Effects of Texture Modifiers on Physicochemical Properties of 3D-Printed Meat Mimics from Pea Protein Isolate-Alginate Gel Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Printing Material
2.3. Modification of Printing Material
2.4. Rheological Properties Measurement of Printing Material
2.5. 3D Printing
2.6. Cooking of 3D-Printed Meat Mimics
2.7. Textural Properties Determination of 3D-Printed Meat Mimics
2.8. Comparison with Animal Meat
2.9. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties of Printing Material
3.2. Appearances of 3D-Printed Meat Mimics
3.3. Textural Properties of 3D-Printed Meat Mimics
3.3.1. Raw Meat Mimics
3.3.2. Cooked Meat Mimics
3.4. Cooking Losses of 3D-Printed Meat Mimics
3.5. Comparison between 3D-Printed Meat Mimics and Animal Meats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006. [Google Scholar]
- Vinnari, M.; Tapio, P. Future images of meat consumption in 2030. Futures 2009, 41, 269–278. [Google Scholar] [CrossRef]
- Elzerman, J.E.; Hoek, A.C.; van Boekel, M.A.J.S.; Luning, P.A. Consumer acceptance and appropriateness of meat substitutes in a meal context. Food Qual Prefer. 2011, 22, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Hoek, A.C.; Luning, P.A.; Weijzen, P.; Engels, W.; Kok, F.J.; de Graaf, C. Replacement of meat by meat substitutes. A survey on person- and product-related factors in consumer acceptance. Appetite 2011, 56, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ associations, perceptions and acceptance of meat and plant-based meat alternatives. Food Qual. Prefer. 2021, 87, 104063. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Bhandari, B. 4D printing of products based on soy protein isolate via microwave heating for flavor development. Food Res. Int. 2020, 137, 109605. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Devahastin, S. Investigation on 3D printing ability of soybean protein isolate gels and correlations with their rheological and textural properties via LF-NMR spectroscopic characteristics. LWT-Food Sci. Technol. 2020, 122, 109019. [Google Scholar] [CrossRef]
