K.R.; Hastings, M.H.; Haydon, P.G.; Rowitch, D.; Shaham, S.; et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron 2021, 109, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.D.; Keles, M.F.; Baz, E.S.; Han, E.; Park, K.; Luu, S.; Issa, H.; Brown, M.; Ho, M.C.W.; Tabuchi, M.; et al. Astroglial Calcium Signaling Encodes Sleep Need in Drosophila. Curr. Biol. 2021, 31, 150–162.e7. [Google Scholar] [CrossRef] [PubMed]
- Ingiosi, A.M.; Hayworth, C.R.; Harvey, D.O.; Singletary, K.G.; Rempe, M.J.; Wisor, J.P.; Frank, M.G. A Role for Astroglial Calcium in Mammalian Sleep and Sleep Regulation. Curr. Biol. 2020, 30, 4373–4383.e7. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, T.V.; Collard, M.; Yokoyama, S.; Reitman, M.E.; Poskanzer, K.E. Cortical astrocytes independently regulate sleep depth and duration via separate GPCR pathways. eLife 2021, 10, e63329. [Google Scholar] [CrossRef]
- Choudhury, M.E.; Miyanishi, K.; Takeda, H.; Tanaka, J. Microglia and the Aging Brain: Are Geriatric Microglia Linked to Poor Sleep Quality? Int. J. Mol. Sci. 2021, 22, 7824. [Google Scholar] [CrossRef]
- Corsi, G.; Picard, K.; di Castro, M.A.; Garofalo, S.; Tucci, F.; Chece, G.; del Percio, C.; Golia, M.T.; Raspa, M.; Scavizzi, F.; et al. Microglia modulates hippocampal synaptic transmission and sleep duration along the light/dark cycle. GLIA 2022, 70, 89–105. [Google Scholar] [CrossRef]
- Barenys, M.; Gassmann, K.; Baksmeier, C.; Heinz, S.; Reverte, I.; Schmuck, M.; Temme, T.; Bendt, F.; Zschauer, T.C.; Rockel, T.D.; et al. Epigallocatechin gallate (EGCG) inhibits adhesion and migration of neural progenitor cells in vitro. Arch. Toxicol. 2017, 91, 827–837. [Google Scholar] [CrossRef]
- Ren, J.L.; Yu, Q.X.; Liang, W.C.; Leung, P.Y.; Ng, T.K.; Chu, W.K.; Pang, C.P.; Chan, S.O. Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci. Rep. 2018, 8, 429. [Google Scholar] [CrossRef]
- Cittadini, M.C.; Garcia-Estevez, I.; Escribano-Bailon, M.T.; Rivas-Gonzalo, J.C.; Valentich, M.A.; Repossi, G.; Soria, E.A. Modulation of Fatty Acids and Interleukin-6 in Glioma Cells by South American Tea Extracts and their Phenolic Compounds. Nutr. Cancer 2018, 70, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Pak, K.; Kim, J.; Kim, K.; Kim, S.J.; Kim, I.J. Sleep and Neuroimaging. Nucl. Med. Mol. Imaging 2020, 54, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.D.; Lee, K.H.; Churgin, M.A.; Hill, A.J.; Van Buskirk, C.; Fang-Yen, C.; Raizen, D.M. FMRFamide-like FLP-13 Neuropeptides Promote Quiescence following Heat Stress in Caenorhabditis elegans. Curr. Biol. 2014, 24, 2406–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanshan, H.; Liyong, L.; Xintong, B.; Rui Hai, L.; Sibo, Z.; Yu, C.; Kang, S.; Jielin, J.; Zhonghua, L.; Liang, Z. Pu-erh tea restored circadian rhythm disruption by regulating tryptophan metabolism. J. Agric. Food Chem. 2022, 70, 5610–5623. [Google Scholar] [CrossRef]
- Ouyang, S.H.; Zhai, Y.J.; Wu, Y.P.; Xie, G.; Wang, G.E.; Mao, Z.F.; Hu, H.H.; Luo, X.H.; Sun, W.Y.; Liang, L.; et al. Theacrine, a Potent Antidepressant Purine Alkaloid from a Special Chinese Tea, Promotes Adult Hippocampal Neurogenesis in Stressed Mice. J. Agric. Food Chem. 2021, 69, 7016–7027. [Google Scholar] [CrossRef]
- Kawada, K.; Kuramoto, N.; Mimori, S. Possibility that the Onset of Autism Spectrum Disorder is Induced by Failure of the Glutamine-Glutamate Cycle. Curr. Mol. Pharmacol. 2021, 14, 170–174. [Google Scholar] [CrossRef]
