QTRAP LC/MS/MS of Garlic Nanoparticles and Improving Sunflower Oil Stabilization during Accelerated Shelf Life Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Preparation of Garlic Nanoparticles (Ga-NPs) and Garlic Extracts
2.3. LC/MS/MS Fingerprint
2.4. Total Phenolics Content (TPC), Total Flavonoids Content (TFC), and Antioxidant Ability of Extracts
2.5. Sunflower Oil (SFO) and Ga-NPs
2.6. Oxidative Stability Parameters of SFO during Storage
2.6.1. Peroxide Value (PV)
2.6.2. p-Anisidine Value (p-AnV) and Totox Value (TV)
2.6.3. Conjugated Dienes (CDs) and Conjugated Trienes (CTs)
2.6.4. Induction Period (IP), Antioxidant Efficiency, and Shelf Life Prediction
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Garlic and Garlic Nanoparticles (Ga-NPs)
3.1.1. Antioxidant Capacity
3.1.2. LC/MS/MS Fingerprint
3.2. Impact of Ga-NPsE on the Oxidative Stability of SFO Samples
3.2.1. Peroxide Value (PV)
3.2.2. p-Anisidine Value (p-AnV)
3.2.3. Totox Value (TV)
3.2.4. Conjugated Dienes (CDs) and Conjugated Trienes (CTs)
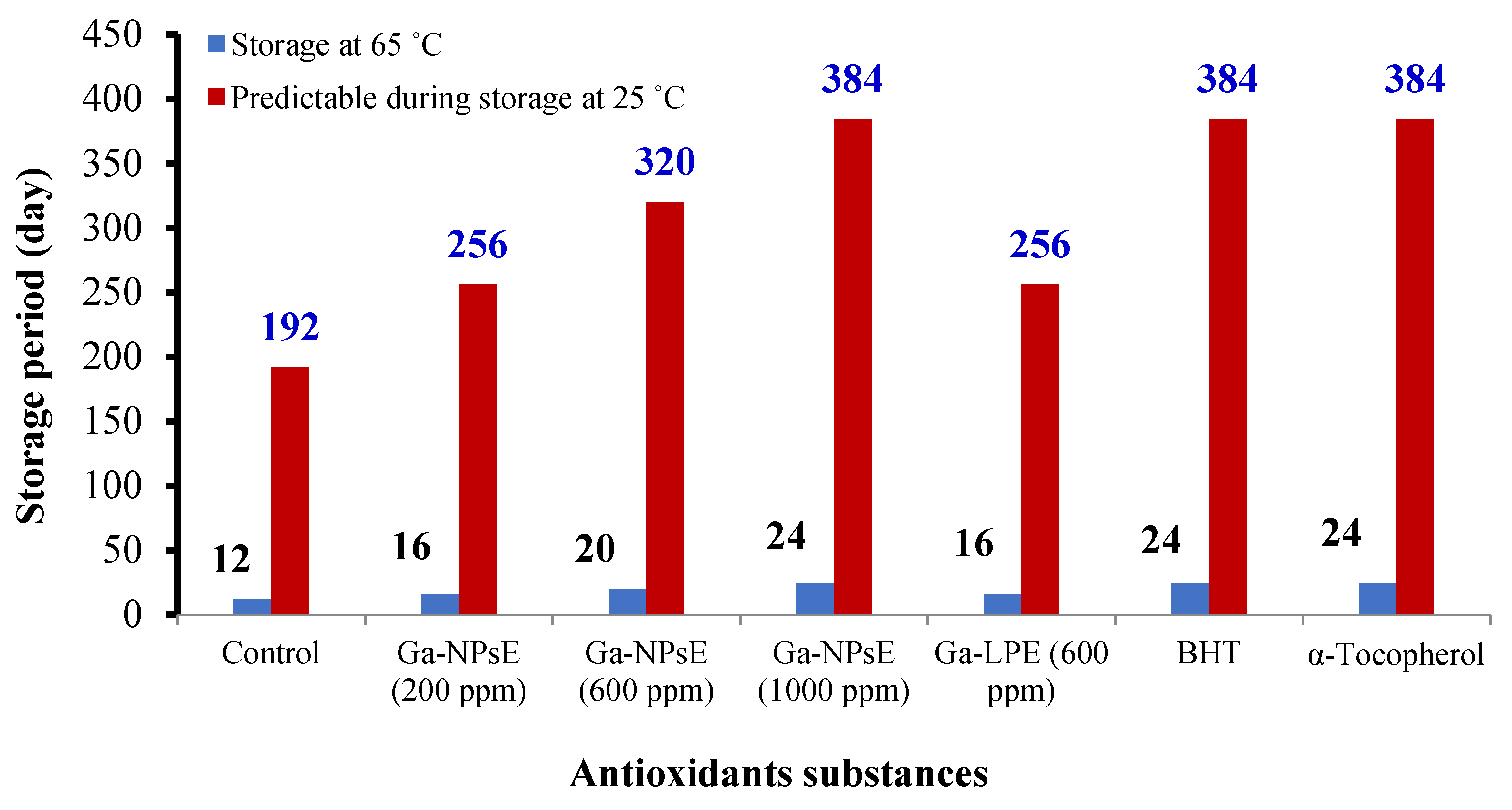
3.3. Induction Period (IP) and Antioxidant Efficiency of Ga-NPsE
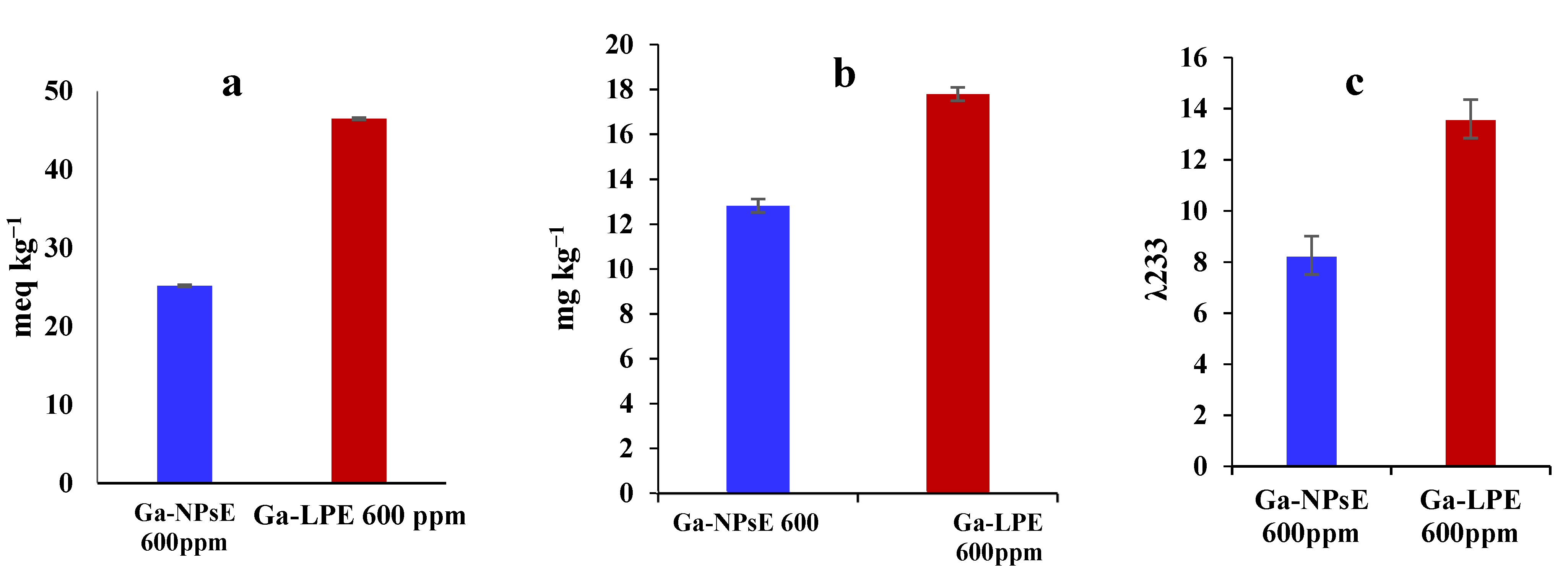
3.4. Impact of Ga-NPsE and/or Ga-LPE (600 ppm) on Stability
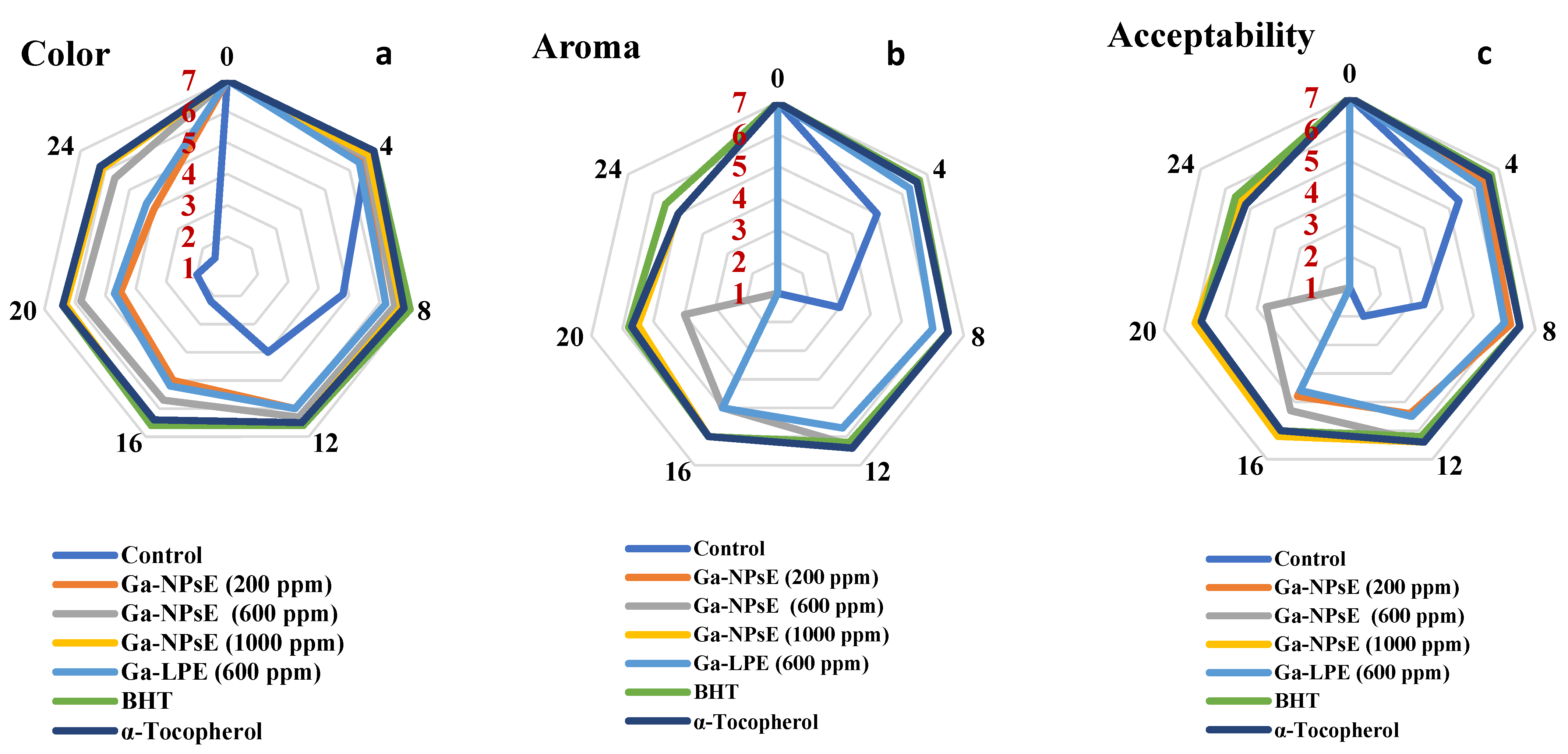
3.5. Sensory Evaluation of SFO Incorporating Ga-NPsE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romanić, R.S.; Lužaić, T.Z.; Radić, B.Đ. Enriched sunflower oil with omega 3 fatty acids from flaxseed oil: Prediction of the nutritive characteristics. LWT 2021, 150, 112064. [Google Scholar] [CrossRef]
- Cherif, A.; Slama, A. Stability and Change in Fatty Acids Composition of Soybean, Corn, and Sunflower Oils during the Heating Process. J. Food Qual. 2022, 2022, 6761029. [Google Scholar] [CrossRef]
- Xu, T.-t.; Li, J.; Fan, Y.-W.; Zheng, T.-W.; Deng, Z.-Y. Comparison of oxidative stability among edible oils under continuous frying conditions. Int. J. Food Prop. 2015, 18, 1478–1490. [Google Scholar] [CrossRef]
