Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review
Abstract
:1. Introduction
2. Nutritional Value of Insects
2.1. Proximate Composition of Matter of Selected Insects
2.2. The Amino Acid Composition of Insect Proteins
2.3. Insect Fat
2.4. Vitamins and Minerals
2.5. Other Components
3. Biological Functions of Insect Active Ingredients
3.1. Anti-Cancer Effect

3.2. Antioxidant Activity
3.3. Antibacterial Activity and Effect on Intestinal Microorganisms
3.4. Anti-Inflammatory Activity
3.5. Regulation of Blood Lipids and Blood Glucose as Well as Anti-Obesity and Anti-Diabetic Activity
3.6. Hypotensive Effect
3.7. Immunomodulatory Effects
3.8. Angiogenesis Inhibition
3.9. The Therapeutic Effects of Several Common Diseases
3.9.1. Alzheimer’s Disease (AD) Therapy Effects
3.9.2. Therapeutic Effects of Parkinson’s Disease (PD)
3.9.3. The Therapeutic Effect of Gastric Ulcers
3.9.4. Therapeutic Effects of Atherosclerosis
3.9.5. Anti-HIV
3.9.6. Therapeutic Effects of External Trauma
3.10. Other Functions
4. Factors to Consider in the Consumption of Edible Insects
4.1. Acceptance of Insects
4.2. The Difference between Wild and Farmed Insects
4.3. Novel Strategies for Farming Insects
4.4. Sustainability of Edible Insects
4.5. The Safety of Edible Insects
4.5.1. Allergic Reactions
4.5.2. Contamination by Pathogenic Microorganisms
4.5.3. Pesticide Residues
4.5.4. Heavy Metal Content Exceeds the Standard
4.5.5. Other Security Concerns
4.6. Processing of Edible Insects
4.7. Purity and Stability of Insect Extract Components
5. Edible Insects and Human Life
6. Current Legislation on Edible Insects
7. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos-Elorduy, J. Anthropo-entomophagy: Cultures, evolution and sustainability. Entomol. Res. 2009, 39, 271–288. [Google Scholar] [CrossRef]
- Barennes, H.; Phimmasane, M.; Rajaonarivo, C. Insect Consumption to Address Undernutrition, a National Survey on the Prevalence of Insect Consumption among Adults and Vendors in Laos. PLoS ONE 2015, 10, e0136458. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Orkusz, A. Edible Insects versus Meat—Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- Liceaga, A.M.; Aguilar-Toalá, J.E.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Insects as an Alternative Protein Source. Annu. Rev. Food Sci. Technol. 2022, 13, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- van Huis, A. Edible Insects. In Handbook of Eating and Drinking: Interdisciplinary Perspectives; Meiselman, H.L., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 965–980. [Google Scholar]
- Sosa, D.A.T.; Fogliano, V. Potential of Insect-Derived Ingredients for Food Applications. In Insect Physiology and Ecology; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yang, D.; Liao, H.; Sun, H.; Liu, C.; Wei, L.; Li, F. Edible insects as a food source: A review. Food Prod. Process. Nutr. 2019, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Azambuja, P.; Mello, C.B. Recent Advances in Developing Insect Natural Products as Potential Modern Day Medicines. Evid. Based Complement. Altern. Med. 2014, 2014, 904958. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, N.A.; Mello, C.B.; Garcia, E.S.; Butt, T.M.; Azambuja, P. Insect natural products and processes: New treatments for human disease. Insect Biochem. Mol. Biol. 2011, 41, 747–769. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, M.; He, Z.; Chen, Z.; Sun, L. Research and utilization of medicinal insects in China. Entomol. Res. 2009, 39, 313–316. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Nutrition and health of edible insects. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on insects as food and feed: A global comparison. J. Insects Food Feed. 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Legendre, T.S.; Baker, M.A. Legitimizing Edible Insects for Human Consumption: The Impacts of Trust, Risk–Benefit, and Purchase Activism. J. Hosp. Tour. Res. 2020, 46, 467–489. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Kromhout, D.; Spaaij, C.J.K.; de Goede, J.; Weggemans, R.M.; Committee Dutch Dietary Guidelines. The 2015 Dutch food-based dietary guidelines. Eur. J. Clin. Nutr. 2016, 70, 869–878. [Google Scholar] [CrossRef]
- Payne, C.L.R.; Scarborough, P.; Rayner, M.; Nonaka, K. Are edible insects more or less ‘healthy’ than commonly consumed meats? A comparison using two nutrient profiling models developed to combat over- and undernutrition. Eur. J. Clin. Nutr. 2016, 70, 285–291. [Google Scholar] [CrossRef]
- Elemo, B.O.; Elemo, G.N.; Makinde, M.A.; Erukainure, O.L. Chemical evaluation of African palm weevil, Rhychophorus phoenicis, larvae as a food source. J. Insect Sci. 2011, 11, 146. [Google Scholar] [CrossRef]
- Fogang Mba, A.R.; Kansci, G.; Viau, M.; Ribourg, L.; Fogoh Muafor, J.; Hafnaoui, N.; Le Gall, P.; Genot, C. Growing conditions and morphotypes of African palm weevil (Rhynchophorus phoenicis) larvae influence their lipophilic nutrient but not their amino acid compositions. J. Food Compos. Anal. 2018, 69, 87–97. [Google Scholar] [CrossRef]
- Chinarak, K.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Chaijan, M. Improved long-chain omega-3 polyunsaturated fatty acids in sago palm weevil (Rhynchophorus ferrugineus) larvae by dietary fish oil supplementation. Food Chem. 2022, 393, 133354. [Google Scholar] [CrossRef] [PubMed]
- Chinarak, K.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Chaijan, M. A Novel Strategy for the Production of Edible Insects: Effect of Dietary Perilla Seed Supplementation on Nutritional Composition, Growth Performance, Lipid Metabolism, and Δ6 Desaturase Gene Expression of Sago Palm Weevil (Rhynchophorus ferrugineus) Larvae. Foods 2022, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Akande, O.A.; Falade, O.O.; Badejo, A.A.; Adekoya, I. Assessment of Mulberry Silkworm Pupae and African Palm Weevil larvae as alternative protein sources in snack fillings. Heliyon 2020, 6, e03754. [Google Scholar] [CrossRef]
- Ayensu, J.; Lutterodt, H.; Annan, R.A.; Edusei, A.; Loh, S.P. Nutritional composition and acceptability of biscuits fortified with palm weevil larvae (Rhynchophorus phoenicis Fabricius) and orange-fleshed sweet potato among pregnant women. Food Sci. Nutr. 2019, 7, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The potential of insects as food sources–A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3642–3652. [Google Scholar] [CrossRef]
- Zimian, D.; Yonghua, Z.; Xiwu, G. Medicinal insects in China. Ecol. Food Nutr. 1997, 36, 209–220. [Google Scholar] [CrossRef]
- Chantawannakul, P. From entomophagy to entomotherapy. Front. Biosci. Landmark 2020, 25, 179–200. [Google Scholar] [CrossRef]
- Williams, J.P.; Williams, J.R.; Kirabo, A.; Chester, D.; Peterson, M. Chapter 3-Nutrient Content and Health Benefits of Insects. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 61–84. [Google Scholar]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Zhou, J.; Han, D. Proximate, amino acid and mineral composition of pupae of the silkworm Antheraea pernyi in China. J. Food Compos. Anal. 2006, 19, 850–853. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.-J.; Xu, M.-L.; Shi, S.-S. Soybean hawkmoth (Clanis bilineata tsingtauica) as food ingredients: A review. CyTA J. Food 2021, 19, 341–348. [Google Scholar] [CrossRef]
- Pérez-Ramírez, R.; Torres-Castillo, J.A.; Barrientos-Lozano, L.; Almaguer-Sierra, P.; Torres-Acosta, R.I. Schistocerca piceifrons piceifrons (Orthoptera: Acrididae) as a Source of Compounds of Biotechnological and Nutritional Interest. J. Insect Sci. 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Vanqa, N.; Mshayisa, V.V.; Basitere, M. Proximate, Physicochemical, Techno-Functional and Antioxidant Properties of Three Edible Insect (Gonimbrasia belina, Hermetia illucens and Macrotermes subhylanus) Flours. Foods 2022, 11, 976. [Google Scholar] [CrossRef]
- Payne, C.L.R.; Scarborough, P.; Rayner, M.; Nonaka, K. A systematic review of nutrient composition data available for twelve commercially available edible insects, and comparison with reference values. Trends Food Sci. Technol. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Banjo, A.D.; Lawal, O.A.; Songonuga, E.A. The nutritional value of fourteen species of edible insects in southwestern Nigeria. Afr. J. Biotechnol. 2006, 5, 298–301. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Siulapwa, N.; Mwambungu, A.; Lungu, E.; Sichilima, W. Nutritional value of four common edible insects in Zambia. Int. J. Sci. Res. 2014, 3, 876–884. [Google Scholar]
- Rapatsa, M.M.; Moyo, N.A.G. Evaluation of Imbrasia belina meal as a fishmeal substitute in Oreochromis mossambicus diets: Growth performance, histological analysis and enzyme activity. Aquac. Rep. 2017, 5, 18–26. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of frozen and dried formulations from migratory locust (Locusta migratoria) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06667. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of frozen and freeze-dried formulations of the lesser mealworm (Alphitobius diaperinus larva) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07325. [Google Scholar] [CrossRef] [PubMed]
- Chinarak, K.; Chaijan, M.; Panpipat, W. Farm-raised sago palm weevil (Rhynchophorus ferrugineus) larvae: Potential and challenges for promising source of nutrients. J. Food Compos. Anal. 2020, 92, 103542. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of four species of feeder insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Y.; Zhang, H.; Chen, Z. Review of the Nutritive Value of Edible Insects; Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 2010; pp. 85–92. [Google Scholar]
- Liceaga, A.M. Chapter Four-Edible insects, a valuable protein source from ancient to modern times. In Advances in Food and Nutrition Research; Wu, J., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 101, pp. 129–152. [Google Scholar]
- Schlüter, O.; Rumpold, B.; Holzhauser, T.; Roth, A.; Vogel, R.F.; Quasigroch, W.; Vogel, S.; Heinz, V.; Jäger, H.; Bandick, N.; et al. Safety aspects of the production of foods and food ingredients from insects. Mol. Nutr. Food Res. 2017, 61, 1600520. [Google Scholar] [CrossRef]
- Bukkens, S.G.F. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Tang, Y.; Debnath, T.; Choi, E.-J.; Kim, Y.W.; Ryu, J.P.; Jang, S.; Chung, S.U.; Choi, Y.-J.; Kim, E.-K. Changes in the amino acid profiles and free radical scavenging activities of Tenebrio molitor larvae following enzymatic hydrolysis. PLoS ONE 2018, 13, e0196218. [Google Scholar] [CrossRef] [PubMed]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species-Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Energy Supplied by Edible Insects from Mexico and their Nutritional and Ecological Importance. Ecol. Food Nutr. 2008, 47, 280–297. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M. A Comprehensive Look at the Possibilities of Edible Insects as Food in Europe—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Roos, N. Insects and Human Nutrition. In Edible Insects in Sustainable Food Systems; Halloran, A., Flore, R., Vantomme, P., Roos, N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 83–91. [Google Scholar]
- Lee, H.; Park, W.J. Unsaturated Fatty Acids, Desaturases, and Human Health. J. Med. Food 2014, 17, 189–197. [Google Scholar] [CrossRef] [PubMed]
- DeFoliart, G.R. Insect fatty acids: Similar to those of poultry and fish in their degree of unsaturation, but higher in the polyunsaturates. Food Insects Newsl. 1991, 4, 13907994. [Google Scholar]