- Deb, S.; Dutta, A.; Phukan, B.C.; Manivasagam, T.; Thenmozhi, A.J.; Bhattacharya, P.; Paul, R.; Borah, A. Neuroprotective attributes of L-theanine, a bioactive amino acid of tea, and its potential role in Parkinson’s disease therapeutics. Neurochem. Int. 2019, 129, 104478. [Google Scholar] [CrossRef]
- Raj, K.; Gupta, G.D.; Singh, S. l-Theanine ameliorates motor deficit, mitochondrial dysfunction, and neurodegeneration against chronic tramadol induced rats model of Parkinson’s disease. Drug Chem Toxicol 2021, 45, 2097–2108. [Google Scholar] [CrossRef]
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef] [Green Version]
- Wagner-Skacel, J.; Dalkner, N.; Moerkl, S.; Kreuzer, K.; Farzi, A.; Lackner, S.; Painold, A.; Reininghaus, E.Z.; Butler, M.I.; Bengesser, S. Sleep and Microbiome in Psychiatric Diseases. Nutrients 2020, 12, 2198. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Yao, Z.W.; Zhao, B.C.; Yang, X.; Lei, S.H.; Jiang, Y.M.; Liu, K.X. Relationships of sleep disturbance, intestinal microbiota, and postoperative pain in breast cancer patients: A prospective observational study. Sleep Breath. 2021, 25, 1655–1664. [Google Scholar] [CrossRef]
- Zhang, R.R.; Gao, S.J.; Wang, S.H.; Zhang, J.W.; Bai, Y.Y.; He, S.; Zhao, P.; Zhang, H.J. Gut Microbiota in Patients with Type 1 Narcolepsy. Nat. Sci. Sleep 2021, 13, 2007–2018. [Google Scholar] [CrossRef]
- Valentini, F.; Evangelisti, M.; Arpinelli, M.; Di Nardo, G.; Borro, M.; Simmaco, M.; Villa, M.P. Gut microbiota composition in children with obstructive sleep apnoea syndrome: A pilot study. Sleep Med. 2020, 76, 140–147. [Google Scholar] [CrossRef]
- Szentirmai, E.; Massie, A.R.; Kapas, L. Lipoteichoic acid, a cell wall component of Gram-positive bacteria, induces sleep and fever and suppresses feeding. Brain Behav. Immun. 2021, 92, 184–192. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Z.; Qian, Y.; Li, X.; Wang, J.; Ma, J.; Guo, J.; Fu, F. Effects of Different Concentrations of Ganpu Tea on Fecal Microbiota and Short Chain Fatty Acids in Mice. Nutrients 2021, 13, 3715. [Google Scholar] [CrossRef]
- Li, B.Y.; Mao, Q.Q.; Zhou, D.D.; Luo, M.; Gan, R.Y.; Li, H.Y.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Li, H.B. Effects of Tea against Alcoholic Fatty Liver Disease by Modulating Gut Microbiota in Chronic Alcohol-Exposed Mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef]
- Gong, Z.P.; Ouyang, J.; Wu, X.L.; Zhou, F.; Lu, D.M.; Zhao, C.J.; Liu, C.F.; Zhu, W.; Zhang, J.C.; Li, N.X.; et al. Dark tea extracts: Chemical constituents and modulatory effect on gastrointestinal function. Biomed. Pharmacother. 2020, 130, 110514. [Google Scholar] [CrossRef]
- Cao, P.Q.; Li, X.P.; Jian, O.Y.; Jiang, R.G.; Huang, F.F.; Wen, B.B.; Zhang, X.N.; Huang, J.A.; Liu, Z.H. The protective effects of yellow tea extract against loperamide-induced constipation in mice. Food Funct. 2021, 12, 5621–5636. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii, a Bacteria Increased With Green Tea Consumption, Promotes Recovery From Acute Colitis in Mice via Suppression of IL-17. Front. Nutr. 2020, 7, 610946. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Navajas-Porras, B.; Lopez-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufian-Henares, J.A. Green Tea and Its Relation to Human Gut Microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The beta-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Chen, J.; He, Q.; Li, S.; Jian, L.; Xie, F.; Dong, C.; Bai, G.Y.; Wang, Z.R.; Zou, T.D.; et al. Oral L-theanine administration promotes fat browning and prevents obesity in mice fed high-fat diet associated with the modulation of gut microbiota. J. Funct. Foods 2021, 81, 104476. [Google Scholar] [CrossRef]