- Viana da Silva, M.; Santos, M.R.C.; Alves Silva, I.R.; Macedo Viana, E.B.; Dos Anjos, D.A.; Santos, I.A.; Barbosa de Lima, N.G.; Wobeto, C.; Jorge, N.; Lannes, S.C.D.S. Synthetic and natural antioxidants used in the oxidative stability of edible oils: An overview. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F.C. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Morsy, M.K.; Morsy, O.M.; Elbarbary, H.A.; Saad, M.A. Enhancing of oxidative stability and quality attributes of olive oil using spirulina (Arthospira platensis) nanoparticles. LWT 2019, 101, 444–455. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E. Essential oils from herbs and spices as natural antioxidants: Diversity of promising food applications in the past decade. Food Rev. Int. 2021, 1–31. [Google Scholar] [CrossRef]
- Morsy, M.K.; Sami, R.; Algarni, E.; Al-Mushhin, A.A.; Benajiba, N.; Almasoudi, A.G.; Mekawi, E. Phytochemical Profile and Antioxidant Activity of Sesame Seed (Sesamum indicum) By-Products for Stability and Shelf Life Improvement of Refined Olive Oil. Antioxidants 2022, 11, 338. [Google Scholar] [CrossRef]
- Akbarpour, A.; Kavoosi, B.; Hosseinifarahi, M.; Tahmasebi, S.; Gholipour, S. Evaluation of yield and phytochemical content of different Iranian garlic (Allium sativum L.) ecotypes. Int. J. Hortic. Sci. Technol. 2021, 8, 1–16. [Google Scholar]
- Kovarovič, J.; Bystricka, J.; Vollmannova, A.; Toth, T.; Brindza, J. Biologically valuable substances in garlic (Allium sativum L.)–A review. J. Cent. Eur. Agric. 2019, 20, 292–304. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, B.; Yagoub, A.E.A.; Ma, H.; Sun, Y.; Xu, X.; Yu, X.; Zhou, C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Misha, G.; Malaiya, A.; Jain, A.; Mody, N.; Raichur, A.M. Antimicrobial application potential of phytoconstituents from turmeric and garlic. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 409–435. [Google Scholar]
