Bioaccessibility and Intestinal Transport of Tebuconazole in Table Grape by Using In Vitro Digestion Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Tebuconazole Residue Analysis
2.3.1. Sample Extraction and Purification
2.3.2. Tebuconazole Residue Analysis
2.4. Bioaccessibility Analysis of In Vitro Simulation
2.5. Impact of In Vitro Digestion Model Conditions on Tebuconazole Bioaccessibility
2.6. Influence of Food Matrix Composition on Tebuconazole Bioaccessibility
2.7. Transport of Tebuconazole in the Caco-2 Cell Model
2.7.1. Cell Culture
2.7.2. Cytotoxicity Assays
2.7.3. Transport Studies
2.8. Data Analysis
3. Results and Discussion
3.1. Method Validation
3.2. Bioaccessibility of Various In Vitro Simulated Digestion Methods
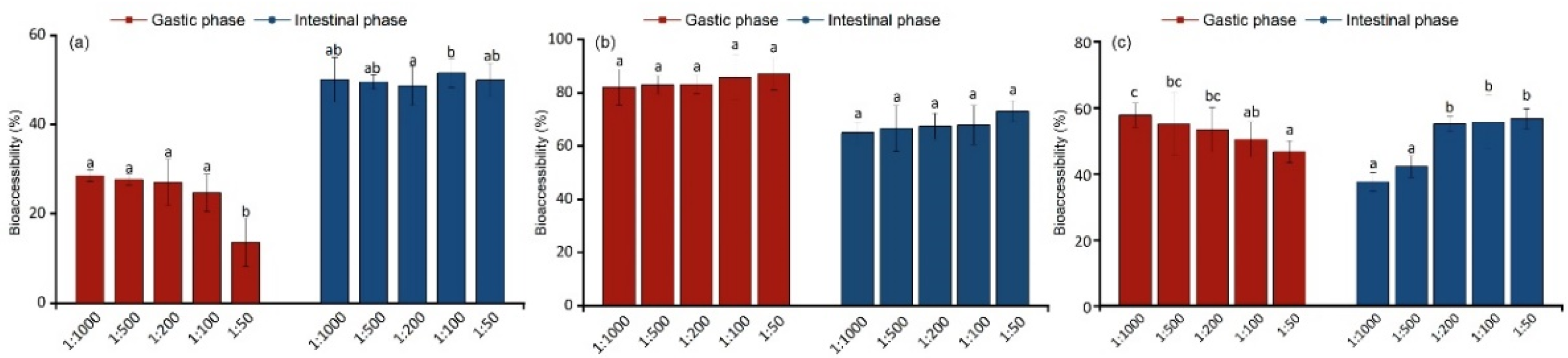
3.3. Factors Affecting Bioaccessibility Based on the SBRC Method
3.4. Changes in Bioaccessibility Caused by the Addition of Dietary Ingredients
3.5. Caco-2 Cell Model Analysis of Intestinal Absorption of Tebuconazole
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurt, A.; Torun, H.; Colak, N.; Seiler, G.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Nutrient profiles of the hybrid grape cultivar ‘Isabel’ during berry maturation and ripening. J. Sci. Food Agric. 2017, 97, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Hazelrigg, A.L.; Bradshaw, T.L.; Maia, G.S. Disease Susceptibility of Interspecific Cold-Hardy Grape Cultivars in Northeastern U.S.A. Horticulturae 2021, 7, 216. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Seem, R.C.; Pearson, R.C.; Wilcox, W.F.; Dunst, R.M. Effects of Powdery Mildew on Vine Growth, Yield, and Quality of Concord Grapes. Plant Dis. 2001, 85, 137–140. [Google Scholar] [CrossRef]
- Kundu, C.; Goon, A.; Bhattacharyya, A. Persistence behaviour of fungicide tebuconazole in a viticulture application. Bull. Environ. Contam. Toxicol. 2014, 92, 415–419. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Torrado-Agrasar, A.; Simal-Gándara, J.; Mazaira-Pérez, J. Evolution of tebuconazole residues through the winemaking process of Mencía grapes. Food Chem. 2009, 117, 529–537. [Google Scholar] [CrossRef]
- Lu, M.; Li, G.; Yang, Y.; Yu, Y. A review on in-vitro oral bioaccessibility of organic pollutants and its application in human exposure assessment. Sci. Total Environ. 2021, 752, 142001. [Google Scholar] [CrossRef]
- Crépet, A.; Luong, T.M.; Baines, J.; Boon, P.E.; Ennis, J.; Kennedy, M.; Massarelli, I.; Miller, D.; Nako, S.; Reuss, R.; et al. An international probabilistic risk assessment of acute dietary exposure to pesticide residues in relation to codex maximum residue limits for pesticides in food. Food Control 2021, 121, 107563. [Google Scholar] [CrossRef]
- Grimm, H.; Olsson, I.A.S.; Sandøe, P. Harm-benefit analysis—What is the added value? A review of alternative strategies for weighing harms and benefits as part of the assessment of animal research. Lab. Anim. 2019, 53, 17–27. [Google Scholar] [CrossRef]
- Toms, L.M.; Hearn, L.; Mueller, J.F.; Harden, F.A. Assessing infant exposure to persistent organic pollutants via dietary intake in Australia. Food Chem. Toxicol. 2016, 87, 166–171. [Google Scholar] [CrossRef]
- Hack, A.; Selenka, F. Mobilization of PAH and PCB from contaminated soil using a digestive tract model. Toxicol. Lett. 1996, 88, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.R.; Basta, N.T.; Casteel, S.W.; Pace, L.W. An In Vitro Gastrointestinal Method To Estimate Bioavailable Arsenic in Contaminated Soils and Solid Media. Environ. Sci. Technol. 1999, 33, 642–649. [Google Scholar] [CrossRef]
- Li, H.B.; Li, M.Y.; Zhao, D.; Li, J.; Li, S.W.; Juhasz, A.L.; Basta, N.T.; Luo, Y.M.; Ma, L.Q. Oral Bioaccessibility of As, Pb, and Cd in Contaminated Soils, Dust, and Foods based on Animal Bioassays: A Review. Environ. Sci. Technol. 2019, 53, 10545–10559. [Google Scholar] [CrossRef] [PubMed]