- de Castro, R.J.S.; Ohara, A.; Aguilar, J.G.d.S.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Oranut, S.; Subhachai, B.; Shen, L.-r.; Li, D. Lipids and Fatty Acid Composition of Dried Edible Red and Black Ants. Agric. Sci. China 2010, 9, 1072–1077. [Google Scholar] [CrossRef]
- Kinyuru, J.N.; Mogendi, J.B.; Riwa, C.A.; Ndung’u, N.W. Edible insects—A novel source of essential nutrients for human diet: Learning from traditional knowledge. Anim. Front. 2015, 5, 14–19. [Google Scholar] [CrossRef]
- Fritz, J.; Walia, C.; Elkadri, A.; Pipkorn, R.; Dunn, R.K.; Sieracki, R.; Goday, P.S.; Cabrera, J.M. A Systematic Review of Micronutrient Deficiencies in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Rempel, J.; Grover, K.; El-Matary, W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients 2021, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Haddad, L.; Hawkes, C.; Webb, P.; Thomas, S.; Beddington, J.; Waage, J.; Flynn, D. A new global research agenda for food. Nature 2016, 540, 30–32. [Google Scholar] [CrossRef]
- Hicks, C.C.; Cohen, P.J.; Graham, N.A.J.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 2019, 574, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, N.; Van Huis, A. Consuming insects: Are there health benefits? J. Insects Food Feed. 2017, 3, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Call, L.-M.; Macheiner, L.; Mayer, H.K. Determination of vitamin B12 in four edible insect species by immunoaffinity and ultra-high performance liquid chromatography. Food Chem. 2019, 281, 124–129. [Google Scholar] [CrossRef]
- Okamoto, N.; Nagao, F.; Umebayashi, Y.; Bito, T.; Prangthip, P.; Watanabe, F. Pseudovitamin B12 and factor S are the predominant corrinoid compounds in edible cricket products. Food Chem. 2021, 347, 129048. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Dierenfeld, E.S. An Investigation into the Chemical Composition of Alternative Invertebrate Prey. Zoo Biol. 2012, 31, 40–54. [Google Scholar] [CrossRef]
- Kinyuru, J.N.; Kenji, G.M.; Njoroge, S.M.; Ayieko, M. Effect of Processing Methods on the In Vitro Protein Digestibility and Vitamin Content of Edible Winged Termite (Macrotermes subhylanus) and Grasshopper (Ruspolia differens). Food Bioprocess Technol. 2010, 3, 778–782. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.A.B.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; van Loon, J.J.A. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Assoc. J. 2005, 173, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Mølgaard, C.; Girma, T.; Friis, H. Choice of Foods and Ingredients for Moderately Malnourished Children 6 Months to 5 Years of Age. Food Nutr. Bull. 2009, 30, S343–S404. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.L.; Orech, F.O.; Mungai, M.N.; Larsen, T.; Friis, H.; Aagaard-Hansen, J. Entomophagy among the Luo of Kenya: A potential mineral source? Int. J. Food Sci. Nutr. 2006, 57, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Yang, W.; Vera Aviles, M. In Vitro Iron Availability from Insects and Sirloin Beef. J. Agric. Food Chem. 2016, 64, 8420–8424. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-K.; Yong, H.I.; Kim, Y.-B.; Kim, H.-W.; Choi, Y.-S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Deng, P.; Chen, F.; Cao, Y.; Sun, H.; Liao, H. The exploration and utilization of functional substances in edible insects: A review. Food Prod. Process. Nutr. 2022, 4, 11. [Google Scholar] [CrossRef]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan production with larval exoskeletons derived from the insect protein production. J. Biotechnol. 2020, 310, 62–67. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leão Gouveia Costa, H.; Martín López, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Sharma, A.K.; Sandhu, S.S.; Kashyap, D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013, 93, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.-K.; Jeong, C.H.; Yong, H.I.; Cha, J.Y.; Kim, B.-K.; Choi, Y.-S. Biological activity and processing technologies of edible insects: A review. Food Sci. Biotechnol. 2021, 30, 1003–1023. [Google Scholar] [CrossRef]
- Chudzinski-Tavassi, A.M.; De-Sá-Júnior, P.L.; Simons, S.M.; Maria, D.A.; de Souza Ventura, J.; de Fátima Correia Batista, I.; Faria, F.; Durães, E.; Reis, E.M.; Demasi, M. A new tick Kunitz type inhibitor, Amblyomin-X, induces tumor cell death by modulating genes related to the cell cycle and targeting the ubiquitin-proteasome system. Toxicon 2010, 56, 1145–1154. [Google Scholar] [CrossRef]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H.; Ahn, Y.J.; Kim, J.Y.; Song, M.J.; Song, H.S.; et al. Cancer Cell Growth Inhibitory Effect of Bee Venom via Increase of Death Receptor 3 Expression and Inactivation of NF-kappa B in NSCLC Cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Kim, B.J.; Kim, H.J.; Jin, J.M.; Yoon, H.J.; Hwang, J.S.; Park, K.-K. Anti-cancer effect of dung beetle glycosaminoglycans on melanoma. BMC Cancer 2019, 19, 9. [Google Scholar] [CrossRef]
- Wang, F.-x.; Wu, N.; Wei, J.-t.; Liu, J.; Zhao, J.; Ji, A.-g.; Lin, X.-k. A novel protein from Eupolyphaga sinensis inhibits adhesion, migration, and invasion of human lung cancer A549 cells. Biochem. Cell Biol. 2013, 91, 244–251. [Google Scholar] [CrossRef]
- Hu, D.; Liu, Q.; Cui, H.; Wang, H.; Han, D.; Xu, H. Effects of amino acids from selenium-rich silkworm pupas on human hepatoma cells. Life Sci. 2005, 77, 2098–2110. [Google Scholar] [CrossRef]
- Ji, X.; Wang, J.; Ma, A.; Feng, D.; He, Y.; Yan, W. Effects of silkworm pupa protein on apoptosis and energy metabolism in human colon cancer DLD-1 cells. Food Sci. Hum. Wellness 2022, 11, 1171–1176. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Chen, Y.; Lang, M.; Chen, Y.; Shi, L. Silkworm Pupa Protein Hydrolysate Induces Mitochondria-Dependent Apoptosis and S Phase Cell Cycle Arrest in Human Gastric Cancer SGC-7901 Cells. Int. J. Mol. Sci. 2018, 19, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weixin, L.; Lixia, M.; Leiyan, W.; Yuxiao, Z.; Haifeng, Z.; Sentai, L. Effects of silkworm pupa protein hydrolysates on mitochondrial substructure and metabolism in gastric cancer cells. J. Asia Pac. Entomol. 2019, 22, 387–392. [Google Scholar] [CrossRef]
- Carneiro-Lobo, T.C.; Konig, S.; Machado, D.E.; Nasciutti, L.E.; Forni, M.F.; Francischetti, I.M.B.; Sogayar, M.C.; Monteiro, R.Q. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J. Thromb. Haemost. 2009, 7, 1855–1864. [Google Scholar] [CrossRef]
- Chukiatsiri, S.; Siriwong, S.; Thumanu, K. Pupae protein extracts exert anticancer effects by downregulating the expression of IL-6, IL-1β and TNF-α through biomolecular changes in human breast cancer cells. Biomed. Pharmacother. 2020, 128, 110278. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sargin, I.; Sabeckis, I.; Noreikaite, D.; Erdonmez, D.; Salaberria, A.M.; Labidi, J.; Baublys, V.; Tubelytė, V. Biological, mechanical, optical and physicochemical properties of natural chitin films obtained from the dorsal pronotum and the wing of cockroach. Carbohydr. Polym. 2017, 163, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Socarras, K.M.; Theophilus, P.A.S.; Torres, J.P.; Gupta, K.; Sapi, E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, expression, purification and functional characterization of novel antimicrobial peptide genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saviane, A.; Romoli, O.; Bozzato, A.; Freddi, G.; Cappelletti, C.; Rosini, E.; Cappellozza, S.; Tettamanti, G.; Sandrelli, F. Intrinsic antimicrobial properties of silk spun by genetically modified silkworm strains. Transgenic Res. 2018, 27, 87–101. [Google Scholar] [CrossRef]
- Nenadić, M.; Soković, M.; Glamočlija, J.; Ćirić, A.; Perić-Mataruga, V.; Ilijin, L.; Tešević, V.; Todosijević, M.; Vujisić, L.; Vesović, N.; et al. The pygidial gland secretion of the forest caterpillar hunter, Calosoma (Calosoma) sycophanta: The antimicrobial properties against human pathogens. Appl. Microbiol. Biotechnol. 2017, 101, 977–985. [Google Scholar] [CrossRef]
- Bílikova, K.; Huang, S.-C.; Lin, I.P.; Šimuth, J.; Peng, C.-C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef]
- Battampara, P.; Nimisha Sathish, T.; Reddy, R.; Guna, V.; Nagananda, G.S.; Reddy, N.; Ramesha, B.S.; Maharaddi, V.H.; Rao, A.P.; Ravikumar, H.N.; et al. Properties of chitin and chitosan extracted from silkworm pupae and egg shells. Int. J. Biol. Macromol. 2020, 161, 1296–1304. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Mizell, R.F. An insect pupal cell with antimicrobial properties that suppress an entomopathogenic fungus. J. Invertebr. Pathol. 2015, 124, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.R.; Gauri, S.S.; Ghosh, T.; Halder, S.K.; DasMohapatra, P.K.; Mondal, K.C.; Ghosh, A.K. Elucidation of structural and functional integration of a novel antimicrobial peptide from Antheraea mylitta. Bioorganic Med. Chem. Lett. 2017, 27, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ray, M.; Gangopadhyay, D. Evaluation of proximate composition and antioxidant properties in silk-industrial byproduct. LWT 2020, 132, 109900. [Google Scholar] [CrossRef]
- Wu, S.; Lu, M.; Wang, S. Antiageing activities of water-soluble chitosan from Clanis bilineata larvae. Int. J. Biol. Macromol. 2017, 102, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xu, X.; Zhang, Q.; Rui, X.; Wu, J.; Dong, M. Ultrasonic-assisted Aqueous Extraction and Physicochemical Characterization of Oil from Clanis bilineata. J. Oleo Sci. 2018, 67, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Jena, K.; Pandey, J.P.; Kumari, R.; Sinha, A.K.; Gupta, V.P.; Singh, G.P. Free radical scavenging potential of sericin obtained from various ecoraces of tasar cocoons and its cosmeceuticals implication. Int. J. Biol. Macromol. 2018, 120, 255–262. [Google Scholar] [CrossRef]
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Long, X.; Song, J.; Zhao, X.; Zhang, Y.; Wang, H.; Liu, X.; Suo, H. Silkworm pupa oil attenuates acetaminophen-induced acute liver injury by inhibiting oxidative stress-mediated NF-κB signaling. Food Sci. Nutr. 2020, 8, 237–245. [Google Scholar] [CrossRef]
- Ali, M.M.; Arumugam, S.B. Effect of crude extract of Bombyx mori coccoons in hyperlipidemia and atherosclerosis. J. Ayurveda Integr. Med. 2011, 2, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Ying, H.; Tong, F.; Zhang, C.; Quan, Y.; Zhang, Y. Protective effect of the silkworm protein 30Kc6 on human vascular endothelial cells damaged by oxidized low density lipoprotein (Ox-LDL). PLoS ONE 2013, 8, e68746. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, S.; Kim, S.I.; Bekker, G.; Pleskach, V.A.; Filatova, N.A.; Anikin, V.B.; Platonov, V.G.; Bulet, P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 2002, 99, 12628–12632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, M.Y.; Han, J.W.; Hwang, J.S.; Yun, E.Y.; Lee, B.M. Anti-inflammatory effect of glycosaminoglycan derived from Gryllus bimaculatus (a type of cricket, insect) on adjuvant-treated chronic arthritis rat model. J. Toxicol. Environ. Health Part A 2014, 77, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.-J.; Jin, X.-B.; Zhu, J.-Y. Housefly Maggots (Musca domestica) Protein-enriched Fraction/ extracts (PE) Inhibit Lipopolysaccharide-induced Atherosclerosis Pro-inflammatory Responses. J. Atheroscler. Thromb. 2011, 18, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotsyfakis, M.; Sá-Nunes, A.; Francischetti, I.M.B.; Mather, T.N.; Andersen, J.F.; Ribeiro, J.M.C. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J. Biol. Chem. 2006, 281, 26298–26307. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.-J.; Fang, P.; Xia, H.-L.; Tu, Z.-C.; Hou, B.-Y.; Yan, Y.-M.; Di, L.; Zhang, L.; Cheng, Y.-X. Constituents from the edible Chinese black ants (Polyrhachis dives) showing protective effect on rat mesangial cells and anti-inflammatory activity. Food Res. Int. 2015, 67, 163–168. [Google Scholar] [CrossRef]