- Rauf, A.; Abu-Izneid, T.; Thiruvengadam, M.; Imran, M.; Olatunde, A.; Shariati, M.A.; Bawazeer, S.; Naz, S.; Shirooie, S.; Sanches-Silva, A. Garlic (Allium sativum L.): Its chemistry, nutritional composition, toxicity, and anticancer properties. Curr. Top. Med. Chem. 2022, 22, 957–972. [Google Scholar] [CrossRef]
- Mancini, S.; Nuvoloni, R.; Pedonese, F.; Paci, G. Effects of garlic powder and salt additions in rabbit meat burgers: Preliminary evaluation. J. Food Process. Preserv. 2019, 43, e13894. [Google Scholar] [CrossRef]
- Khan, S.; Guha, P. Effect of Garlic Essential Oil on Performance of Rice Bran Oil During Deep Fat Frying. Biosci. Biotechnol. Res. Asia 2017, 14, 473–481. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef]
- Rawat, K. Nanotechnology in Food Production. In Nanotechnology; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2021; pp. 333–368. [Google Scholar]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014, 79, M675–M684. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of nanotechnology in food packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Shehzad, Q.; Rehman, A.; Jafari, S.M.; Zuo, M.; Khan, M.A.; Ali, A.; Khan, S.; Karim, A.; Usman, M.; Hussain, A. Improving the oxidative stability of fish oil nanoemulsions by co-encapsulation with curcumin and resveratrol. Colloids Surf. B Biointerfaces 2021, 199, 111481. [Google Scholar] [CrossRef]
- Saeed, A.; Shabbir, A.; Khan, A. Stabilization of sunflower oil by using potato peel extract as a natural antioxidant. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Poiana, M.-A. Enhancing oxidative stability of sunflower oil during convective and microwave heating using grape seed extract. Int. J. Mol. Sci. 2012, 13, 9240–9259. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zu, Y.; Yang, L.; Lu, Q.; Wang, W. Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. Int. J. Food Sci. Technol. 2014, 49, 385–391. [Google Scholar] [CrossRef]