- Wang, S.; Lui, S. 3D printing of soy protein- and gluten-based gels facilitated by thermosensitive cocoa butter in a model study. ACS Food Sci. Technol. 2021, 1, 1990–1996. [Google Scholar] [CrossRef]
- Mackay, M.E. The importance of rheological behavior in the additive manufacturing technique material extrusion. J. Rheol. 2018, 62, 1549. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Stability of 3D printing using a mixture of pea protein and alginate: Precision and application of additive layer manufacturing simulation approach for stress distribution. J. Food Eng. 2021, 288, 110127. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J. Food Biochem. 2020, 44, 13157. [Google Scholar] [CrossRef] [PubMed]
- Barać, M.; Cabrilo, S.; Pešić, M.; Stanojević, S.; Pavlićević, M.; Maćej, O.; Ristić, N. Functional properties of pea (Pisum sativum, L.) protein isolates modified with chymosin. Int. J. Mol. Sci. 2011, 12, 8372–8387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.W.; Lee, I.J.; Park, S.M.; Lee, J.H.; Nguyen, M.-H.; Park, H.J. Effect of hydrocolloid addition on dimensional stability in post-processing of 3D printable cookie dough. LWT-Food Sci. Technol. 2019, 101, 69–75. [Google Scholar] [CrossRef]
- Carvajal-Piñero, J.M.; Ramos, M.; Jiménez-Rosado, M.; Perez-Puyana, V.; Romero, A. Development of pea protein bioplastics by a thermomoulding process: Effect of the mixing stage. J. Polym. Environ. 2019, 27, 968–978. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.-G.; Lee, E.Y. Alginate lyase: Structure, property and application. Biotechnol. Bioprocess Eng. 2011, 16, 843. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Su, S.; Qiu, J.; Wang, S. Ion-linked double-network hydrogel with high toughness and stiffness. J. Mater. Sci. 2015, 50, 5458–5465. [Google Scholar] [CrossRef]