- Danneels, E.L.; Gerlo, S.; Heyninck, K.; Van Craenenbroeck, K.; De Bosscher, K.; Haegeman, G.; de Graaf, D.C. How the venom from the ectoparasitoid wasp Nasonia vitripennis exhibits anti-inflammatory properties on mammalian cell lines. PLoS ONE 2014, 9, e96825. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Palm, N.W.; Rosenstein, R.K.; Yu, S.; Schenten, D.D.; Florsheim, E.; Medzhitov, R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 2013, 39, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.F.Z.; Yasin, I.A.; Ohta, T.; Hashizume, A.; Ido, A.; Takahashi, T.; Miura, C.; Miura, T. The silkrose of Bombyx mori effectively prevents vibriosis in penaeid prawns via the activation of innate immunity. Sci. Rep. 2018, 8, 8836. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification, Identification and Functional Analysis of a Novel Immunomodulatory Peptide from Silkworm Pupa Protein. Int. J. Pept. Res. Ther. 2020, 26, 243–249. [Google Scholar] [CrossRef]
- Tszydel, M.; Zabłotni, A.; Wojciechowska, D.; Michalak, M.; Krucińska, I.; Szustakiewicz, K.; Maj, M.; Jaruszewska, A.; Strzelecki, J. Research on possible medical use of silk produced by caddisfly larvae of Hydropsyche angustipennis (Trichoptera, Insecta). J. Mech. Behav. Biomed. Mater. 2015, 45, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Hwang, J.S.; Kim, M.-J.; Park, K.-K. Antilipidemic effects and gene expression profiling of the glycosaminoglycans from cricket in rats on a high fat diet. Arch. Pharmacal Res. 2016, 39, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Lee, H.J.; Suh, H.J. Silk protein hydrolysate increases glucose uptake through up-regulation of GLUT 4 and reduces the expression of leptin in 3T3-L1 fibroblast. Nutr. Res. 2011, 31, 937–943. [Google Scholar] [CrossRef]
- Ryu, S.P. Silkworm pupae powder ingestion increases fat metabolism in swim-trained rats. J. Exerc. Nutr. Biochem. 2014, 18, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Hyun, C.-K.; Kim, I.-Y.; Frost, S.C. Soluble Fibroin Enhances Insulin Sensitivity and Glucose Metabolism in 3T3-L1 Adipocytes. J. Nutr. 2004, 134, 3257–3263. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Park, D.; Yang, G.; Bae, D.-K.; Yang, Y.-H.; Kim, T.K.; Kim, D.; Kyung, J.; Yeon, S.; Koo, K.C.; et al. Silk and silkworm pupa peptides suppress adipogenesis in preadipocytes and fat accumulation in rats fed a high-fat diet. Eur. J. Nutr. 2012, 51, 1011–1019. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Zhang, Y. Antihypertensive properties on spontaneously hypertensive rats of peptide hydrolysates from silkworm pupae protein. Food Nutr. Sci. 2014, 5, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shen, S.; Chen, Q.; Tang, B.; He, G.; Ruan, H.; Das, U.N. Hydrolyzates of silkworm pupae (Bombyx mori) protein is a new source of angiotensin I-converting enzyme inhibitory peptides (ACEIP). Curr. Pharm. Biotechnol. 2008, 9, 307–314. [Google Scholar] [CrossRef]
- Fukumoto, S.; Sakaguchi, T.; You, M.; Xuan, X.; Fujisaki, K. Tick troponin I-like molecule is a potent inhibitor for angiogenesis. Microvasc. Res. 2006, 71, 218–221. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; Mather, T.N.; Ribeiro, J.M.C. Tick saliva is a potent inhibitor of endothelial cell proliferation and angiogenesis. Thromb. Haemost. 2005, 94, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izuta, H.; Shimazawa, M.; Tsuruma, K.; Araki, Y.; Mishima, S.; Hara, H. Bee products prevent VEGF-induced angiogenesis in human umbilical vein endothelial cells. BMC Complement. Altern. Med. 2009, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, D.H.; Kim, J.K.; Kim, J.Y.; Jeong, H.Y.; Keum, K.S.; Han, S.H.; Rho, Y.I.; Woo, W.H.; Jung, K.Y.; Choi, B.K.; et al. Anti-angiogenic activities of Cnidium officinale Makino and Tabanus bovinus. J. Ethnopharmacol. 2002, 81, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Tsuji, N.; Miyoshi, T.; Alim, M.A.; Huang, X.; Hatta, T.; Fujisaki, K. The Kunitz-like modulatory protein haemangin is vital for hard tick blood-feeding success. PLoS Pathog. 2009, 5, e1000497. [Google Scholar] [CrossRef] [Green Version]
- Baik, J.E.; Rhee, W.J. Anti-apoptotic effects of the alpha-helix domain of silkworm storage protein 1. Biotechnol. Bioprocess Eng. 2017, 22, 671–678. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, H.J.; Park, T.H. Inhibition of apoptosis by recombinant 30K protein originating from silkworm hemolymph. Biochem. Biophys. Res. Commun. 2003, 308, 523–528. [Google Scholar] [CrossRef]
- Deori, M.; Boruah, D.C.; Devi, D.; Devi, R. Antioxidant and antigenotoxic effects of pupae of the muga silkworm Antheraea assamensis. Food Biosci. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Majtan, J.; Majtan, V. Is manuka honey the best type of honey for wound care? J. Hosp. Infect. 2010, 74, 305–306. [Google Scholar] [CrossRef]
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 2010, 19, e73–e79. [Google Scholar] [CrossRef]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, Y.-Y.; Ju, W.-T.; Kim, K.-Y.; Kweon, H. Effects of silkworm and its by-products on muscle mass and exercise performance in ICR mice. Int. J. Ind. Entomol. 2019, 39, 34–38. [Google Scholar] [CrossRef]
- Vangsoe, M.T.; Joergensen, M.S.; Heckmann, L.-H.L.; Hansen, M. Effects of Insect Protein Supplementation during Resistance Training on Changes in Muscle Mass and Strength in Young Men. Nutrients 2018, 10, 335. [Google Scholar] [CrossRef] [Green Version]
- Han, S.M.; Hong, I.P.; Woo, S.O.; Chun, S.N.; Park, K.K.; Nicholls, Y.M.; Pak, S.C. The beneficial effects of honeybee-venom serum on facial wrinkles in humans. Clin. Interv. Aging 2015, 10, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, L.; Pastorelli, R.; Viti, C.; Gasco, L.; Parisi, G. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 2018, 487, 56–63. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [Green Version]
- Uzair, B.; Bushra, R.; Khan, B.A.; Zareen, S.; Fasim, F. Potential uses of venom proteins in treatment of HIV. Protein Pept. Lett. 2018, 25, 619–625. [Google Scholar] [CrossRef]
- Alvarez-Fischer, D.; Noelker, C.; Vulinović, F.; Grünewald, A.; Chevarin, C.; Klein, C.; Oertel, W.H.; Hirsch, E.C.; Michel, P.P.; Hartmann, A. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE 2013, 8, e61700. [Google Scholar] [CrossRef] [Green Version]
- Wattanathorn, J.; Muchimapura, S.; Boosel, A.; Kongpa, S.; Kaewrueng, W.; Tong-Un, T.; Wannanon, P.; Thukhammee, W. Silkworm Pupae Protect Against Alzheimer’s Disease. Am. J. Agric. Biol. Sci. 2012, 7, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Jin, Y.; Wang, C.; Chen, J.; Yu, W.; Jin, Y.; Lv, Z. Effects of a 15-amino-acid isoform of amyloid- β expressed by silkworm pupae on B6C3-Tg Alzheimer’s disease transgenic mice. J. Biotechnol. 2019, 296, 83–92. [Google Scholar] [CrossRef]
- Kwon, M.-G.; Kim, D.-S.; Lee, J.-H.; Park, S.-W.; Choo, Y.-K.; Han, Y.-S.; Kim, J.-S.; Hwang, K.-A.; Ko, K.; Ko, K. Isolation and analysis of natural compounds from silkworm pupae and effect of its extracts on alcohol detoxification. Entomol. Res. 2012, 42, 55–62. [Google Scholar] [CrossRef]
- Long, X.; Zhao, X.; Wang, W.; Zhang, Y.; Wang, H.; Liu, X.; Suo, H. Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J. Sci. Food Agric. 2019, 99, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Hwang, U.W. Beneficial Effects of Fermented Cricket Powder as a Hair Growth Promoting Agent in a Mice Model. J. Life Sci. 2022, 32, 196–201. [Google Scholar] [CrossRef]
- Tonk, M.; Vilcinskas, A.; Rahnamaeian, M. Insect antimicrobial peptides: Potential tools for the prevention of skin cancer. Appl. Microbiol. Biotechnol. 2016, 100, 7397–7405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Lv, D.; Wang, X.; Wang, Y.; Hou, C.; Gao, K.; Guo, X. Inhibitory effects of Bombyx mori antimicrobial peptide cecropins on esophageal cancer cells. Eur. J. Pharmacol. 2020, 887, 173434. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Paik, H.D. Anticancer and immunomodulatory activity of egg proteins and peptides: A review. Poult. Sci. 2019, 98, 6505–6516. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Shi, W.; Liu, X.; Zhao, X.; Zhang, Z. Anticancer Action and Mechanism of Ergosterol Peroxide from Paecilomyces cicadae Fermentation Broth. Int. J. Mol. Sci. 2018, 19, 3935. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, T.; Ishibashi, J.; Tanaka, H.; Sato, M.; Asaoka, A.; Taylor, D.; Yamakawa, M. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides 2009, 30, 660–668. [Google Scholar] [CrossRef]
- Kang, B.-R.; Kim, H.; Nam, S.-H.; Yun, E.-Y.; Kim, S.-R.; Ahn, M.-Y.; Chang, J.-S.; Hwang, J.-S. CopA3 peptide from Copris tripartitus induces apoptosis in human leukemia cells via a caspase-independent pathway. Bmb Rep. 2012, 45, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Saido-Sakanaka, H.; Ishibashi, J.; Momotani, E.; Amano, F.; Yamakawa, M. In vitro and in vivo activity of antimicrobial peptides synthesized based on the insect defensin. Peptides 2004, 25, 19–27. [Google Scholar] [CrossRef]
- Kim, I.-W.; Lee, J.H.; Kwon, Y.-N.; Yun, E.-Y.; Nam, S.-H.; Ahn, M.-Y.; Kang, D.-C.; Hwang, J.S. Anticancer activity of a synthetic peptide derived from harmoniasin, an antibacterial peptide from the ladybug Harmonia axyridis. Int. J. Oncol. 2013, 43, 622–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghouts, C.; Kunz, C.; Groner, B. Current strategies for the development of peptide-based anti-cancer therapeutics. J. Pept. Sci. 2005, 11, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Barbault, F.; Landon, C.; Guenneugues, M.; Meyer, J.-P.; Schott, V.; Dimarcq, J.-L.; Vovelle, F. Solution Structure of Alo-3: A New Knottin-Type Antifungal Peptide from the Insect Acrocinus longimanus. Biochemistry 2003, 42, 14434–14442. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-c.; Hao, D.-j.; Zhang, Q.; An, J.; Zhao, J.-j.; Chen, B.; Zhang, L.-l.; Yang, H. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother. Pharmacol. 2016, 78, 1113–1130. [Google Scholar] [CrossRef]
- Park, M.H.; Choi, M.S.; Kwak, D.H.; Oh, K.-W.; Yoon, D.Y.; Han, S.B.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate 2011, 71, 801–812. [Google Scholar] [CrossRef]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef]
- Ip, S.-W.; Chu, Y.-L.; Yu, C.-S.; Chen, P.-Y.; Ho, H.-C.; Yang, J.-S.; Huang, H.-Y.; Chueh, F.-S.; Lai, T.-Y.; Chung, J.-G. Bee venom induces apoptosis through intracellular Ca2+-modulated intrinsic death pathway in human bladder cancer cells. Int. J. Urol. 2012, 19, 61–70. [Google Scholar] [CrossRef]