- Pezeshky, A.; Khakbaz Heshmati, M.; Abutalebi, Z. Effect of thyme and ginger metanoic extraction on the oxidative stability of sunflower oil. J. Food Res. 2020, 30, 107–121. [Google Scholar]
- Khataee, A.; Fathinia, S.; Fathinia, M. Production of pyrite nanoparticles using high energy planetary ball milling for sonocatalytic degradation of sulfasalazine. Ultrason. Sonochem. 2017, 34, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Zhu, Q.; Kakino, K.; Nogami, C.; Ohnuki, K.; Shimizu, K. An LC-MS/MS-SRM method for simultaneous quantification of four representative organosulfur compounds in garlic products. Food Anal. Methods 2016, 9, 3378–3384. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem. 2010, 118, 656–662. [Google Scholar] [CrossRef]
- Chong, Y.M.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci. 2015, 12, 18–25. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 1998. [Google Scholar]
- Paquot, C. Standard Methods for the Analysis of Oils, Fats and Derivatives; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Läubli, M.W.; Bruttel, P.A. Determination of the oxidative stability of fats and oils: Comparison between the active oxygen method (AOCS Cd 12-57) and the rancimat method. J. Am. Oil Chem. Soc. 1986, 63, 792–795. [Google Scholar] [CrossRef]
- Bandonien, D.; Pukalskas, A.; Venskutonis, P.; Gruzdien, D. Preliminary screening of antioxidant activity of some plant extracts in rapeseed oil. Food Res. Int. 2000, 33, 785–791. [Google Scholar] [CrossRef]
- Steele, R. Understanding and Measuring the Shelf-Life of Food; Woodhead Publishing: Cambridge, UK, 2004. [Google Scholar]