- Ruby, M.V.; Schoof, R.; Brattin, W.; Goldade, M.; Post, G.; Harnois, M.; Mosby, D.E.; Casteel, S.W.; Berti, W.; Carpenter, M.; et al. Advances in Evaluating the Oral Bioaccessibility of Inorganics in Soil for Use in Human Health Risk Assessment. Environ. Sci. Technol. 1999, 33, 3697–3705. [Google Scholar] [CrossRef]
- Van de Wiele, T.R.; Verstraete, W.; Siciliano, S.D. Polycyclic aromatic hydrocarbon release from a soil matrix in the in vitro gastrointestinal tract. J. Environ. Qual. 2004, 33, 1343–1353. [Google Scholar] [CrossRef]
- Oomen, A.G.; Hack, A.; Minekus, M.; Zeijdner, E.; Cornelis, C.; Schoeters, G.; Verstraete, W.; Van de Wiele, T.; Wragg, J.; Rompelberg, C.J.; et al. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.; Basta, N.; Brandon, E.; Casteel, S.; Denys, S.; Gron, C.; Oomen, A.; Reimer, K.; Tack, K.; et al. An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Sci. Total. Environ. 2011, 409, 4016–4030. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Smith, E.; Nelson, C.; Thomas, D.J.; Bradham, K. Variability associated with as in vivo-in vitro correlations when using different bioaccessibility methodologies. Environ. Sci. Technol. 2014, 48, 11646–11653. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Recio, I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protocols 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Yu, Y.; Han, S.; Zhang, D.; Van de Wiele, T.; Lu, M.; Wang, D.; Yu, Z.; Wu, M.; Sheng, G.; Fu, J. Factors affecting the bioaccessibility of polybrominated diphenylethers in an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 133–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Pignatello, J.J.; Tao, S. Bioaccessibility of PAHs and PAH derivatives in a fuel soot assessed by an in vitro digestive model with absorptive sink: Effects of aging the soot in a soil-water mixture. Sci. Total Environ. 2018, 615, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Xiao, J.J.; Feng, R.P.; Liu, Y.Y.; Liao, M.; Wu, X.W.; Hua, R.M.; Cao, H.Q. In-vitro bioaccessibility of five pyrethroids after human ingestion and the corresponding gastrointestinal digestion parameters: A contribution for human exposure assessments. Chemosphere 2017, 182, 517–524. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Rose, H.E.; Quarterman, J. Dietary fibers and heavy metal retention in the rat. Environ. Res. 1987, 42, 166–175. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Wang, J.; Lin, K.; Taylor, A.; Gan, J. In vitro assessment of pyrethroid bioaccessibility via particle ingestion. Environ. Int. 2018, 119, 125–132. [Google Scholar] [CrossRef]
- Li, K.; Li, C.; Yu, N.Y.; Juhasz, A.L.; Cui, X.Y.; Ma, L.Q. In vivo bioaccessibility and in vitro bioaccessibility of perfluorooctanoic acid (PFOA) in food matrices: Correlation analysis and method development. Environ. Sci. Technol. 2015, 49, 150–158. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Shi, Y.H.; Xiao, J.J.; Liu, Y.Y.; Deng, Y.J.; Feng, W.Z.; Wei, D.; Liao, M.; Cao, H.Q. Gut microbiota influence on oral bioaccessibility and intestinal transport of pesticides in Chaenomeles speciosa. Food Chem. 2021, 339, 127985. [Google Scholar] [CrossRef]