- Moreno, M.; Giralt, E. Three Valuable Peptides from Bee and Wasp Venoms for Therapeutic and Biotechnological Use: Melittin, Apamin and Mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef] [Green Version]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Lu, H.; Tan, H.; Sun, N.; Wang, K.; He, R.; Luo, L.; Ma, H.; Li, Z. Caspase 3-mediated cytotoxicity of mealworm larvae (Tenebrio molitor) oil extract against human hepatocellular carcinoma and colorectal adenocarcinoma. J. Ethnopharmacol. 2020, 250, 112438. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Wang, Y.; Zhang, J.; Ouyang, X.; Peng, R.; Yang, J. Antitumor and immunostimulatory activity of a polysaccharide–protein complex from Scolopendra subspinipes mutilans L. Koch in tumor-bearing mice. Food Chem. Toxicol. 2012, 50, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [Green Version]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Foroudi, S.; Potter, A.S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking Orange Juice Increases Total Antioxidant Status and Decreases Lipid Peroxidation in Adults. J. Med. Food 2014, 17, 612–617. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Serafini, M.; Estruch, R.; Lamuela-Raventós, R.M.; Martínez-González, M.A.; Salas-Salvadó, J.; Fiol, M.; Lapetra, J.; Arós, F.; Covas, M.I.; et al. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: Evidence for a mechanism of antioxidant tuning. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1167–1174. [Google Scholar] [CrossRef]
- Li, H.; Inoue, A.; Taniguchi, S.; Yukutake, T.; Suyama, K.; Nose, T.; Maeda, I. Multifunctional biological activities of water extract of housefly larvae (Musca domestica). PharmaNutrition 2017, 5, 119–126. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Zhang, A.-J.; Li, X.; Zhang, J.-H.; Qin, Q.-L.; Wu, Y.-J. Antioxidant activities of protein hydrolysates obtained from the housefly larvae. Acta Biol. Hung. Acta Biol. Hung. 2016, 67, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.-Y.; Jia, J.-Q.; Tan, G.-X.; Xu, J.-L.; Gui, Z.-Z. Physicochemical properties of silkworm larvae protein isolate and gastrointestinal hydrolysate bioactivities. Afr. J. Biotechnol. 2011, 10, 6145–6153. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, S.; Liu, J.; Zou, Y.; Liao, S. Antioxidant Activity and Stability Study of Peptides from Enzymatically Hydrolyzed Male Silkmoth. J. Food Process. Preserv. 2017, 41, e13081. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [Green Version]
- Pattarayingsakul, W.; Nilavongse, A.; Reamtong, O.; Chittavanich, P.; Mungsantisuk, I.; Mathong, Y.; Prasitwuttisak, W.; Panbangred, W. Angiotensin-converting enzyme inhibitory and antioxidant peptides from digestion of larvae and pupae of Asian weaver ant, Oecophylla smaragdina, Fabricius. J. Sci. Food Agric. 2017, 97, 3133–3140. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and biological activities of chitosan from the larvae of housefly, Musca domestica. Carbohydr. Polym. 2008, 72, 419–423. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- D’Antonio, V.; Serafini, M.; Battista, N. Dietary modulation of oxidative stress from edible insects: A mini-review. Front. Nutr. 2021, 8, 642551. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Mitri, S.; Richard Foster, K. The Genotypic View of Social Interactions in Microbial Communities. Annu. Rev. Genet. 2013, 47, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between gut microbiota and antimicrobial peptides. Anim. Nutr. 2020, 6, 389–396. [Google Scholar] [CrossRef]
- Kim, S.R.; Hong, M.Y.; Park, S.W.; Choi, K.H.; Yun, E.Y.; Goo, T.W.; Kang, S.W.; Suh, H.J.; Kim, I.; Hwang, J.S. Characterization and cDNA cloning of a cecropin-like antimicrobial peptide, papiliocin, from the swallowtail butterfly, Papilio xuthus. Mol. Cells 2010, 29, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Quadroni, S.; Caramella, S.; Brivio, M.F. The Silkworm as a Source of Natural Antimicrobial Preparations: Efficacy on Various Bacterial Strains. Antibiotics 2021, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Cytryńska, M.; Mak, P.; Zdybicka-Barabas, A.; Suder, P.; Jakubowicz, T. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 2007, 28, 533–546. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Cytryńska, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Wiesner, J.; Twyman, R.M.; Zuchner, T.; Sadd, B.M.; Regoes, R.R.; Schmid-Hempel, P.; et al. Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteria. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150293. [Google Scholar] [CrossRef] [Green Version]
- Seufi, A.M.; Hafez, E.E.; Galal, F.H. Identification, phylogenetic analysis and expression profile of an anionic insect defensin gene, with antibacterial activity, from bacterial-challenged cotton leafworm, Spodoptera littoralis. BMC Mol. Biol. 2011, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Thirumalaisamy, G.; Malik, P.K.; Trivedi, S.; Kolte, A.P.; Bhatta, R. Effect of Long-Term Supplementation with Silkworm Pupae Oil on the Methane Yield, Ruminal Protozoa, and Archaea Community in Sheep. Front. Microbiol. 2022, 13, 780073. [Google Scholar] [CrossRef]
- Lanng, S.K.; Zhang, Y.; Christensen, K.R.; Hansen, A.K.; Nielsen, D.S.; Kot, W.; Bertram, H.C. Partial Substitution of Meat with Insect (Alphitobius diaperinus) in a Carnivore Diet Changes the Gut Microbiome and Metabolome of Healthy Rats. Foods 2021, 10, 1814. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Walton, G.E.; Poveda, C.G.; Silva, S.N.; Amorim, M.; Madureira, A.R.; Pintado, M.E.; Gibson, G.R.; Jauregi, P. Study of in vitro digestion of Tenebrio molitor flour for evaluation of its impact on the human gut microbiota. J. Funct. Foods 2019, 59, 101–109. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol. Interact. 2019, 313, 108824. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Wong, N.A.K.; Chae, M.; Auh, J.-H. Comparative Characterization of Protein Hydrolysates from Three Edible Insects: Mealworm Larvae, Adult Crickets, and Silkworm Pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Joung, O.; Lee, H.-Y.; Shin, J.-C.; Choi, W.-S.; Lee, T.H.; Hwang, J.-S.; Nam, S.-H.; Son, H.-U.; Lee, S.-H. Anti-oxidative Fraction of Lycorma delicatula Alleviates Inflammatory Indicators. Nat. Prod. Commun. 2018, 13, 1934578X1801300413. [Google Scholar] [CrossRef] [Green Version]
- Bais, S.; Patel, N.J. In vitro anti diabetic and anti obesity effect of J. communis extract on 3T3L1 mouse adipocytes: A possible role of MAPK/ERK activation. Obes. Med. 2020, 18, 100219. [Google Scholar] [CrossRef]
- Lee, J.-E.; Min, S.H.; Lee, D.-H.; Oh, T.J.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Comprehensive assessment of lipoprotein subfraction profiles according to glucose metabolism status, and association with insulin resistance in subjects with early-stage impaired glucose metabolism. Int. J. Cardiol. 2016, 225, 327–331. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Zhu, S.-S.; He, M.-J.; Luo, F.; Fu, C.-Z.; Zou, T.-B. Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect. Mar. Drugs 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.; Goo, T.-W.; Chung, M.Y.; Baek, M.; Hwang, J.-S.; Kim, M.-A.; Yun, E.-Y. Tenebrio molitor larvae inhibit adipogenesis through AMPK and MAPKs signaling in 3T3-L1 adipocytes and obesity in high-fat diet-induced obese mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.-I.; Chung, M.Y.; Hwang, J.-S.; Han, M.S.; Goo, T.-W.; Yun, E.-Y. Allomyrina dichotoma (Arthropoda: Insecta) larvae confer resistance to obesity in mice fed a high-fat diet. Nutrients 2015, 7, 1978–1991. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.Y.; Yoon, Y.-I.; Hwang, J.-S.; Goo, T.-W.; Yun, E.-Y. Anti-obesity effect of Allomyrina dichotoma (Arthropoda: Insecta) larvae ethanol extract on 3T3-L1 adipocyte differentiation. Entomol. Res. 2014, 44, 9–16. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, J.; Wu, S. Hypolipidemic activity of the chitooligosaccharides from Clanis bilineata (Lepidoptera), an edible insect. Int. J. Biol. Macromol. 2013, 59, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, S.; Duan, H.; Wang, J.; Yan, W. Silkworm Pupae: A Functional Food with Health Benefits for Humans. Foods 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, K.G.; Kirubha, M.H.B. Gliptins: A new class of oral antidiabetic agents. Indian J. Pharm. Sci. 2009, 71, 608. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.M.E.; Dávalos Terán, I.; Fogliano, V.; Wichers, H.J. Investigation into the potential of commercially available lesser mealworm (A. diaperinus) protein to serve as sources of peptides with DPP-IV inhibitory activity. Int. J. Food Sci. Technol. 2019, 54, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Han, J.S. Oxya Chinensis Sinuosa Mishchenko Extract: Potent Glycosidase Inhibitor Alleviates Postprandial Hyperglycemia in Diabetic Mice. J. Life Sci. 2020, 30, 1054–1062. [Google Scholar] [CrossRef]
- Coates, D. The angiotensin converting enzyme (ACE). Int. J. Biochem. Cell Biol. 2003, 35, 769–773. [Google Scholar] [CrossRef]
- Antonios, T.F.; MacGregor, G.A. Angiotensin converting enzyme inhibitors in hypertension: Potential problems. J. Hypertens. Suppl. 1995, 13, S11–S16. [Google Scholar] [CrossRef]
- Dai, C.; Ma, H.; Luo, L.; Yin, X. Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 681–689. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Zhou, Y.; Zhang, Y.; Xu, L.; Xu, J.; Feng, F.; He, G. Isolation of a novel peptide from silkworm pupae protein components and interaction characteristics to angiotensin I-converting enzyme. Eur. Food Res. Technol. 2011, 232, 29–38. [Google Scholar] [CrossRef]
- Vercruysse, L.; Smagghe, G.; Beckers, T.; Camp, J.V. Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm, Spodoptera littoralis. Food Chem. 2009, 114, 38–43. [Google Scholar] [CrossRef]
- Vercruysse, L.; Smagghe, G.; Matsui, T.; Van Camp, J. Purification and identification of an angiotensin I converting enzyme (ACE) inhibitory peptide from the gastrointestinal hydrolysate of the cotton leafworm, Spodoptera littoralis. Process Biochem. 2008, 43, 900–904. [Google Scholar] [CrossRef]
- Clark, K.D. Insect Hemolymph Immune Complexes. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Hoeger, U., Harris, J.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 123–161. [Google Scholar]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, M.; Wang, S.; Cao, B.; Li, C.; Li, G. Novel Angiogenic Regulators and Anti-Angiogenesis Drugs Targeting Angiogenesis Signaling Pathways: Perspectives for Targeting Angiogenesis in Lung Cancer. Front. Oncol. 2022, 12, 842960. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Wada, J.; Sato, Y. Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat. Rev. Nephrol. 2020, 16, 289–303. [Google Scholar] [CrossRef]
- Deveza, L.; Choi, J.; Yang, F. Therapeutic Angiogenesis for Treating Cardiovascular Diseases. Theranostics 2012, 2, 801–814. [Google Scholar] [CrossRef]
- Kim, J.-I.; Yang, E.J.; Lee, M.S.; Kim, Y.-S.; Huh, Y.; Cho, I.-H.; Kang, S.; Koh, H.-K. Bee Venom Reduces Neuroinflammation in the MPTP-Induced Model of Parkinson’s Disease. Int. J. Neurosci. 2011, 121, 209–217. [Google Scholar] [CrossRef]
- Doo, A.-R.; Kim, S.-T.; Kim, S.-N.; Moon, W.; Yin, C.S.; Chae, Y.; Park, H.-K.; Lee, H.; Park, H.-J. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurol. Res. 2010, 32, 88–91. [Google Scholar] [CrossRef]
- Molan, P.C. The Evidence Supporting the Use of Honey as a Wound Dressing. Int. J. Low. Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Eleftherianos, I.; Zhang, W.; Heryanto, C.; Mohamed, A.; Contreras, G.; Tettamanti, G.; Wink, M.; Bassal, T. Diversity of insect antimicrobial peptides and proteins-A functional perspective: A review. Int. J. Biol. Macromol. 2021, 191, 277–287. [Google Scholar] [CrossRef]
- Fellows, P.; Halloran, A.; Muenke, C.; Vantomme, P.; van Huis, A. Insects in the human food chain: Global status and opportunities. Food Chain 2014, 4, 103–118. [Google Scholar] [CrossRef]
- Cherniack, E.P. Bugs as Drugs, Part 1: Insects. The "New" Alternative Medicine for the 21 st Century? Alternative Medicine Review LLC: Miami, FL, USA, 2010; Volume 15, pp. 124–135. [Google Scholar]