- Tsai., C.-R.; Lin, Y.-K. Artificial steak: A 3D printable hydrogel composed of egg albumen, pea protein, gellan gum, sodium alginate and rice mill by-products. Future Foods 2022, 5, 100121. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm. Bowel Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef]
- Sarteshnizi, R.; Hosseini, H.; Mousavi Khaneghah, A.; Karimi, N. A review on application of hydrocolloids in meat and poultry products. Int. Food Res. J. 2015, 22, 872–887. [Google Scholar]
- Setiadi, S.W.I.; Alisha, N. The influences of transglutaminase enzyme dosage on the meat characteristic from restructuring the animal and vegetable protein sources. E3S Web Conf. 3rd Int. Trop. Renew. Energy Conf. 2018, 67, 03043. [Google Scholar] [CrossRef]
- Tunnarut, D.; Nopwinyuwong, A.; Tanakamolpradit, T. Quality improvement of plant-based patty using methylcellulose, κ-carrageenan and xanthan gum. JCST 2022, 12, 327–335. [Google Scholar] [CrossRef]
- Ruiz de Huidobro, F.; Miguel, E.; Blázquez, B.; Onega, E. A comparison between two methods (Warner-Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef]
- Szczesniak, A. Classification of textural characteristics. J. Food Sci. 1963, 29, 385–389. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3D printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liang, X.; Saeed, A.; Lan, W.; Qin, W. 2019. Properties of 3D printed dough and optimization of printing parameters. Innov. Food Sci. Emerg. Technol. 2019, 54, 9–18. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Devahastin, S. 3D extrusion-based printability evaluation of selected cereal grains by computational fluid dynamic simulation. J. Food Eng. 2020, 286, 110113. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Rossa, P.N.; de Sá, E.M.F.; Burin, V.M.; Bordignon-Luiz, M.T. Optimization of microbial transglutaminase activity in ice cream using response surface methodology. LWT-Food Sci. Technol. 2011, 44, 29–34. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bandyopadhyay, P. Polysaccharide-protein interactions and their relevance in food colloids. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; IntechOpen: London, UK, 2012; pp. 395–408. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zhang, M.; Bhandari, B.; Liu, Y. Investigation on lemon juice gel as food material for 3D printing and optimization of printing parameters. LWT-Food Sci. Technol. 2018, 87, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Samaranayaka, A.G.P.; Pegg, R.B. Maillard reaction and browning. In Encyclopedia of Meat Sciences, 2nd ed.; Dikeman, M., Devine, C., Eds.; Academic Press: Amsterdam, Netherlands, 2014; pp. 391–403. [Google Scholar]
- Hiller, B.; Lorenzen, P.C. Effect of phosphatase/transglutaminase treatment on molar mass distribution and techno-functional properties of sodium caseinate. LWT-Food Sci. Technol. 2009, 42, 87–92. [Google Scholar] [CrossRef]
- Pietrasik, Z. Binding and textural properties of beef gels processed with κ-carrageenan, egg albumin and microbial transglutaminase. Meat Sci. 2003, 63, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Ramsey, S.R. Rheological and water-holding properties of gelled meat batters containing iota carrageenan, kappa carrageenan or xanthan gum. J. Food Sci. 1987, 52, 549–553. [Google Scholar] [CrossRef]
- Vu, G.; Zhou, H.; McClements, D.J. Impact of cooking method on properties of beef and plant-based burgers: Appearance, texture, thermal properties and shrinkage. J. Agric. Food Res. 2022, 9, 100355. [Google Scholar] [CrossRef]