- Cao, J.; Herman, A.B.; West, G.B.; Poe, G.; Savage, V.M. Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development. Sci. Adv. 2020, 6, eaba0398. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Gunter, M.; Schuhmacher, J.; Bieber, K.; Poschel, S.; Schutz, M.; Engelhardt, B.; Oster, H.; Sina, C.; Lange, T.; et al. Sleep enhances numbers and function of monocytes and improves bacterial infection outcome in mice. Brain Behav. Immun. 2020, 87, 329–338. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Du, K.; Xie, T.; Wang, B.; Chen, C.; Reiter, R.J.; Cen, B.; Yuan, Y. Pan-cancer analyses reveal genomics and clinical characteristics of the melatonergic regulators in cancer. J. Pineal Res. 2021, 71, e12758. [Google Scholar] [CrossRef]
- Sinner, M.P.; Masurat, F.; Ewbank, J.J.; Pujol, N.; Bringmann, H. Innate Immunity Promotes Sleep through Epidermal Antimicrobial Peptides. Curr. Biol. 2021, 31, 27. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; You, P.N.; SenGupta, T.; Nilsen, H.; Sharma, K. Crosstalk between Different DNA Repair Pathways Contributes to Neurodegenerative Diseases. Biology 2021, 10, 163. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Miao, L.; Daozhou, L.; Ying, C.; Qibing, M.; Siyuan, Z. A resveratrol-loaded nanostructured lipid carrier hydrogel to enhance the anti-UV irradiation and anti-oxidant efficacy. Colloids Surf. B Biointerfaces 2021, 204, 111786. [Google Scholar] [CrossRef]
- Hou, X.; Shao, C.; Sun, K.; Li, R.; Gao, L.; Meng, Y.; Jing, Y.; Wei, L. Autophagy deficiency downregulates O(6)methylguanine-DNA methyltransferase and increases chemosensitivity of liver cancer cells. Aging 2021, 13, 14289–14303. [Google Scholar] [CrossRef]
- Rodriguez-Rocha, H.; Garcia-Garcia, A.; Panayiotidis, M.I.; Franco, R. DNA damage and autophagy. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2011, 711, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Kansal, V.; Agarwal, A.; Harbour, A.; Farooqi, H.; Singh, V.K.; Prasad, R. Regular Intake of Green Tea Polyphenols Suppresses the Development of Nonmelanoma Skin Cancer through miR-29-Mediated Epigenetic Modifications. J. Clin. Med. 2022, 11, 398. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Lin, E.; He, P.; Ru, G. Oxidative damage-induced hyperactive ribosome biogenesis participates in tumorigenesis of offspring by cross-interacting with the Wnt and TGF-beta1 pathways in IVF embryos. Exp. Mol. Med. 2021, 53, 1792–1806. [Google Scholar] [CrossRef]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure-activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef]
- Zada, D.; Sela, Y.; Matosevich, N.; Monsonego, A.; Lerer-Goldshtein, T.; Nir, Y.; Appelbaum, L. Parp1 promotes sleep, which enhances DNA repair in neurons. Mol. Cell 2021, 81, 4979–4993.e7. [Google Scholar] [CrossRef]
- DeBardeleben, H.K.; Lopes, L.E.; Nessel, M.P.; Raizen, D.M. Stress-Induced Sleep After Exposure to Ultraviolet Light Is Promoted by p53 in Caenorhabditis elegans. Genetics 2017, 207, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Cheung, V.; Yuen, V.M.; Wong, G.T.C.; Choi, S.W. The effect of sleep deprivation and disruption on DNA damage and health of doctors. Anaesthesia 2019, 74, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Zada, D.; Bronshtein, I.; Lerer-Goldshtein, T.; Garini, Y.; Appelbaum, L. Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Sellegounder, D.; Liu, Y.; Wibisono, P.; Chen, C.