- Cavret, S.; Feidt, C.; Laurent, F. Differential Transfer of Organic Micropollutants through Intestinal Barrier Using Caco-2 Cell Line. J. Agric. Food Chem. 2005, 53, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Ilboudo, S.; Fouche, E.; Rizzati, V.; Toé, A.M.; Gamet-Payrastre, L.; Guissou, P.I. In vitro impact of five pesticides alone or in combination on human intestinal cell line Caco-2. Toxicol. Rep. 2014, 1, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Xiao, J.J.; Feng, R.P.; Liu, Y.Y.; Liao, M.; Wu, X.W.; Cao, H.Q. Factors Affecting the Bioaccessibility and Intestinal Transport of Difenoconazole, Hexaconazole, and Spirodiclofen in Human Caco-2 Cells Following in Vitro Digestion. J. Agric. Food Chem. 2017, 65, 9139–9146. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Yang, X.D.; Wang, Y.; Ma, L.; Zhang, Y.; Yang, X.G.; Wang, K. Establishment of Caco-2 cell monolayer model and standard operation procedure for assessing intestinal absorption of chemical components of traditional Chinese medicine. Zhong Xi Yi Jie He Xue Bao 2007, 5, 634–641. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A.K. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorganic Med. Chem. 2017, 25, 4595–4613. [Google Scholar] [CrossRef]
- Oomen, A.G.; Sips, A.J.A.M.; Groten, J.P.; Sijm, D.T.H.M.; Tolls, J. Mobilization of PCBs and Lindane from Soil during in Vitro Digestion and Their Distribution among Bile Salt Micelles and Proteins of Human Digestive Fluid and the Soil. Environ. Sci. Technol. 2000, 34, 297–303. [Google Scholar] [CrossRef]
- Mennah-Govela, Y.A.; Singh, R.P.; Bornhorst, G.M. Buffering capacity of protein-based model food systems in the context of gastric digestion. Food Funct. 2019, 10, 6074–6087. [Google Scholar] [CrossRef]
- Tian, M.; Han, J.; Ye, A.; Liu, W.; Xu, X.; Yao, Y.; Li, K.; Kong, Y.; Wei, F.; Zhou, W. Structural characterization and biological fate of lactoferrin-loaded liposomes during simulated infant digestion. J. Sci. Food Agric. 2019, 99, 2677–2684. [Google Scholar] [CrossRef]
- Sanchez, C.L.; Souders, C.L.; Pena-Delgado, C.J.; Nguyen, K.T.; Kroyter, N.; Ahmadie, N.E.; Aristizabal-Henao, J.J.; Bowden, J.A.; Martyniuk, C.J. Neurotoxicity assessment of triazole fungicides on mitochondrial oxidative respiration and lipids in differentiated human SH-SY5Y neuroblastoma cells. Neurotoxicology 2020, 80, 76–86. [Google Scholar] [CrossRef]


| Simulation Methods | Bioaccessibility (%) | |
|---|---|---|
| Gastric Phase (%) | Intestinal Phase (%) | |
| DIN | 27.47 ± 1.53 | 72.90 ± 7.77 |
| IVG | 33.32 ± 3.56 | 69.48 ± 3.89 |
| PBET | 33.53 ± 5.98 | 70.00 ± 2.61 |
| SBRC | 50.02 ± 8.95 | 76.73 ± 2.80 |
| SHIME | 31.13 ± 1.78 | 54.83 ± 5.58 |
| Time (h) | Apparent Permeability Coefficient (Papp) | |
|---|---|---|
| Papp AP-BL (1 × 10−5 cm2/s) | Papp BL-AP (1 × 10−5 cm2/s) | |
| 0.5 | 1.36 ± 0.14 | 1.71 ± 0.03 |
| 1 | 1.61 ± 0.13 | 1.79 ± 0.13 |
| 1.5 | 2.13 ± 0.07 | 2.00 ± 0.40 |
| 2 | 2.15 ± 0.05 | 2.62 ± 0.09 |
| 3 | 2.66 ± 1.08 | 3.07 ± 0.44 |
| 4 | 3.84 ± 0.55 | 3.09 ± 0.12 |
| 6 | 6.37 ± 0.65 | 5.72 ± 1.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Han, Y.; Xiao, O.; Cui, W.; Chen, J.; Dai, X.; Li, M.; Kong, Z. Bioaccessibility and Intestinal Transport of Tebuconazole in Table Grape by Using In Vitro Digestion Models. Foods 2022, 11, 3926. https://doi.org/10.3390/foods11233926
Liu X, Han Y, Xiao O, Cui W, Chen J, Dai X, Li M, Kong Z. Bioaccessibility and Intestinal Transport of Tebuconazole in Table Grape by Using In Vitro Digestion Models. Foods. 2022; 11(23):3926. https://doi.org/10.3390/foods11233926
Chicago/Turabian StyleLiu, Xiaowei, Ying Han, Ouli Xiao, Weiye Cui, Jieyin Chen, Xiaofeng Dai, Minmin Li, and Zhiqiang Kong. 2022. "Bioaccessibility and Intestinal Transport of Tebuconazole in Table Grape by Using In Vitro Digestion Models" Foods 11, no. 23: 3926. https://doi.org/10.3390/foods11233926
APA StyleLiu, X., Han, Y., Xiao, O., Cui, W., Chen, J., Dai, X., Li, M., & Kong, Z. (2022). Bioaccessibility and Intestinal Transport of Tebuconazole in Table Grape by Using In Vitro Digestion Models. Foods, 11(23), 3926. https://doi.org/10.3390/foods11233926