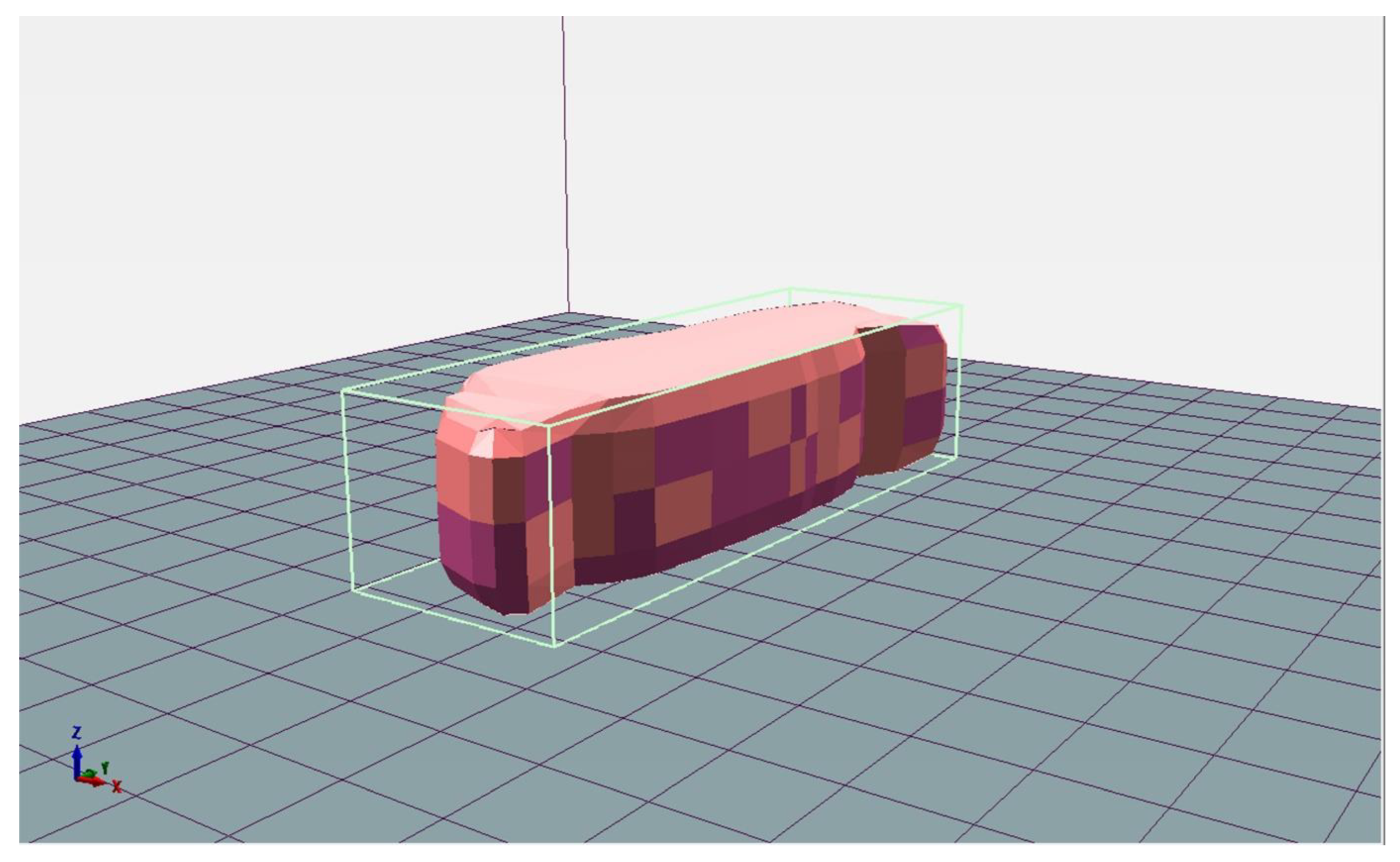

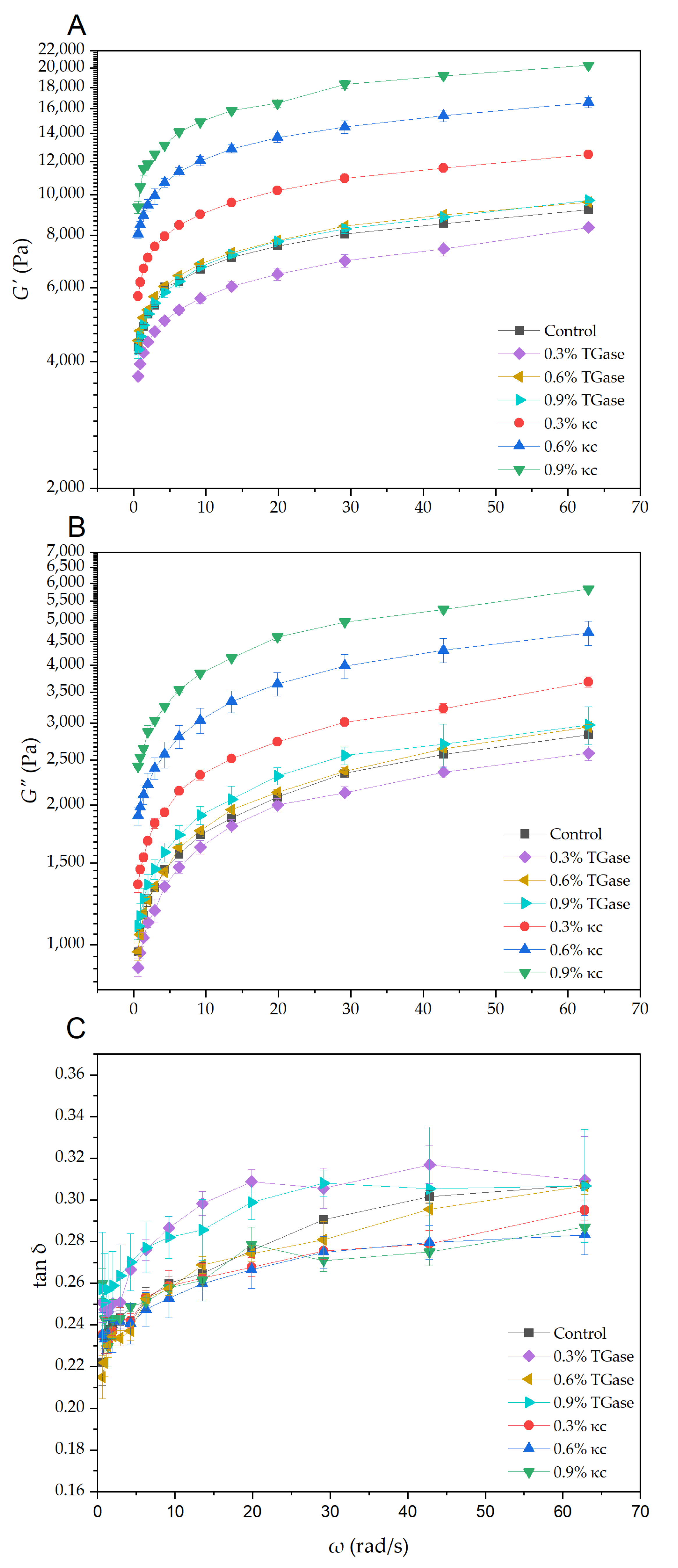

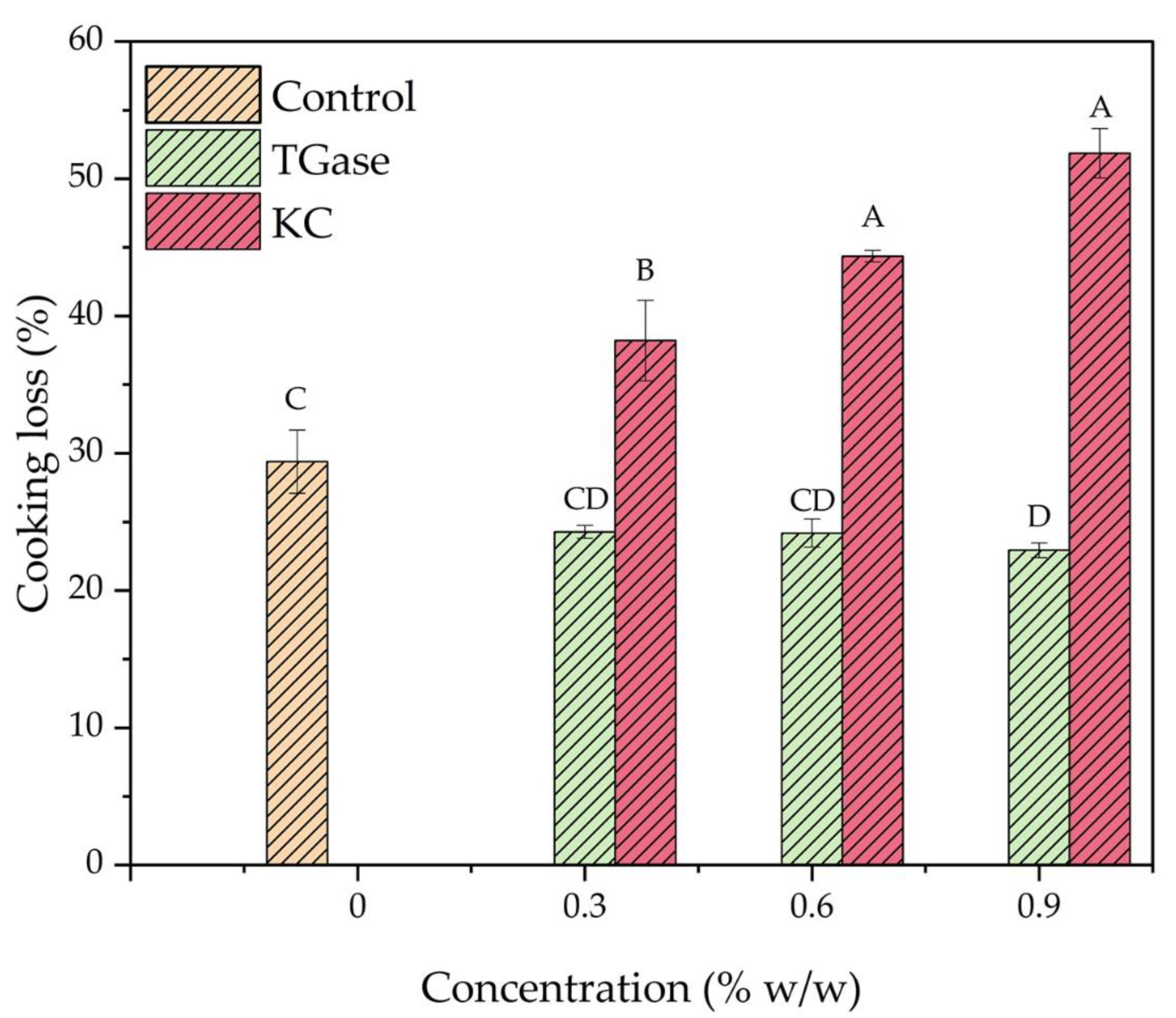
| Sample | Hardness (N) | Chewiness (N) | Springiness | Adhesiveness (N·s) | Cohesiveness |
|---|---|---|---|---|---|
| Control | 7.30 ± 1.19 e | 173.08 ± 20.01 c | 0.49 ± 0.04 a | −17.22 ± 4.11 d | 0.47 ± 0.02 a |
| 0.3% TGase | 8.10 ± 1.01 e | 57.38 ± 17.67 c | 0.24 ± 0.05 b | −9.64 ± 1.71 c | 0.29 ± 0.03 b |
| 0.6% TGase | 8.74 ± 0.61 de | 51.34 ± 8.71 c | 0.23 ± 0.03 b | −7.84 ± 3.05 bc | 0.25 ± 0.03 bc |
| 0.9% TGase | 10.48 ± 1.09 de | 61.27 ± 7.26 c | 0.25 ± 0.20 b | −6.20 ± 2.02 bc | 0.23 ± 0.04 bc |
| 0.3% κc | 6.23 ± 0.66 e | 41.96 ± 5.38 c | 0.24 ± 0.02 b | −6.67 ± 1.01 bc | 0.28 ± 0.01 b |
| 0.6% κc | 5.51 ± 0.56 e | 31.75 ± 8.67 c | 0.21 ± 0.03 b | −5.71 ± 0.42 bc | 0.27 ± 0.04 b |
| 0.9% κc | 5.76 ± 0.53 e | 27.83 ± 14.79 c | 0.19 ± 0.07 b | −4.43 ± 0.72 ab | 0.24 ± 0.04 bc |
| Pork tenderloin | 149.89 ± 11.78 a | 1573.03 ± 275.68 a | 0.48 ± 0.13 b | −0.67 ± 0.16 a | 0.25 ± 0.09 b |
| Chicken breast | 79.06 ± 8.41 b | 515.65 ± 135.69 b | 0.34 ± 0.04 b | −0.91 ± 0.25 a | 0.17 ± 0.03 cd |
| Salmon | 16.45 ± 1.81 d | 49.82 ± 11.41 c | 0.26 ± 0.05 b | −0.97 ± 0.73 a | 0.11 ± 0.01 d |
| Spanish mackerel | 25.38 ± 2.19 c | 109.97 ± 32.84 c | 0.25 ± 0.08 b | −3.76 ± 0.65 ab | 0.15 ± 0.02 d |
| Sample | Hardness (N) | Chewiness (N) | Springiness | Adhesiveness (N·s) | Cohesiveness |
|---|---|---|---|---|---|
| Control | 20.07 ± 0.95 e | 150.04 ± 31.53 d | 0.34 ± 0.07 a | −2.65 ± 0.87 cd | 0.22 ± 0.02 d |
| 0.3% TGase | 11.32 ± 2.48 e | 59.32 ± 19.50 d | 0.20 ± 0.04 b | −2.04 ± 0.99 bcd | 0.21 ± 0.02 d |
| 0.6% TGase | 15.59 ± 2.50 e | 63.68 ± 18.34 d | 0.21 ± 0.03 ab | −1.90 ± 0.50 bcd | 0.21 ± 0.02 d |