- Sadeghi, E.; Karami, F.; Etminan, A. The effect of Ferulago angulata (Schlecht) Boiss essential oil on stabilization of sunflower oil during accelerated storage. J. Food Process. Preserv. 2017, 41, e12745. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach; McGraw-Hill Kogakusha, Ltd.: Tokyo, Japan, 1980. [Google Scholar]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Soto, V.; Gonzalez, R.E.; Sance, M.M.; Galmarini, C.R. Organosulfur and phenolic content of garlic (Allium sativum L.) and onion (Allium cepa L.) and its relationship with antioxidant activity. In Proceedings of the VII International Symposium on Edible Alliaceae, Nigde, Turkey, 21–25 May 2015; Volume 1143, pp. 277–290. [Google Scholar]
- Della Pelle, F.; Compagnone, D. Nanomaterial-based sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Bhanger, M. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007, 100, 246–254. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre-and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Leelarungrayub, N.; Rattanapanone, V.; Chanarat, N.; Gebicki, J.M. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition 2006, 22, 266–274. [Google Scholar] [CrossRef]
- Tavares, L.; Barros, H.L.B.; Vaghetti, J.C.P.; Noreña, C.P.Z. Microencapsulation of garlic extract by complex coacervation using whey protein isolate/chitosan and gum arabic/chitosan as wall materials: Influence of anionic biopolymers on the physicochemical and structural properties of microparticles. Food Bioprocess Technol. 2019, 12, 2093–2106. [Google Scholar] [CrossRef]
- Prati, P.; Henrique, C.M.; Souza, A.S.d.; Silva, V.S.N.d.; Pacheco, M.T.B. Evaluation of allicin stability in processed garlic of different cultivars. Food Sci. Technol. 2014, 34, 623–628. [Google Scholar] [CrossRef]
- Ilić, J.D.; Nikolovski, B.G.; Petrović, L.B.; Kojić, P.S.; Lončarević, I.S.; Petrović, J.S. The garlic (A. sativum L.) extracts food grade W1/O/W2 emulsions prepared by homogenization and stirred cell membrane emulsification. J. Food Eng. 2017, 205, 1–11. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C.; Márquez-Ruiz, G. Oxidative rancidity in foods and food quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–32. [Google Scholar]