-H.; Leap, D.; Sun, J. Neuronal GPCR NPR-8 regulates C. elegans defense against pathogen infection. Sci. Adv. 2019, 5, eaaw4717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, J.C.; Antoniades, W.; Okalova, J.; Roos, M.M.; Grimsey, N.J. Atypical p38 Signaling, Activation, and Implications for Disease. Int. J. Mol. Sci. 2021, 22, 4183. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Efimova, T.; Balasubramanian, S.; Crish, J.F.; Bone, F.; Dashti, S. p38 Mitogen-activated protein kinases on the body surface—A function for p38 delta. J. Investig. Dermatol. 2003, 120, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Enslen, H.; Raingeaud, J.; Davis, R.J. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 1998, 273, 1741–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Wang, M.; Zhang, J.; Xu, R. p38 MAPK: A Potential Target of Chronic Pain. Curr. Med. Chem. 2014, 21, 4405–4418. [Google Scholar] [CrossRef]
- Sarikaya, D.P.; Cridland, J.; Tarakji, A.; Sheehy, H.; Davis, S.; Kochummen, A.; Hatmaker, R.; Khan, N.; Chiu, J.; Begun, D.J. Phenotypic coupling of sleep and starvation resistance evolves in D. melanogaster. BMC Evol. Biol. 2020, 20, 126. [Google Scholar] [CrossRef]
- Szentirmai, E.; Kapas, L. Sleep and body temperature in TNF alpha knockout mice: The effects of sleep deprivation, beta 3-AR stimulation and exogenous TNF alpha. Brain Behav. Immun. 2019, 81, 260–271. [Google Scholar] [CrossRef]
- Nguyen, J.; Gibbons, C.M.; Dykstra-Aiello, C.; Ellingsen, R.; Koh, K.M.S.; Taishi, P.; Krueger, J.M. Interleukin-1 receptor accessory proteins are required for normal homeostatic responses to sleep deprivation. J. Appl. Physiol. 2019, 127, 770–780. [Google Scholar] [CrossRef]
- Xia, X.; Xu, J.; Wang, X.; Wang, H.; Lin, Z.; Shao, K.; Fang, L.; Zhang, C.; Zhao, Y. Jiaogulan tea (Gpostemma pentaphyllum) potentiates the antidiabetic effect of white tea via the AMPK and PI3K pathways in C57BL/6 mice. Food Funct. 2020, 11, 4339–4355. [Google Scholar] [CrossRef]
- Xiong, L.G.; Pan, L.Y.; Gong, Y.S.; Huang, J.A.; Liu, Z.H. Fuzhuan Tea protects Caenorhabditis elegans from glucose and advanced glycation end products via distinct pathways. J. Funct. Foods 2019, 59, 148–155. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Liu, Y.; Sun, K.; Luo, L.; Zeng, L. Aged Ripe Pu-erh Tea Reduced Oxidative Stress-Mediated Inflammation in Dextran Sulfate Sodium-Induced Colitis Mice by Regulating Intestinal Microbes. J. Agric. Food Chem. 2021, 69, 10592–10605. [Google Scholar] [CrossRef]
- Cai, X.; Quan, H.; Chang, D.; Bi, J.; Zhang, K. Metabolic rate and substrate oxidation of young males with obesity at the different sleep stages. Obes. Res. Clin. Pract. 2022, 16, 17–22. [Google Scholar] [CrossRef]
- Szentirmai, E.; Kapas, L. Brown adipose tissue plays a central role in systemic inflammation-induced sleep responses. PLoS ONE 2018, 13, e0197409. [Google Scholar] [CrossRef] [Green Version]
- Juozaityte, V.; Pladevall-Morera, D.; Podolska, A.; Norgaard, S.; Neumann, B.; Pocock, R. The ETS-5 transcription factor regulates activity states in Caenorhabditis elegans by controlling satiety. Proc. Natl. Acad. Sci. USA 2017, 114, E1651–E1658. [Google Scholar] [CrossRef] [Green Version]
- Wilms, B.; Leineweber, E.M.; Molle, M.; Chamorro, R.; Pommerenke, C.; Salinas-Riester, G.; Sina, C.; Lehnert, H.; Oster, H.; Schmid, S.M. Sleep Loss Disrupts Morning-to-Evening Differences in Human White Adipose Tissue Transcriptome. J. Clin. Endocrinol. Metab. 2019, 104, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Liu, X.; Zhou, W.; Wu, S.; Wang, X. Night sleep duration and risk of each lipid profile abnormality in a Chinese population: A prospective cohort study. Lipids Health Dis. 2020, 19, 185. [Google Scholar] [CrossRef]