| 0.9% TGase | 19.11 ± 1.36 e | 87.58 ± 10.86 d | 0.26 ± 0.02 ab | −1.83 ± 0.93 bcd | 0.17 ± 0.01 d |
| 0.3% κc | 19.48 ± 0.78 e | 68.17 ± 12.01 d | 0.19 ± 0.03 b | −1.21 ± 0.41 abc | 0.18 ± 0.02 d |
| 0.6% κc | 16.44 ± 1.28 e | 57.31 ± 12.18 d | 0.18 ± 0.01 b | −1.55 ± 0.85 bc | 0.19 ± 0.05 d |
| 0.9% κc | 37.14 ± 3.85 de | 156.16 ± 18.29 d | 0.21 ± 0.05 ab | −1.26 ± 0.13 abc | 0.21 ± 0.04 d |
| Pork tenderloin | 246.42 ± 30.89 a | 6510.83 ± 1509.93 a | 0.49 ± 0.07 ab | −1.59 ± 0.89 bc | 0.51 ± 0.02 a |
| Chicken breast | 175.71 ± 10.18 b | 2360.66 ± 385.27 c | 0.41 ± 0.06 ab | −3.15 ± 0.91 d | 0.29 ± 0.02 c |
| Salmon | 47.55 ± 10.84 d | 932.36 ± 567.65 cd | 0.42 ± 0.10 ab | −0.88 ± 0.33 ab | 0.41 ± 0.06 b |
| Spanish mackerel | 149.02 ± 2.70 c | 4241.37 ± 727.12 b | 0.25 ± 0.08 ab | −0.02 ± 0.02 a | 0.49 ± 0.03 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leelapunnawut, S.; Ngamwonglumlert, L.; Devahastin, S.; Derossi, A.; Caporizzi, R.; Chiewchan, N. Effects of Texture Modifiers on Physicochemical Properties of 3D-Printed Meat Mimics from Pea Protein Isolate-Alginate Gel Mixture. Foods 2022, 11, 3947. https://doi.org/10.3390/foods11243947
Leelapunnawut S, Ngamwonglumlert L, Devahastin S, Derossi A, Caporizzi R, Chiewchan N. Effects of Texture Modifiers on Physicochemical Properties of 3D-Printed Meat Mimics from Pea Protein Isolate-Alginate Gel Mixture. Foods. 2022; 11(24):3947. https://doi.org/10.3390/foods11243947
Chicago/Turabian StyleLeelapunnawut, Supanut, Luxsika Ngamwonglumlert, Sakamon Devahastin, Antonio Derossi, Rossella Caporizzi, and Naphaporn Chiewchan. 2022. "Effects of Texture Modifiers on Physicochemical Properties of 3D-Printed Meat Mimics from Pea Protein Isolate-Alginate Gel Mixture" Foods 11, no. 24: 3947. https://doi.org/10.3390/foods11243947