- El-Tantawy, N.L. Helminthes and insects: Maladies or therapies. Parasitol. Res. 2015, 114, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.H.; Lieberoth, A. We will eat disgusting foods together–Evidence of the normative basis of Western entomophagy-disgust from an insect tasting. Food Qual. Prefer. 2019, 72, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Gere, A.; Székely, G.; Kovács, S.; Kókai, Z.; Sipos, L. Readiness to adopt insects in Hungary: A case study. Food Qual. Prefer. 2017, 59, 81–86. [Google Scholar] [CrossRef]
- La Barbera, F.; Verneau, F.; Amato, M.; Grunert, K. Understanding Westerners’ disgust for the eating of insects: The role of food neophobia and implicit associations. Food Qual. Prefer. 2018, 64, 120–125. [Google Scholar] [CrossRef]
- Vanhonacker, F.; Van Loo, E.J.; Gellynck, X.; Verbeke, W. Flemish consumer attitudes towards more sustainable food choices. Appetite 2013, 62, 7–16. [Google Scholar] [CrossRef]
- Chen, P.P.; Wongsiri, S.; Jamyanya, T.; Rinderer, T.E.; Vongsamanode, S.; Matsuka, M.; Sylvester, H.A.; Oldroyd, B.P. Honey Bees and other Edible Insects Used as Human Food in Thailand. Am. Entomol. 1998, 44, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Verspoor, R.L.; Soglo, M.; Adeoti, R.; Djouaka, R.; Edwards, S.; Fristedt, R.; Langton, M.; Moriana, R.; Osborne, M.; Parr, C.L.; et al. Mineral analysis reveals extreme manganese concentrations in wild harvested and commercially available edible termites. Sci. Rep. 2020, 10, 6146. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, R.; Akala, N.; van der Bank, F.H. Heavy Metal Concentrations in Two Populations of Mopane Worms (Imbrasia belina) in the Kruger National Park Pose a Potential Human Health Risk. Bull. Environ. Contam. Toxicol. 2014, 93, 316–321. [Google Scholar] [CrossRef]
- Labu, S.; Subramanian, S.; Cheseto, X.; Akite, P.; Kasangaki, P.; Chemurot, M.; Tanga, C.M.; Salifu, D.; Egonyu, J.P. Agrochemical contaminants in six species of edible insects from Uganda and Kenya. Curr. Res. Insect Sci. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Illgner, P.; Nel, E. The Geography of Edible Insects in Sub-Saharan Africa: A study of the Mopane Caterpillar. Geogr. J. 2000, 166, 336–351. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Żuk-Gołaszewska, K.; Gałęcki, R.; Obremski, K.; Smetana, S.; Figiel, S.; Gołaszewski, J. Edible Insect Farming in the Context of the EU Regulations and Marketing & mdash; An Overview. Insects 2022, 13, 446. [Google Scholar] [CrossRef]
- Aquino, J.C.d.; Souza, C.F.C.; Santos, J.R.d.J.; Joachim-Bravo, I.S. Adding guarana powder to medfly diets: An alternative for improving the Sterile Insect Technique. Sci. Agric. 2016, 73, 294–298. [Google Scholar] [CrossRef]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect Rearing: Potential, Challenges, and Circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Stull, V.; Patz, J. Research and policy priorities for edible insects. Sustain. Sci. 2020, 15, 633–645. [Google Scholar] [CrossRef]
- Marberg, A.; van Kranenburg, H.; Korzilius, H. The big bug: The legitimation of the edible insect sector in the Netherlands. Food Policy 2017, 71, 111–123. [Google Scholar] [CrossRef]
- Verbeke, W. Profiling consumers who are ready to adopt insects as a meat substitute in a Western society. Food Qual. Prefer. 2015, 39, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Kok, R. Preliminary project design for insect production: Part 4—Facility considerations. J. Insects Food Feed. 2021, 7, 541–551. [Google Scholar] [CrossRef]
- Bertola, M.; Mutinelli, F. A Systematic Review on Viruses in Mass-Reared Edible Insect Species. Viruses 2021, 13, 2280. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanga, C.M.; Egonyu, J.P.; Beesigamukama, D.; Niassy, S.; Emily, K.; Magara, H.J.O.; Omuse, E.R.; Subramanian, S.; Ekesi, S. Edible insect farming as an emerging and profitable enterprise in East Africa. Curr. Opin. Insect Sci. 2021, 48, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M. Edible insects: Cricket farming and processing as an emerging market. J. Insects Food Feed. 2020, 6, 211–220. [Google Scholar] [CrossRef]
- Ashizawa, R.; Rubio, N.; Letcher, S.; Parkinson, A.; Dmitruczyk, V.; Kaplan, D.L. Entomoculture: A Preliminary Techno-Economic Assessment. Foods 2022, 11, 3037. [Google Scholar] [CrossRef]
- Berggren, Å.; Jansson, A.; Low, M. Approaching Ecological Sustainability in the Emerging Insects-as-Food Industry. Trends Ecol. Evol. 2019, 34, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Klüppel, H.-J. The Revision of ISO Standards 14040-3-ISO 14040: Environmental management–Life cycle assessment–Principles and framework-ISO 14044: Environmental management–Life cycle assessment–Requirements and guidelines. Int. J. Life Cycle Assess. 2005, 10, 165. [Google Scholar] [CrossRef]
- International Organization for Standardization. Environmental Management: Life Cycle Assessment; Requirements and Guidelines; ISO: Geneva, Switzerland, 2006; Volume 14044. [Google Scholar]
- Wade, M.; Hoelle, J. A review of edible insect industrialization: Scales of production and implications for sustainability. Environ. Res. Lett. 2020, 15, 123013. [Google Scholar] [CrossRef]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- de Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Sousa-Pinto, B.; Fonseca, J.; Fonseca, S.C.; Cunha, L.M. Edible insects and food safety: Allergy. J. Insects Food Feed. 2021, 7, 833–847. [Google Scholar] [CrossRef]
- Kamemura, N.; Sugimoto, M.; Tamehiro, N.; Adachi, R.; Tomonari, S.; Watanabe, T.; Mito, T. Cross-allergenicity of crustacean and the edible insect Gryllus bimaculatus in patients with shrimp allergy. Mol. Immunol. 2019, 106, 127–134. [Google Scholar] [CrossRef]
- Doi, H.; Gałęcki, R.; Mulia, R.N. The merits of entomophagy in the post COVID-19 world. Trends Food Sci. Technol. 2021, 110, 849–854. [Google Scholar] [CrossRef]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S.; et al. Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible insects: Cross-recognition of IgE from crustacean- and house dust mite allergic patients, and reduction of allergenicity by food processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, L.; Mainente, F.; Leonardi, M.; Scheurer, S.; Wangorsch, A.; Mahler, V.; Pilolli, R.; Sorio, D.; Zoccatelli, G. Allergenicity assessment of the edible cricket Acheta domesticus in terms of thermal and gastrointestinal processing and IgE cross-reactivity with shrimp. Food Chem. 2021, 359, 129878. [Google Scholar] [CrossRef]
- Evans, J.; Müller, A.; Jensen, A.B.; Dahle, B.; Flore, R.; Eilenberg, J.; Frøst, M.B. A descriptive sensory analysis of honeybee drone brood from Denmark and Norway. J. Insects Food Feed. 2016, 2, 277–283. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Durst, P.B.; Johnson, D.V.; Leslie, R.N.; Shono, K. Forest insects as food: Humans bite back. RAP Publ. 2010, 1, 1–241. [Google Scholar]
- Gao, Y.; Wang, H.; Qin, F.; Xu, P.; Lv, X.; Li, J.; Guo, B. Enantiomerization and Enantioselective Bioaccumulation of Metalaxyl in Tenebrio molitor Larvae. Chirality 2014, 26, 88–94. [Google Scholar] [CrossRef]
- Houbraken, M.; Spranghers, T.; De Clercq, P.; Cooreman-Algoed, M.; Couchement, T.; De Clercq, G.; Verbeke, S.; Spanoghe, P. Pesticide contamination of Tenebrio molitor (Coleoptera: Tenebrionidae) for human consumption. Food Chem. 2016, 201, 264–269. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food Safety Issues Related to Uses of Insects for Feeds and Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Huis, A. Prospects of insects as food and feed. Org. Agric. 2021, 11, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Handley, M.A.; Hall, C.; Sanford, E.; Diaz, E.; Gonzalez-Mendez, E.; Drace, K.; Wilson, R.; Villalobos, M.; Croughan, M. Globalization, Binational Communities, and Imported Food Risks: Results of an Outbreak Investigation of Lead Poisoning in Monterey County, California. Am. J. Public Health 2007, 97, 900–906. [Google Scholar] [CrossRef]
- Boye, J.I.; Danquah, A.O.; Lam Thang, C.; Zhao, X. Food Allergens. In Food Biochemistry and Food Processing; Blackwell: New Jerssey, NJ, USA, 2012; pp. 798–819. [Google Scholar]
- Kachapulula, P.W.; Akello, J.; Bandyopadhyay, R.; Cotty, P.J. Aflatoxin Contamination of Dried Insects and Fish in Zambia. J. Food Prot. 2018, 81, 1508–1518. [Google Scholar] [CrossRef]
- Idowu, A.B.; Oliyide, E.O.; Ademolu, K.O.; Bamidele, J.A. Nutritional and anti-nutritional evaluation of three edible insects consumed by the Abeokuta community in Nigeria. Int. J. Trop. Insect Sci. 2019, 39, 157–163. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.-J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Okolie, C.L.; Akanbi, T.O.; Mason, B.; Udenigwe, C.C.; Aryee, A.N.A. Influence of conventional and recent extraction technologies on physicochemical properties of bioactive macromolecules from natural sources: A review. Food Res. Int. 2019, 116, 827–839. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.D.; Wong, N.A.K.; Auh, J.-H. Defatting and sonication enhances protein extraction from edible insects. Korean J. Food Sci. Anim. Resour. 2017, 37, 955. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef] [Green Version]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Hall, F.; Liceaga, A. Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Ward, N.; Sahebkar, A.; Banach, M.; Watts, G. Recent perspectives on the role of nutraceuticals as cholesterol-lowering agents. Curr. Opin. Lipidol. 2017, 28, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kolarič, L.; Šimko, P. Effect of processing conditions on measure of cholesterol removal from milk and cream. Mon. Chem. Chem. Mon. 2022, 153, 1069–1075. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes-Extraction and Functional Properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-K.; Yong, H.I.; Chun, H.H.; Lee, M.-A.; Kim, Y.-B.; Choi, Y.-S. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps. J. Asia Pac. Entomol. 2020, 23, 298–305. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.; Agyekum, A.A.; Dabbour, M.; Golly, M.K.; Ma, H. Edible insect protein for food applications: Extraction, composition, and functional properties. J. Food Process Eng. 2020, 43, e13362. [Google Scholar] [CrossRef]
- van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef] [Green Version]
- DeFoliart, G.R. Insects as human food: Gene DeFoliart discusses some nutritional and economic aspects. Crop Prot. 1992, 11, 395–399. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Khanafari, A.; Marandi, R.E.Z.A.; SANATI, S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran. J. Health Environ. 2008, 5, 19–24. [Google Scholar]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of deproteinization and demineralization using natural deep eutectic solvents for production of insect chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225, 115255. [Google Scholar] [CrossRef] [PubMed]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.; Altintas, Z. Ultrasound-Assisted Alcoholic Extraction of Lesser Mealworm Larvae Oil: Process Optimization, Physicochemical Characteristics, and Energy Consumption. Antioxidants 2022, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Sete da Cruz, R.M.; da Silva, C.; da Silva, E.A.; Hegel, P.; Barão, C.E.; Cardozo-Filho, L. Composition and oxidative stability of oils extracted from Zophobas morio and Tenebrio molitor using pressurized n-propane. J. Supercrit. Fluids 2022, 181, 105504. [Google Scholar] [CrossRef]