- Tasali, E.; Wroblewski, K.; Kahn, E.; Kilkus, J.; Schoeller, D.A. Effect of Sleep Extension on Objectively Assessed Energy Intake Among Adults With Overweight in Real-life Settings: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 365–374. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Sun, L.; Lai, X.; Li, Q.; Zhang, W.; Xiang, L.; Sun, S.; Cao, F. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019, 10, 2061–2074. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Liu, S.; Zhang, B.W.; Hu, Y.Z.; Ma, H.; Wang, S. Green tea leaf powder prevents dyslipidemia in high-fat diet-fed mice by modulating gut microbiota. Food Nutr. Res. 2020, 64, 3672. [Google Scholar] [CrossRef]
- Huang, F.; Wang, S.; Zhao, A.; Zheng, X.; Zhang, Y.; Lei, S.; Ge, K.; Qu, C.; Zhao, Q.; Yan, C.; et al. Pu-erh Tea Regulates Fatty Acid Metabolism in Mice Under High-Fat Diet. Front. Pharmacol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Zhou, M.X.; Tian, X.; Wu, Z.Q.; Li, K.; Li, Z.J. Fuzhuan brick tea supplemented with areca nuts: Effects on serum and gut microbiota in mice. J. Food Biochem. 2021, 45, e13737. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lang, H.; Zhou, M.; Huang, L.; Hui, S.; Wang, X.; Chen, K.; Mi, M. The Preventive Effects of Pterostilbene on the Exercise Intolerance and Circadian Misalignment of Mice Subjected to Sleep Restriction. Mol. Nutr. Food Res. 2020, 64, e1900991. [Google Scholar] [CrossRef] [PubMed]
- Yurgel, M.E.; Kakad, P.; Zandawala, M.; Nassel, D.R.; Godenschwege, T.A.; Keene, A.C. A single pair of leucokinin neurons are modulated by feeding state and regulate sleep-metabolism interactions. PLoS Biol. 2019, 17, e2006409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funato, H.; Miyoshi, C.; Fujiyama, T.; Kanda, T.; Kanda, T.; Sato, M.; Wang, Z.; Ma, J.; Nakane, S.; Tomita, J.; et al. Forward-genetics analysis of sleep in randomly mutagenized mice. Nature 2016, 539, 378–383. [Google Scholar] [CrossRef]
- Park, M.; Miyoshi, C.; Fujiyama, T.; Kakizaki, M.; Ikkyu, A.; Honda, T.; Choi, J.; Asano, F.; Mizuno, S.; Takahashi, S.; et al. Loss of the conserved PKA sites of SIK1 and SIK2 increases sleep need. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Iwasaki, K.; Fujiyama, T.; Nakata, S.; Park, M.; Miyoshi, C.; Hotta-Hirashima, N.; Ikkyu, A.; Kakizaki, M.; Sugiyama, F.; Mizuno, S.; et al. Induction of Mutant Sik3(Sleepy) Allele in Neurons in Late Infancy Increases Sleep Need. J. Neurosci. 2021, 41, 2733–2746. [Google Scholar] [CrossRef]
- Grubbs, J.J.; Lopes, L.E.; van der Linden, A.M.; Raizen, D.M. A salt-induced kinase is required for the metabolic regulation of sleep. PLoS Biol. 2020, 18, e3000220. [Google Scholar] [CrossRef] [Green Version]
- An, R.; Wen, S.; Li, D.L.; Li, Q.H.; Lai, X.F.; Zhang, W.J.; Chen, R.H.; Cao, J.X.; Li, Z.G.; Huang, Q.S.; et al. Mixtures of Tea and Citrus maxima (pomelo) Alleviate Lipid Deposition in HepG2 Cells Through the AMPK/ACC Signaling Pathway. J. Med. Food 2020, 23, 943–951. [Google Scholar] [CrossRef]
- Mika, M.; Wikiera, A.; Antonczyk, A.; Grabacka, M. The impact of catechins included in high fat diet on AMP-dependent protein kinase in apoE knock-out mice. Int. J. Food Sci. Nutr. 2021, 72, 348–356. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, H.T.; Chen, D.; Guo, D.D.; Wang, X.Y. Targeting at cancer energy metabolism and lipid droplet formation as new treatment strategies for epigallocatechin-3-gallate (EGCG) in colorectal cancer cells. J. Funct. Foods 2021, 83, 104570. [Google Scholar] [CrossRef]
- Karp, X. Hormonal Regulation of Diapause and Development in Nematodes, Insects, and Fishes. Front. Ecol. Evol. 2021, 9, 735924. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Wang, L.L.; Song, L.S. An insulin-like peptide serves as a regulator of glucose metabolism in the immune response of Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2020, 108, 103686. [Google Scholar] [CrossRef]
- Li, J.T.S.; Hon, K.L.; Leung, A.K.C.; Lee, V.W.Y. Pharmacologic Evidence of Green Tea in Targeting Tyrosine Kinases. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 239–246. [Google Scholar] [CrossRef]
- Ni, D.; Ai, Z.; Munoz-Sandoval, D.; Suresh, R.; Ellis, P.R.; Yuqiong, C.; Sharp, P.A.; Butterworth, P.J.; Yu, Z.; Corpe, C.P. Inhibition of the facilitative sugar transporters (GLUTs) by tea extracts and catechins. FASEB J. 2020, 34, 9995–10010. [Google Scholar] [CrossRef]
- Tian, J.; Geiss, C.; Zarse, K.; Madreiter-Sokolowski, C.T.; Ristow, M. Green tea catechins EGCG and ECG enhance the fitness and lifespan of Caenorhabditis elegans by complex I inhibition. Aging 2021, 13, 22629–22648. [Google Scholar] [CrossRef]
- Hirano, N.; Sakamoto, K. Linalool odor stimulation improves heat stress tolerance and decreases fat accumulation in nematodes. Biosci. Biotechnol. Biochem. 2019, 83, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Toyoura, M.; Toyota, Y.; Takaoka, Y. Serum concentrations of insulin-like growth factor-1 as a biomarker of improved circadian rhythm sleep-wake disorder in school-aged children. J. Clin. Sleep Med. 2020, 16, 2073–2078. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez de Sevilla, M.E.; Fernandez, A.M.; Munive, V.; Martinez-Rachadell, L.; Nunez, A.; Torres Aleman, I. Insulin-like growth factor I modulates sleep through hypothalamic orexin neurons. FASEB J. 2020, 34, 15975–15990. [Google Scholar] [CrossRef]
- Brown, E.B.; Shah, K.D.; Faville, R.; Kottler, B.; Keene, A.C. Drosophila insulin-like peptide 2 mediates dietary regulation of sleep intensity. PLoS Genet. 2020, 16, e1008270. [Google Scholar] [CrossRef]
- He, Q.; Du, J.; Wei, L.; Zhao, Z. AKH-FOXO pathway regulates starvation-induced sleep loss through remodeling of the small ventral lateral neuron dorsal projections. PLoS Genet. 2020, 16, e1009181. [Google Scholar] [CrossRef]
- Kelly, M.L.; Collins, S.P.; Lesku, J.A.; Hemmi, J.M.; Collin, S.P.; Radford, C.A. Energy conservation characterizes sleep in sharks. Biol. Lett. 2022, 18, 20210259. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Masurat, F.; Preis, J.; Bringmann, H. Sleep Counteracts Aging Phenotypes to Survive Starvation-Induced Developmental Arrest in C. elegans. Curr. Biol. 2018, 28, 3610–3624.e8. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.K.; Kim, H.H. The functions of mTOR in ischemic diseases. BMB Rep. 2011, 44, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams, R.; Ito, Y.; Miyatake, H. Mapping of mTOR drug targets: Featured platforms for anti-cancer drug discovery. Pharmacol. Ther. 2021, 232, 108012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, D.J.; Han, L.R.; Rensing, N.; Satoh, A.; Wong, M. Hypothalamic orexin and mechanistic target of rapamycin activation mediate sleep dysfunction in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2019, 134, 104615. [Google Scholar] [CrossRef]