- Haber, M.; Mishyna, M.; Martinez, J.J.I.; Benjamin, O. The influence of grasshopper (Schistocerca gregaria) powder enrichment on bread nutritional and sensorial properties. LWT 2019, 115, 108395. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and Volatilome characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef]
- da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Biró, B.; Fodor, R.; Szedljak, I.; Pásztor-Huszár, K.; Gere, A. Buckwheat-pasta enriched with silkworm powder: Technological analysis and sensory evaluation. LWT 2019, 116, 108542. [Google Scholar] [CrossRef]
- Park, Y.-S.; Choi, Y.-S.; Hwang, K.-E.; Kim, T.-K.; Lee, C.-W.; Shin, D.-M.; Han, S.G. Physicochemical properties of meat batter added with edible silkworm pupae (Bombyx mori) and transglutaminase. Korean J. Food Sci. Anim. Resour. 2017, 37, 351. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.; Jones, O.G.; Kim, Y.H.B. Effect of House Cricket (Acheta domesticus) Flour Addition on Physicochemical and Textural Properties of Meat Emulsion Under Various Formulations. J. Food Sci. 2017, 82, 2787–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.M.M.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Kewuyemi, Y.O.; Kesa, H.; Chinma, C.E.; Adebo, O.A. Fermented Edible Insects for Promoting Food Security in Africa. Insects 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, N.; Liu, C.; Jin, J. Effect of Silkworm Pupae Peptide on the Fermentation and Quality of Yogurt. J. Food Processing Preserv. 2017, 41, e12893. [Google Scholar] [CrossRef]
- Cho, J.-H.; Zhao, H.-L.; Kim, J.-S.; Kim, S.-H.; Chung, C.-H. Characteristics of fermented seasoning sauces using Tenebrio molitor larvae. Innov. Food Sci. Emerg. Technol. 2018, 45, 186–195. [Google Scholar] [CrossRef]
- Reverberi, M. The new packaged food products containing insects as an ingredient. J. Insects Food Feed. 2021, 7, 901–908. [Google Scholar] [CrossRef]
- Hanboonsong, Y.; Jamjanya, T.; Durst, P.B. Six-Legged Livestock: Edible Insect Farming, Collection and Marketing in Thailand; Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 2013; Volume 3, pp. 8–21. [Google Scholar]
- Usman, H.S.; Yusuf, A.A. Legislation and legal frame work for sustainable edible insects use in Nigeria. Int. J. Trop. Insect Sci. 2021, 41, 2201–2209. [Google Scholar] [CrossRef]
- Mariod, A.A. The Legislative Status of Edible Insects in the World. In African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Adam Mariod, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 141–148. [Google Scholar]
- Van Huis, A. Edible crickets, but which species? J. Insects Food Feed. 2020, 6, 91–94. [Google Scholar] [CrossRef]
- Halloran, A.; Vantomme, P.; Hanboonsong, Y.; Ekesi, S. Regulating edible insects: The challenge of addressing food security, nature conservation, and the erosion of traditional food culture. Food Secur. 2015, 7, 739–746. [Google Scholar] [CrossRef]
- Vandeweyer, D. Microbiological Quality of Raw Edible Insects and Impact of Processing and Preservation; Katholieke Universiteit Leuven: Leuven, Belgium, 2018. [Google Scholar]
- Laurenza, E.C.; Carreño, I. Edible Insects and Insect-based Products in the EU: Safety Assessments, Legal Loopholes and Business Opportunities. Eur. J. Risk Regul. 2015, 6, 288–292. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of frozen and dried formulations from whole house crickets (Acheta domesticus) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06779. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of partially defatted house cricket (Acheta domesticus) powder as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07258. [Google Scholar] [CrossRef] [PubMed]
- German Federal Institute for Risk Assessment (BfR); National Reference Laboratory for Animal Protein in Feed; NRL-AP; Garino, C.; Zagon, J.; Braeuning, A. Insects in food and feed–Allergenicity risk assessment and analytical detection. EFSA J. 2019, 17, e170907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilderspin, D.E.; Halloran, A. The Effects of Regulation, Legislation and Policy on Consumption of Edible Insects in the Global South. In Edible Insects in Sustainable Food Systems; Halloran, A., Flore, R., Vantomme, P., Roos, N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 443–455. [Google Scholar]
- Lähteenmäki-Uutela, A.; Hénault-Ethier, L.; Marimuthu, S.B.; Talibov, S.; Allen, R.N.; Nemane, V.; Vandenberg, G.W.; Józefiak, D. The impact of the insect regulatory system on the insect marketing system. J. Insects Food Feed. 2018, 4, 187–198. [Google Scholar] [CrossRef]
- van Huis, A.; Dicke, M.; van Gurp, H. The Insect Cookbook: Food for a Sustainable Planet; Columbia Scholarship Online: New York, NY, USA, 2014. [Google Scholar] [CrossRef]






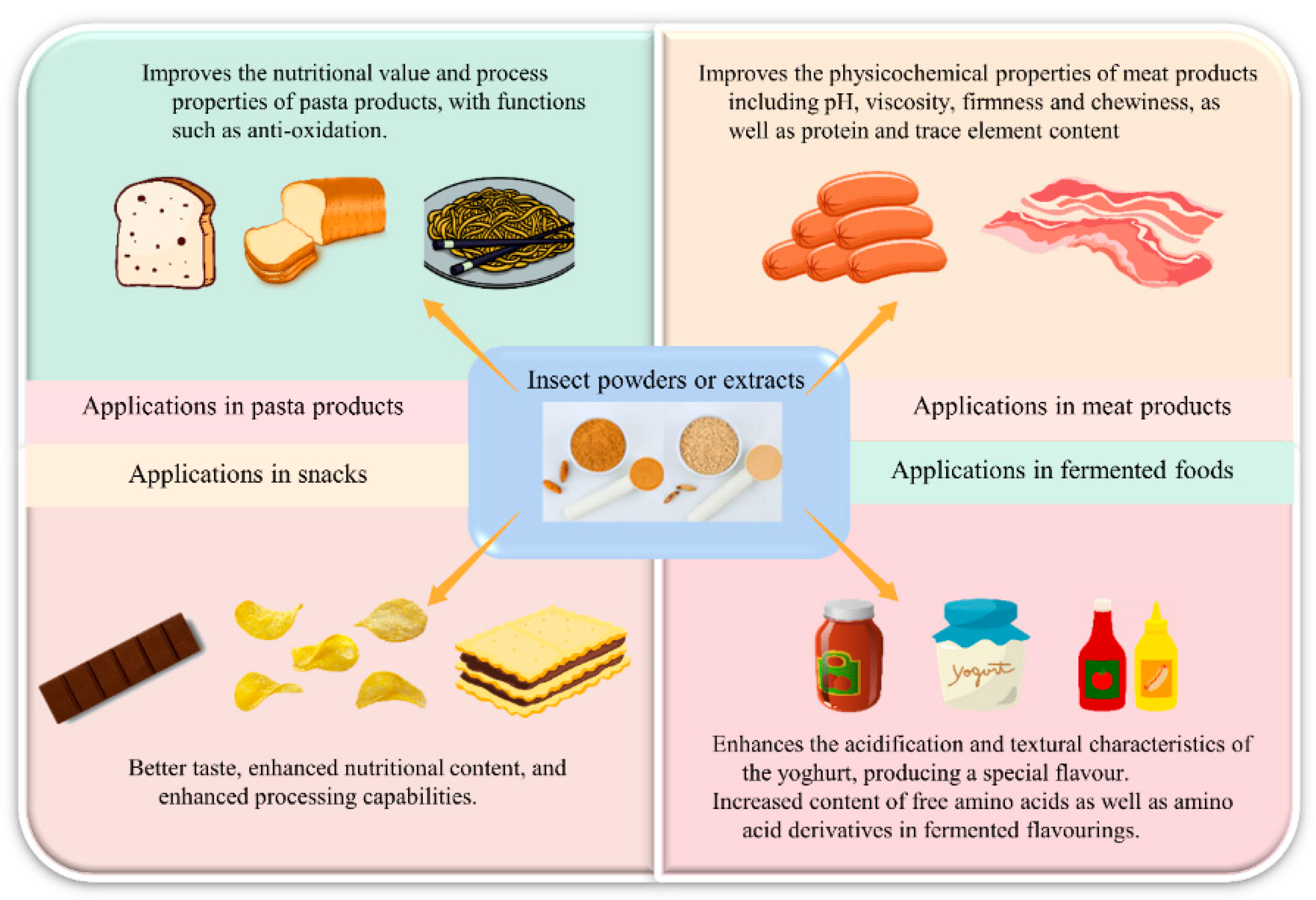
| Scientific Name | Moisture | Protein | Fat | Ash | Fiber | Reference |
|---|---|---|---|---|---|---|
| Antheraea pernyi | 7.6 a | 71.9 a | 20.1 a | 4 a | NA | [34] |
| Clanis bilineata tsingtauica | NA | 65.5 a | 23.68 a | 2.17 a | 3.77 a | [35] |
| Oxya chinensis | NA | 20.8 a | 2.2 a | 1.2 a | 1.2 a | [35] |
| Schistocerca piceifrons piceifrons | NA | 80.26 a | 6.21 a | 3.25 a | 12.56 a | [36] |
| Gryllus bimaculatus | NA | 58.32 a | 11.88 a | 9.69 a | 9.53 a | [37] |
| Gonimbrasia belina | 5.68 a | 46.7 a | 14.04 a | 11.38 a | NA | [38,39] |
| Hermetia illucens | 5.76 a | 34.9 a | 27.93 a | 7.5 a | NA | [38] |
| Macrotermes subhylanus | 6.40 a | 52.74 a | 6.36 a | 6.41 a | NA | [38] |
| Macrotermes bellicosus | 2.82 a | 20.4 a | NA | 2.9 a | 2.7 a | [40] |
| Macrotermes notalensis | 2.98 a | 22.1 a | NA | 1.9 a | 2.2 a | [40] |
| Brachytrypes spp. | 3.41 a | 6.25 a | NA | 1.82 a | 1.01 a | [40] |
| Cytacanthacris aeruginosus unicolor | 2.56 a | 12.1 a | NA | 2.1 a | 1.5 a | [40] |
| Zonocerus variegatus | 2.61 a | 26.8 a | NA | 1.2 a | 2.4 a | [40] |
| Analeptes trifasciata | 2.19 a | 29.62 a | NA | 4.21 a | 1.96 a | [40] |
| Anaphe infracta | 2.73 a | 20 a | NA | 1.6 a | 2.4 a | [40] |
| Anaphe recticulata | 3.21 a | 23 a | NA | 2.5 a | 3.1 a | [40] |
| Anaphe spp. | 2.52 a | 18.9 a | NA | 4.1 a | 1.68 a | [40] |
| Anaphe venata | 3.34 a | 25.7 a | NA | 3.2 a | 2.3 a | [40] |
| Cirina forda | 4.40 a | 20.2 a | NA | 1.5 a | 1.8 a | [40,41] |
| Apis mellifera | 3.82 a | 21 a | 14.5 a | 2.2 a | 2 a | [39,40,42] |
| Analeptes trifasciata | 2.65 a | 20.1 a | NA | 1.5 a | 3.3 a | [40] |
| Oryctes boas | 1.91 a | 26 a | NA | 1.5 a | 3.4 a | [40] |
| Rhynchophorus phoenicis | 2.74 a | 28.42 a | NA | 2.7 a | 2.82 a | [40,41] |
| Gynanisa maja | 9.2 a | 55.92 a | 12.1 a | 7.4 a | NA | [43] |
| Macrotermes falciger | 4.1 a | 43.26 a | 43.0 a | 7.3 a | NA | [43] |
| Ruspolia differens | NA | 44.3 a | 46.2 a | 2.6 a | 4.9 a | [41,43] |
| Imbrasia belina | NA | 56.8 a | 12.9 a | 10.4 a | NA | [44] |
| Gryllodes sigillatus | NA | 70 a | 18.23 a | 4.74 a | 3.65 a | [45] |
| Schidtocerca gregaria | NA | 76 a | 12.97 a | 3.33 a | 2.53 a | [45] |
| Locusta migratoria | 4.2 a | 48.7 a | 38.1 a | 2.3 a | 8.8 a | [46] |
| Alphitobius diaperinus | 2.74 a | 58.76 a | 25.9 a | 3.5 a | 6.08 a | [47] |
| Rhynchophorus ferrugineus | 67.9 b | 18.0 a | 58.8 a | 2.4 a | NA | [48] |
| Hermetia illucens | 61.2 b | 17.5 b | 14 b | NA | 6.8 b | [39,49] |
| Chilecomadia moorei | 60.2 b | 15.5 b | 29.4 b | NA | 4 b | [49] |
| Blatta lateralis | 69.1 b | 19 b | 10 b | NA | 5 b | [49] |
| Musca domestica | 74.8 b | 19.7 b | 1.9 b | NA | 6.5 b | [49] |
| Zophobas morio | 57.90 b | 19.70 b | 17.70 b | 1.00 b | 6.60 b | [33,50] |
| Tenebrio molitor | 61.00 b | 18.40 b | 16.80 b | 1.20 b | 5.40 b | [33,50,51] |
| Galleria mellonela | 58.50 b | 14.10 b | 24.90 b | 0.60 b | 12.50 b | [33,50] |
| Bombyx mori | 82.70 b | 9.30 b | 1.40 b | 1.10 b | 2.20 b | [33,41] |
| Acheta domesticus | 69.20 b | 20.50 b | 6.80 b | 1.10 b | 10.00 b | [33,41,50] |
| Oecyphylla smaragdina | 59.50 b | 10.80 b | 10.80 b | NA | NA | [39] |
| Scientific Name | Ile | Leu | Lys | Met | Cys | Phe | Tyr | Thr | Trp | Val | Arg | His | Ala | Asp | Glu | Gly | Pro | Ser | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antheraea pernyi | 79.5 a | 32.4 a | 45.4 a | 14.7 a | 1.5 a | 81 a | 20.6 a | 46.4 a | 40.5 a | 66.3 a | 41.2 a | 29.4 a | 62.6 a | 64.1 a | 127.4 a | 44.2 a | 122.2 a | 46.4 a | [34] |
| Bombyx mori | 57 a | 83 a | 75 a | 46 a | 14 a | 51 a | 54 a | 54 a | 6 a | 56 a | 68 a | 25 a | 55 a | 109 a | 149 a | 46 a | 40 a | 47 a | [34] |
| Gryllodes sigillatus | 25.6 | 57.8 | 38.4 | 15.9 | 11.1 | 22.0 | 31.8 | 36.8 | NA | 47.0 | 46.6 | 17.2 | 58.0 | 72.8 | 106.6 | 40.7 | 54.2 | 40.4 | [45] |
| Schistocerca gregaria | 28.2 | 77.7 | 35.1 | 8.2 | 3.6 | 18.7 | 33.1 | 35.5 | NA | 56.6 | 39.8 | 20.6 | 88.8 | 66.1 | 107.5 | 49.4 | 67.1 | 33.7 | [45] |
| Hermetia illucens | 7.62 b | 12.1 b | 11.9 b | 3.37 b | 1.02 b | 7.56 b | 12.1 b | 6.82 b | 3.00 b | 12.9 b | 12.3 b | 5.94 b | 12.2 b | 16.5 b | 19.7 b | 9.14 b | 10.2 b | 7.02 b | [49] |
| Chilecomadia moorei | 6.51 b | 10.1 b | 8.72 b | 2.49 b | 0.87 b | 5.47 b | 7.95 b | 5.74 b | 1.56 b | 9.71 b | 11.7 b | 4.08 b | 8.67 b | 12.9 b | 16.4 b | 6.53 b | 9.52 b | 7.88 b | [49] |
| Blatta lateralis | 7.73 b | 12 b | 12.8 b | 3.35 b | 1.44 b | 7.67 b | 14.3 b | 7.89 b | 1.66 b | 12.3 b | 14 b | 5.49 b | 16.7 b | 15.1 b | 22.6 b | 12.4 b | 10.6 b | 8.38 b | [49] |
| Musca domestica | 8.1 b | 12.4 b | 12.6 b | 5.84 b | 1.4 b | 7.91 b | 9.26 b | 7.54 b | 2.4 b | 11 b | 12.1 b | 5.71 b | 11.7 b | 16.3 b | 21.1 b | 8.43 b | 8.36 b | 6.97 b | [49] |
| Zophobas morio | 9.3 b | 19.1 b | 10.3 b | 2.1 b | 1.5 b | 6.8 b | 13.7 b | 7.8 b | 1.8 b | 10.3 b | 9.6 b | 6.0 b | 14.3 b | 15.8 b | 24.2 b | 9.5 b | 10.8 b | 9.2 b | [33,50] |
| Tenebrio molitor | 8.6 b | 14.3 b | 11.2 b | 2.6 b | 1.5 b | 7.5 b | 14.3 b | 6.4 b | 1.7 b | 12.2 b | 10.3 b | 6.5 b | 13.7 b | 16.2 b | 22.8 b | 9.9 b | 12.1 b | 9.1 b | [33,50,57] |
| Galleria mellonella | 6.3 b | 12.4 b | 7.9 b | 2.2 b | 1.1 b | 5.3 b | 8.8 b | 5.9 b | 1.2 b | 6.8 b | 7.1 b | 3.3 b | 9.4 b | 13.4 b | 19.5 b | 7.4 b | 9.5 b | 10.5 b | [33,50] |
| Acheta domesticus | 9.4 b | 20.5 b | 11.0 b | 3.0 b | 1.7 b | 6.5 b | 10.0 b | 7.4 b | 1.3 b | 10.7 b | 12.5 b | 4.8 b | 18.0 b | 17.2 b | 21.5 b | 10.4 b | 11.5 b | 10.2 b | [33,50,58] |
| Gryllus bimaculatus | 9.2 b | 16.5 b | 11.4 b | 3.5 b | 1.6 b | 7.4 b | 11.7 b | 8.1 b | 2.2 b | 13.6 b | 11.4 b | 5.2 b | 19.3 b | 19.7 b | 24.4 b | 12.4 b | 12.5 b | 10.5 b | [58] |
| Gonimbrasia belina | 13.0 c | 18.3 c | 25.6 c | 4.1 c | 1.1 c | 13.5 c | 22.3 c | 18.4 c | 4.8 c | 19.1 c | 45.7 c | 18.4 c | 23.6 c | 31.3 c | 43.5 c | 17.9 c | 18.6 c | 17.5 c | [43] |
| Gynanisa maja | 18.8 c | 27.2 c | 40.2 c | 8.2 c | 2.2 c | 19.8 c | 41.7 c | 22.6 c | 7.5 c | 20.9 c | 31.4 c | 25.3 c | 25.5 c | 39.9 c | 52.4 c | 19.9 c | 25.0 c | 23.1 c | [43] |
| Ruspolia differens | 26.1 c | 26.7 c | 57.4 c | 4.3 c | 0.7 c | 26.1 c | 25.3 c | 28.6 c | 0.3 c | 16.4 c | 49.8 c | 44.1 c | 26.6 c | 49.0 c | 84.3 c | 26.0 c | 19.0 c | 25.9 c | [43] |
| Macrotermes falciger | 18.9 c | 31.6 c | 37.2 c | 8.2 c | 1.3 c | 19.7 c | 34.4 c | 19.5 c | 3.5 c | 21.7 c | 30.1 c | 26.5 c | 27.4 c | 37.3 c | 46.8 c | 18.9 c | 19.3 c | 20.8 c | [43] |
| Imbrasia belina | 22.0 c | 35.0 c | 36.0 c | 9.0 c | NA | 25.0 c | 36.0 c | 27.0 c | 7.0 c | NA | 32.0 c | 17.0 c | NA | NA | NA | NA | NA | NA | [44] |
| Apis mellifera | 16.0 c | 25.0 c | 19.0 c | NA | 3.0 c | 2.0 c | 15.0 c | 16.0 c | NA | 17.0 c | 16.0 c | 7.0 c | 16.0 c | 26.0 c | 50.0 c | 14.0 c | NA | 14.0 c | [42] |
| Rhynchophorus ferrugineus | 8 c | 12 c | 11 c | 2 c | 1 c | 7 c | 21 c | 8 c | 1 c | 10 c | 10 c | 4 c | 11 c | 16 c | 25 c | 9 c | 10 c | 9 c | [48] |