- Goetting, D.L.; Soto, R.; Van Buskirk, C. Food-Dependent Plasticity in Caenorhabditis elegans Stress-Induced Sleep Is Mediated by TOR-FOXA and TGF- Signaling. Genetics 2018, 209, 1183–1195. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Wang, Q.; Yang, X. Fu Brick Tea Alleviates Chronic Kidney Disease of Rats with High Fat Diet Consumption through Attenuating Insulin Resistance in Skeletal Muscle. J. Agric. Food Chem. 2019, 67, 2839–2847. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, Y.; Kuramoto, N.; Kawada, K. The role of glutamine in neurogenesis promoted by the green tea amino acid theanine in neural progenitor cells for brain health. Neurochem. Int. 2019, 129, 104505. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Huang, X.; Hong, T.; Zhang, K.; Qi, W.; Guo, M.; Nie, S. Lysosome-Mediated Cytotoxic Autophagy Contributes to Tea Polysaccharide-Induced Colon Cancer Cell Death via mTOR-TFEB Signaling. J. Agric. Food Chem. 2021, 69, 686–697. [Google Scholar] [CrossRef]



| Types of Tea | Degree of Fermentation | Key Processing Technology | Major Health Benefits |
|---|---|---|---|
| Green tea | Non-fermented | Fixing | Antibacterial [34] Suppressing the amyloid beta levels and alleviating cognitive impairment in 5XFAD mice [35] Reducing lipid peroxidation and increasing total antioxidant capacity, and reducing oxidative damage [36] Significantly lowering the risk of developing liver cancer and improving the effect on body mass index, liver enzymes, and lipoprotein [37] Preventing obesity [38,39] |
| Black tea | Fully fermented | Fermentation | Exerting antibacterial activity against major periodontopathogens, attenuating the secretion of IL-8, and inducing hBD secretion in oral epithelial cells [40] Preventing radiation-induced increase of ACE activity and oxidative stress in the aorta [41] Limiting the formation of glycation products [42] |
| Yellow tea | Slightly fermented | Yellowing | Antioxidant and preventing gastric injury [43] Reducing blood glucose levels, increasing glucose tolerance, and preventing fatty liver in diabetes mice [44] |
| Oolong tea | Semi-fermented | Rotating | Neurodegenerative and neurite outgrowth-promoting [45] Inhibiting cancer cell proliferation [46] Providing cardio-protective benefits during hypoxic conditions [47] Prolonging lifespan and improving health span by curtailing the age-related decline in muscle activity and the accumulation of age pigment in C. elegans [48] |
| White tea | Slightly fermented | Withering | Inhibiting PhlP-induced aberrant crypt foci by altering the expression of carcinogen-metabolizing enzymes in rats [49] |
| Dark tea | Post-fermented | Pile fermentation | Decreasing risks of coronary heart disease and diabetes [50] Scavenging of DPPH and ABTS free radicals [51] Regulating the glycolipid metabolic disorders [52] Decreasing body weight and serum triglycerides for SD rats [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Peng, Y.; Gong, Y. New Perspectives on Sleep Regulation by Tea: Harmonizing Pathological Sleep and Energy Balance under Stress. Foods 2022, 11, 3930. https://doi.org/10.3390/foods11233930
Ouyang J, Peng Y, Gong Y. New Perspectives on Sleep Regulation by Tea: Harmonizing Pathological Sleep and Energy Balance under Stress. Foods. 2022; 11(23):3930. https://doi.org/10.3390/foods11233930
Chicago/Turabian StyleOuyang, Jin, Yuxuan Peng, and Yushun Gong. 2022. "New Perspectives on Sleep Regulation by Tea: Harmonizing Pathological Sleep and Energy Balance under Stress" Foods 11, no. 23: 3930. https://doi.org/10.3390/foods11233930
APA StyleOuyang, J., Peng, Y., & Gong, Y. (2022). New Perspectives on Sleep Regulation by Tea: Harmonizing Pathological Sleep and Energy Balance under Stress. Foods, 11(23), 3930. https://doi.org/10.3390/foods11233930