| Scientific Name | C10:0 | C12:0 | C14:0 | C15:0 | C16:0 | C17:0 | C18:0 | C20:0 | SFA | C14:1 | C16:1 | C17:1 | C18:1 | C20:1 | MUFA | C18:2 | C18:3 | PUFA | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zophobas morio | NA | <0.2 a | 1.7 a | 0.4 a | 52.8 a | 0.7 a | 12.6 a | 0.4 a | 68.6 a | NA | 0.7 a | 0.6 a | 66.0 a | NA | 67.3 a | 32.9 a | 1.1 a | 34.0 a | [33,50] |
| Tenebrio molitor | NA | 0.6 a | 5.2 a | 0.2 a | 25.5 a | 0.2 a | 4.0 a | 0.2 a | 35.9 a | NA | 4.8 a | 0.2 a | 66.4 a | NA | 71.4 a | 49.0 a | 2.2 a | 51.2 a | [33,50] |
| Galleria mellonella | NA | <0.2 a | 0.4 a | <0.2 a | 79.6 a | <0.2 a | 3.4 a | 0.3 a | 83.7 a | NA | 5.1 a | 0.3 a | 124.0 a | NA | 129.4 a | 15.2 a | 1.1 a | 16.3 a | [33,50] |
| Bombyx mori | NA | <0.2 a | <0.3 a | <0.4 a | 1.7 a | <0.2 a | 1.2 a | 0.1 a | 3.0 a | NA | 0.1 a | <0.2 a | 3.2 a | NA | 3.3 a | 3.5 a | 1.4 a | 4.9 a | [33] |
| Acheta domesticus | NA | <0.2 a | 0.4 a | <0.2 a | 15.6 a | 0.2 a | 5.8 a | 0.4 a | 22.4 a | NA | 0.9 a | <0.1 a | 15.4 a | NA | 16.3 a | 22.9 a | 0.6 a | 23.5 a | [33,50] |
| Hermetia illucens | 0.69 a | 52.1 a | 12.0 a | 0.12 a | 16.1 a | 0.20 a | 2.45 a | 0.16 a | 83.82 a | 0.12 a | 4.96 a | <0.08 a | 15.6 a | <0.08 a | 20.68 a | 16.9 b | 0.65 a | 17.55 a | [49] |
| Chilecomadia moorei | <0.10 a | 0.93 a | 0.95 a | <0.10 a | 69.3 a | <0.10 a | 2.19 a | 0.24 a | 73.61 a | <0.10 a | 14.7 a | <0.10 a | 149.0 a | 0.19 a | 163.89 a | 6.99 a | 0.45 a | 7.44 a | [49] |
| Blatta lateralis | <0.20 a | <0.20 a | 0.48 a | <0.20 a | 17.4 a | <0.20 a | 4.22 a | <0.20 a | 22.1 a | <0.20 a | 1.21 a | <0.20 a | 40.9 a | <0.25 a | 42.11 a | 21.6 a | 0.71 a | 22.31 a | [49] |
| Musca domestica | <0.01 a | 0.02 a | 0.32 a | 0.17 a | 3.72 a | 0.10 a | 0.40 a | 0.04 a | 4.77 a | 0.02 a | 1.96 a | <0.01 a | 2.89 a | 0.01 a | 4.97 a | 4.15 a | 0.45 a | 4.6 a | [49] |
| Imbrasia belina | NA | <0.1 b | <0.1 b | NA b | 3.0 b | NA b | 1.7 b | <0.1b | 4.9 b | NA | 0.1 b | NA | 1.8 b | NA | 1.7 b | 1.6 b | 3.7 b | 5.4 b | [44] |
| Ruspolia differens | NA | NA | NA | NA | 32.1 c | NA | 5.9 c | NA | 39.1 c | NA | 1.4 c | NA | 24.9 c | NA | 26.3 c | 29.5 c | 4.2 c | 33.8 c | [41] |
| Gryllodes sigillatus | NA | 0.10 c | 1.65 c | 0.24 c | 23.5 c | 0.32 c | 7.35 c | 0.40 c | 33.74 c | 0.09 c | 3.78 c | 0.29 c | 29.14 c | 1.03 c | 34.33 c | 29.78 c | 2.13 c | 31.91 c | [45] |
| Schistocerca gregaria | 0.07 c | 0.23 c | 1.68 c | 0.09 c | 23.26 c | 0.24 c | 9.27 c | 0.40 c | 35.3 c | NA | 1.80 c | 0.20 c | 36.22 c | 0.14 c | 38.35 c | 14.04 c | 11.35 c | 26.28 c | [45] |
| Polyrhachis vicina | NA | 0.7 c | 0.6 c | 0.1 c | 17.5 c | 0.2 c | 4.3 c | 0.3 c | 23.9 c | NA | 8.2 c | 0.4 c | 63.0 c | 0.7 c | 72.4 c | 2.1 c | 0.2 c | 2.5 c | [66] |
| Oecophylla smaragdina | NA | 0.9 c | 2.1 c | 0.2 c | 20.8 c | 0.3 c | 5.8 c | 1.0 c | 31.9 c | NA | 4.3 c | 0.5 c | 52.1 c | 1.6 c | 58.7 c | 7.0 c | 1.0 c | 8.4 c | [66] |
| Apis mellifera | NA | 0.3 c | 2.4 c | NA | 37.3 c | NA | 11.8 c | NA | 51.8 c | NA | 0.7 c | NA | 47.5 c | NA | 48.2 c | NA | NA | NA | [42] |
| Rhynchophorus ferrugi-neus | NA | 1 c | 1.6 c | NA | 49.4 c | NA | 0.1 c | NA | 53 c | NA | NA | NA | 46.9 c | NA | 46.9 c | 0.8 c | 0.5 c | 1.3 c | [48] |
| Scientific Name | A (IU/kg) | B1 (mg/kg) | B2 (mg/kg) | B6 (mg/kg) | B12 (μg/kg) | C (mg/kg) | E (IU/kg) | PP (mg/kg) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Hermetia illucens | <1000 | 7.7 | 16.2 | 6.01 | 55.8 | <10.0 | 6.2 | 71.0 | [49] |
| Chilecomadia moorei | <1000 | <0.01 | 64.5 | 3.29 | 5.1 | 23.0 | 13.0 | 33.6 | [49] |
| Blatta lateralis | <1000 | 0.9 | 15.6 | 3.10 | 237 | <10.0 | <3.3 | 43.8 | [49] |
| Musca domestica | <1000 | 11.3 | 77.2 | 1.72 | 6.0 | <10.0 | 29.7 | 90.5 | [49] |
| Zophobas morio | <1000 | 0.6 | 7.5 | 3.2 | NA | 12.0 | 7.7 | 32.3 | [33,50] |
| Tenebrio molitor | <1000 | 1.2 | 16.1 | 5.8 | NA | 24.0 | <5.0 | 41.3 | [33,50,51] |
| Galleria mellonella | <1000 | 2.3 | 7.3 | 1.3 | NA | <10.0 | 13.3 | 37.5 | [33,50] |
| Bombyx mori | 1580 | 3.3 | 9.4 | 1.6 | NA | <10.0 | 8.9 | 26.3 | [33] |
| Acheta domesticus | <1000 | 0.4 | 34.1 | 2.3 | NA | 30.0 | 19.7 | 38.4 | [33,50] |
| Ruspolia differens | <1000 | NA | 14 | 4.4 | NA | 6.2 | 22.6 | 36.1 | [41,76] |
| Macrotermessubhylanus | <1000 | NA | 41.8 | 2.7 | NA | 7.3 | NA | 28.0 | [76] |
| Oecyphylla smaragdina | NA | 2.25 | 6.75 | NA | NA | 20.0 | NA | NA | [39] |
| Rhynchophorus ferrugi-neus | NA | NA | NA | NA | NA | NA | 18.8 | NA | [48] |
| Scientific Name | Ca | P | Mg | Na | K | Cl | Fe | Zn | Cu | Mn | I | Se | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apis mellifera | 849 a | 7825 a | 1770 a | 584 a | 18,719 a | NA | 133 a | 116 a | 36 a | 12 a | NA | NA | [42] |
| Gryllodes sigillatus | 1300 a | NA | 1010 a | 3330 a | 11,900 a | NA | 42.3 a | 139 a | 47.9 a | NA | NA | NA | [45] |
| Schistocerca gregaria | 700 a | NA | 820 a | 1730 a | 7490 a | NA | 83.8 a | 186 a | 63.2 a | NA | NA | NA | [45] |
| Ruspolia differens | 245 a | NA | 331 a | 1210 a | 2597 a | NA | 2297 a | 130 a | 25 a | 124 a | NA | 5 a | [41,43] |
| Gonimbrasia belina | 1278 a | NA | 697 a | 412 a | 102 a | NA | 267 a | NA | 3 a | 15 a | NA | NA | [43] |
| Gynanisa maja | 1664 a | NA | 1000 a | 324 a | 655 a | NA | 136 a | NA | 3 a | 14 a | NA | NA | [43] |
| Macrotermes falciger | 780 a | NA | 490 a | 127 a | 127 a | NA | 248 a | NA | 7 a | 15 a | NA | NA | [43] |
| Imbrasia belina | 570 a | 240 a | NA | 2670 a | 110 a | NA | 1160 a | NA | NA | NA | NA | NA | [44] |
| Gryllus bimaculatus | 2402 a | 11,696 a | 1467 a | 4530 a | 10,799 a | NA | 97 a | 224 a | 46 a | 104 a | NA | NA | [37] |
| Allomyrina dichotoma | 1234 a | 8607 a | 2836 a | 1484 a | 12,491 a | NA | 143 a | 103 a | 14 a | 86 a | NA | NA | [37] |
| Protaetia brevitarsis | 2587 a | 11,404 a | 3276 a | 2116 a | 20,014 a | NA | 162 a | 119 a | 18 a | 59 a | NA | NA | [37] |
| Teleogryllus emma | 1935 a | 10,854 a | 1525 a | 2782 a | 8955 a | NA | 108 a | 185 a | 22 a | 59 a | NA | NA | [37] |
| Rhynchophorus ferrugineus | 380 a | 2390 a | 1200 a | 380 a | 5680 a | NA | 10 a | 80 a | 11 a | 6 a | NA | NA | [48] |
| Hermetia illucens | 9340 b | 3560 b | 1740 b | 887 b | 4530 b | 1160 b | 66.60 b | 56.20 b | 4.03 b | 61.80 b | 0.26 b | 0.32 b | [49] |
| Chilecomadia moorei | 125 b | 2250 b | 278 b | 198 b | 2590 b | 1160 b | 14 b | 37.70 b | 2.95 b | 0.71 b | 0.10 b | 0.03 b | [49] |
| Blatta lateralis | 385 b | 1760 b | 250 b | 744 b | 2240 b | 1600 b | 14.80 b | 32.70 b | 7.93 b | 2.64 b | 0.30 b | 0.30 b | [49] |
| Musca domestica | 765 b | 3720 b | 806 b | 1380 b | 3030 b | 1760 b | 125 b | 85.80 b | 12.90 b | 26.60 b | 0.10 b | 0.15 b | [49] |
| Zophobas morio | 177 b | 2370 b | 498 b | 475 b | 3160 b | 1520 b | 16.5 b | 30.7 b | 3.6 b | 4.3 b | <0.1 b | 0.14 b | [33,50] |
| Tenebrio molitor | 184 b | 2720 b | 864 b | 489 b | 2970 b | 1750 b | 21.5 b | 44.5 b | 6.4 b | 3.6 b | <0.1 b | 0.13 b | [33,50,51] |
| Galleria mellonella | 234 b | 1950 b | 316 b | 165 b | 2210 b | 640 b | 20.9 b | 25.4 b | 3.8 b | 1.3 b | <0.1 b | 0.11 b | [33,50] |
| Bombyx mori | 177 b | 2370 b | 498 b | 475 b | 3160 b | 620 b | 16.5 b | 30.7 b | 3.6 b | 4.3 b | <0.1 b | 0.14 b | [33] |
| Acheta domesticus | 407 b | 2950 b | 337 b | 1340 b | 3470 b | 2270 b | 19.3 b | 67.1 b | 6.2 b | 11.5 b | 0.21 b | 0.19 b | [33,50] |
| Efficacy | Functional Ingredients | Origin (Latin Name of the Insect) | Cell Line/Animal Model | In Vivo/In Vitro | Effects | References |
|---|---|---|---|---|---|---|
| Anti-cancer | Amblyomin-X | Amblyomma americanum | Human melanoma (SK-Mel-28) and primary pancreatic adenocarcinoma (Mia-PaCa-2) cells | In vitro | Amblyomin-X targets the ubiquitin-proteasome system and cell cycle-related genes to promote tumor cell death. | [92] |
| Bee venom | —— | Human lung cancer cell lines A549 and NCI-H460 | In vitro | By boosting the expression of death receptor 3 and deactivating NF-kappa β in non-small cell lung cancer cells, bee venom reduces the development of cancer cells. | [93] | |
| Glycosaminoglycan | Catharsius molossus | Melanoma mice induced by B16F10 cells | In vivo | By boosting TIMP-2 activity and adhesion activity, glycosaminoglycan can thicken the extracellular matrix, which in turn promotes the invasion and growth of tumor cells. | [94] | |
| 72-kDa anticancer protein (EPS72) | Eupolyphaga sinensis | Human lung cancer A549 cell line | In Vitro | A549 cells are made to detach and undergo apoptosis when exposed to EPS72, which also prevents cell migration and invasion by impairing cell adherence to collagen IV and fibronectin. | [95] | |
| Se-rich amino acids | Ziyang Silkworm pupae | Human hepatoma cells | In Vitro | Se-rich amino acids can inhibit cell viability, induce changes in cell morphology and cycle, and induce apoptosis through the production of ROS. | [96] | |
| Protein | Bombyx mori | Human colon cancer cells DLD-1 | In vitro | The protein from silkworm pupae prevents the growth of cancer cells, encourages apoptosis, and alters the energy metabolism of cancer cells by slowing down glycolysis and mitochondrial respiration. | [97] | |
| Protein hydrolysates | Bombyx mori | Human gastric cancer SGC-7901 cells | In vitro | Protein hydrolysates cause the accumulation of ROS, the depolarization of the mitochondrial membrane potential, and death in cancer cells while also blocking the S-phase cell cycle. | [98] | |
| Protein hydrolysates | Bombyx mori | MGC-803 gastric cancer cells | In vitro | Affects the metabolism of the MGC-803 cell energy supply. | [99] | |
| Ixolaris | Ixodes scapularis | U87-MG human glioblastoma cell lines | In vitro | The inhibitory effect of Ixolaris on tumor growth is associated with the downregulation of VEGF and reduced tumor angiogenesis. | [100] | |
| Protein extracts | Bombyx mori; Samia ricini | Breast cancer cells MCF-7 | In vitro | The extracts dramatically decreased the levels of IL-6, IL-1, and TNF-α in MCF-7 cells as well as their protein and nucleic acid composition. | [101] | |
| Antibacterial | Chitin film | Blaberus giganteus | Aspergillus niger (CBS 554.65) | In vitro | The hydrophobic properties of chitin film prevent microbial development. | [102] |
| Bee venom and melittin | —— | Borrelia burgdorferi | In vitro | All examined species of Borrelia burgdorferi were significantly affected by bee venom and melittin, which also suppressed the Lyme disease that Borrelia burgdorferi causes. | [103] | |
| Trx-stomoxynZH1 | Hermetia illucens | Bacterium | In vitro | Trx-stomoxyn ZH1 exhibits different inhibitory activities against a variety of bacteria. | [104] | |
| Silk, Cecropin B | transgenic Bombyx mori | E. coli (ATCC 25922) | In vitro | It prevents Gram-negative E. coli from growing. | [105] | |
| Pygidial gland secretion | Calosoma sycophanta | E. coli | In vitro | When compared to effective medications, pygidial gland secretion exhibited stronger antifungal activity. | [106] | |
| Royalisin | Apis mellifera | —— | In vitro | Royalisin has antibacterial activity against fungi and gram-positive and gram-negative bacteria. | [107] | |
| Chitin and chitosan | Bombyx mori | Bacillus cereus; Staphylococcus aureus; E. coli; Klebsiella pneumonia | In vitro | Compared to commercially available chitosan, it has stronger antibacterial properties. | [108] | |
| Pupal cell | Curculio caryae | Beauveria bassiana | In vitro | Entomopathogenic fungi are suppressed by pupal cells. | [109] | |
| Peptide fraction II | Antheraea mylitta | MDR Gram-negative bacteria | In vitro | The bacterial outer membrane may become pit-likely deformed as a result of peptide fraction II. | [110] | |
| Antioxidant | Polypheno ls | Bombyx mori | —— | In vitro | It has a strong capacity for scavenging ROS. | [111] |
| water-soluble chitosan | Clanis bilineata | d-galactose-induced-aged mouse model | In vivo | Water-soluble chitosan dramatically boosted the activity of superoxide dismutase and glutathione peroxidase in mouse stomachs demonstrating strong scavenging ability against superoxide anions and hydroxyl radicals and prevented the generation of malondialdehyde in mouse brain and serum. | [112] | |
| extract oil | Clanis bilineata | —— | In vitro | In experiments to prevent β-carotene from bleaching and scavenge DPPH radicals, extract oil demonstrated strong antioxidant activity. | [113] | |
| Sericin | Antheraea mylitta | —— | In vitro | Researchers have discovered that sericin contains anti-tyrosinase, anti-elastase, glutathione-S-transferase activity inhibition, free radical scavenging potential, and inhibits lipid peroxidation properties. | [114] | |
| Protein hydrolysates | Bombyx mori | Hepatic HepG2 cells | In vitro | In HepG2 cells, protein hydrolysates had ROS reduction, SOD expression, and glutathione synthesis effects. | [115] | |
| Hepatoprotection | Oil | Bombyx mori | Acetaminophen-induced acute liver injury Kunming mice model | In vivo | Silkworm oil reduces acute liver damage by blocking the NF-κB signaling pathway that is caused by oxidative stress. | [116] |
| Improves atherosclerosis | Crude extract | Bombyx mori | Male New Zealand white rabbits | in vivo | There are fewer atherosclerotic plaques in histopathology. | [117] |
| Silkworm Protein 30Kc6 | Bombyx mori | In vivo atherosclerosis rabbit model | in vivo | By lowering serum levels of total triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDLC), and total cholesterol (TC), this protein reduces atherosclerosis in rabbits. | [118] | |
| Antiviral | Alloferon | Calliphora vicina | the model of mice lethal pulmonary infection with human influenza viruses A and B | in vivo | Natural killer cells are stimulated by alloferon, which also causes the production of IFN in mice. | [119] |
| Anti-inflammatory | Glycosaminoglycan | Gryllus bimaculatus | Chronic arthritic rat model | in vivo | By inhibiting C-reactive protein (CRP) and rheumatoid factor, this GAG demonstrated a strong anti-edema impact. It also prevented atherogenesis by lowering proinflammatory cytokine levels. | [120] |
| Protein-enriched fraction/extracts (PE) | Musca domestica | Male C57/BL6 inbred mice | In vivo | In experimental atherosclerotic lesions, PE is efficient at inhibiting a range of pro-inflammatory responses in vivo. | [121] | |
| Sialostatin L | Ixodes scapularis | Mouse cell line CTLL-2 | In vitro | Sialostatin L possesses anti-inflammatory properties and prevents cytotoxic T lymphocyte proliferation. | [122] | |
| Non-peptide nitrogen compounds | Polyrhachis dives | Rat mesangial cells | In vitro | Non-peptide nitrogen compounds reduce inflammation by preventing the activity of COX-1, COX-2, and TNF-α. | [123] | |
| Venom | Nasonia vitripennis | Raw264.7 cells, murine fibrosarcoma L929sA cells, human embryonic kidney 293T cells | In vitro | In mammalian cells, it blocks the NF-κB signaling pathway. | [124] | |
| Peptides | Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria | —— | In vitro | The hydrolysates of edible insects include peptide fractions with significant lipoxygenase and cyclooxygenase-2 inhibitory activity. | [125] | |
| Immunomodulatory activity | Bee Venom Phospholipase A2 | Honeybee | BALB/c and C57BL/6 mice | In vivo | Bee Venom Phospholipase A2 Induces a Primary Type 2 Response that Is Dependent on the Receptor ST2 and Confers Protective Immunity | [126] |
| Polysaccharide | Bombyx mori | Penaeid prawns | In vivo | Activated innate immunity in prawns. | [127] | |
| Peptides | Bombyx mori | Mouse spleen cells | In vitro | The expression of immune-related factors is stimulated by active peptides. | [128] | |
| As medical biomaterial | Silk (cocoons) | Hydropsyche angustipennis | —— | —— | Silk is a promising biomaterial for tissue engineering since it may be utilized as a scaffold for cell growth. | [129] |
| Regulation of blood sugar and blood lipids | Glycosaminoglycan | Gryllus bimaculatus | Wistar rats | In vivo | Total cholesterol, phospholipid, and glucose levels decreased in the treated rats in a dose-dependent way, as did abdominal and epididymal fat. | [130] |
| Protein hydrolysates | Bombyx mori | 3T3-L1 cells | In vitro | Protein hydrolysates can boost leptin levels and boost GLUT4 levels to promote glucose uptake and decrease fat storage, respectively. | [131] | |
| Oil | Bombyx mori | Sprague– Dawley rats | In vivo | Silkworm pupa oil increases fat metabolism, which decreases blood lipid levels. | [132] | |
| Soluble fibroin | Bombyx mori | 3T3-L1 adipocyte | In vitro | It improves glucose uptake and metabolism. | [133] | |
| Protein | Bombyx mori | Male C57BL/6mice | In vivo | Silkworm pupae protein dramatically lowers blood glucose levels in mice. | [134] | |
| Blood pressure reduction | Peptide hydrolysates | Silkworm pupae | Spontaneously hypertensive rats | In vivo | In the treatment group, hypertensive mice’s systolic blood pressure dropped and was dose-dependent. | [135] |
| Protein hydrolysates | Bombyx mori | RP- HPLC | In vitro | Silkworm protein hydrolysates have an inhibiting effect on the angiotensin I-converting enzyme. | [136] | |
| Angiogenesis inhibition | Troponin I-like | Haemaphysalis longicornis | Human vascular endothelial cells | In vitro | Troponin I-like compounds effectively prevented human vascular endothelial cells from forming capillaries. There was evidence of a dose-dependent inhibition. | [137] |
| Salivary gland extracts | Ixodes scapularis | microvascular endothelial cell | In vitro | Salivary gland extracts are negative regulators of angiogenesis-dependent wound healing and tissue repair and suppress the proliferation of microvascular endothelial cells in a dose-dependent way. | [138] | |
| Caffeic acid phenethyl ester | Apis mellifera | Human umbilical vein endothelial cells | In vitro | A potent inhibitor of vascular endothelial growth factor-induced angiogenesis is the caffeine acid phenethyl ester. | [139] | |
| Crude whole body extracts | Tabanus bovinus | Rat corneal model | In vivo | Extracts greatly decreased the length of blood vessels in the neovascularized cornea and the thick vascular networks emanating from the corneoscleral limbus. | [140] | |
| Haemangin | Haemaphysalis longicornis | Rabbits | In vivo | By preventing vascular endothelial cells from proliferating and triggering death, haemangin can impair angiogenesis and wound healing. | [141] | |
| Anti-apoptotic | Silkworm Protein 30Kc6 | Bombyx mori | The in vitro cell apoptosis model of HUVEC was induced by oxidized low-density lipoprotein. | In vitro | By obstructing the MAPK signaling pathway, 30Kc6 inhibits the death of HUVEC cells brought on by oxidized LDL. | [118] |
| Storage protein 1 | —— | HeLa cells | In vitro | Storage protein 1 may operate as an upstream inhibitor of apoptosis since it decreases the loss of mitochondrial membrane potential and prevents caspase-3 activation. | [142] | |
| Recombinant 30 K protein | Bombyx mori | HeLa cells; Spodoptera Frugiperda (Sf9) cells | In vitro | In human and insect cells, the 30 K protein inhibits the apoptosis that is brought on by viruses or toxins. | [143] | |
| Anti-genotoxic | Pupae extract | Antheraea assamensis | Normal human leukocytes | In vitro | DNA damage brought on by hydrogen peroxide was stopped by pupa extract at 1 mg/mL. | [144] |
| Wound healing | Honey | Apis mellifera | —— | In vitro | A strong non-antibacterial chemical found in honey promotes the cells responsible for wound healing. | [145] |
| Royal jelly protein 1 | Honeybee | keratinocytes | In vitro | Keratinocytes are activated by royal jelly protein 1. | [146] | |
| Anti-allergy | Royal jelly protein 3 | Honeybee | OVA/alum-immunized mice | In vivo | Royal jelly protein 3 suppresses IL-4 production by activating splenocytes with anti-CD3 receptors. | [147] |
| Anti-fatigue agents | Silk powder | Bombyx mori | Imprinting Control Region (ICR) mice | In vivo | In mice, the silkworm powder was able to prolong swimming time and muscle mass while lowering exhaustion. | [148] |
| Protein isolate | Buffalo larvae | Healthy young men | In vivo | Consumption of insect protein isolates improves individuals’ muscular strength. | [149] | |
| Improves skin wrinkles | Honeybee-venom serum | A. mellifera L. | Healthy women | In vitro | By reducing the total wrinkle area, total wrinkle counts, and average wrinkle depth, bee venom serum can clinically improve facial wrinkles. | [150] |
| Regulation of intestinal flora | Whole worm | Hermetiaillucens | Laying hens | In vivo | The species and relative abundance of gut bacteria are changed by consuming Hermetia illucens. | [151] |
| Partially defatted meal of larvae | Rainbow trout | Rainbow trout | In vivo | The diversity of the gut flora has risen, and its community organization has changed. | [152] | |
| Whole cricket powder | Cricket | Healthy adults | In vivo | Consuming cricket powder can help probiotics grow and minimize inflammatory responses. | [153] | |
| Anti-HIV | Venom peptide | Honeybees | HIV cells | In vitro | HIV-infected cells that absorb bee venom peptides exhibit decreased HIV gene expression and replication. | [154] |
| Treatment of Parkinson’s disease | Bee Venom | Honeybee | Chronic mouse model of MPTP/probenecid | In vivo | In an animal model modeling the chronic degenerative process of Parkinson’s disease, bee venom offers long-lasting protection. | [155] |
| Anti-Alzheimer’s disease | Silkworm pupae Powder | Bombyx mori | Male Wistar rats | In vivo | In vivo, hippocampus memory impairments and hippocampal neuron density in mice were both considerably enhanced by silkworm pupae powder. | [156] |
| Silkworm pupa vaccine | Bombyx mori | Transgenic mouse model of AD | In vivo | In AD mice, it enhances memory and cognitive function. | [157] | |
| Alcohol detoxification | Extracts | Bombyx mori | ICR mice | In vivo | Alcohol dehydrogenase activity in the liver was dramatically boosted by oral administration of silkworm pupa extract at 0.5 mg/mL. | [158] |
| Treatment of gastric ulcers | Oil | Bombyx mori | Hydrochloric acid/ethanol-induced gastric ulcers Kunming mice model | In vivo | Gastric ulcers can be treated with silkworm pupa oil by shrinking the ulcer and lessening the inflammatory response. | [159] |
| Promotes hair growth | Fermented cricket powder | Gryllus bimaculatus | Male C57BL/6 mice | In vivo | By controlling the expression of growth factors, the amino acids and other trace components in fermented cricket powder enhance hair development. | [160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, D.; Zhou, S.; Duan, H.; Guo, J.; Yan, W. Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review. Foods 2022, 11, 3961. https://doi.org/10.3390/foods11243961
Zhou Y, Wang D, Zhou S, Duan H, Guo J, Yan W. Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review. Foods. 2022; 11(24):3961. https://doi.org/10.3390/foods11243961
Chicago/Turabian StyleZhou, Yaxi, Diandian Wang, Shiqi Zhou, Hao Duan, Jinhong Guo, and Wenjie Yan. 2022. "Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review" Foods 11, no. 24: 3961. https://doi.org/10.3390/foods11243961
APA StyleZhou, Y., Wang, D., Zhou, S., Duan, H., Guo, J., & Yan, W. (2022). Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review. Foods, 11(24), 3961. https://doi.org/10.3390/foods11243961