- Nyam, K.; Wong, M.; Long, K.; Tan, C. Oxidative stability of sunflower oils supplemented with kenaf seeds extract, roselle seeds extract and roselle extract, respectively under accelerated storage. Int. Food Res. J. 2013, 20, 695–701. [Google Scholar]
- Carelli, A.A.; Franco, I.C.; Crapiste, G.H. Effectiveness of added natural antioxidants in sunflower oil. Grasas Aceites 2005, 56, 303–310. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Mamouni, R.; Matthäus, B.; Charrouf, Z. Chemical Characterization and Kinetic parameter determination under Rancimat test conditions of four monovarietal virgin olive oils grown in Morocco. Ocl 2016, 23, A401. [Google Scholar] [CrossRef]

| Sample | TPC (mg g−1 dw Extract) | TFC (mg g−1 dw Extract) | DPPH IC50 (μg mL−1) | ABTS assay (μg mL−1) | R |
|---|---|---|---|---|---|
| Balady | 27.25 ± 0.45 b | 1.52 ± 0.14 b | 11.87 ± 0.76 d | 10.88 ± 1.51 e | 1 |
| Italian Red | 25.35 ± 0.88 c | 0. 91 ± 0.65 c | 12.14 ± 0.41 d | 11.34 ± 0.87 d | 1 |
| Sids-40 | 24.37 ± 1.07 c | 0.65 ± 0.33 d | 14.41 ± 0.32 c | 13.11 ± 0.23 c | 1 |
| Chinese | 23.30 ± 0.78 d | 0.74 ± 0.65 d | 17.77 ± 2.06 a | 15.55 ± 0.22 b | 1 |
| Brazilian Hozan | 22.76 ± 1.56 d | 0.72 ± 1.33 d | 18.30 ± 1.65 a | 17.17 ± 0.44 a | 1 |
| BHT (200 ppm) | - | - | 5.05 ± 0.87 f | 3.79 ± 1.42 g | |
| α-Tocopherol (200 ppm) | - | - | 16.15 ± 1.61 b | 13.21 ± 0.55 c |
| Sample | Storage Period (day) | ||||||
|---|---|---|---|---|---|---|---|
| Time Zero | 4 | 8 | 12 | 16 | 20 | 24 | |
| Peroxide value (meq kg−1) | |||||||
| Control | 1.20 ± 0.02 a G | 20.22 ± 0.15 a F | 25.15 ± 0.33 b E | 40.54 ± 0.21a D | 78.00 ± 0.02 a C | 170.11 ± 0.56 a A | 166.16 ± 0.34 a B |
| Ga-NPsE (200 ppm) | 1.03 ± 0.01 a G | 17.39 ± 0.21b F | 24.09 ± 0.04 b E | 31.16 ± 0.43 c D | 35.25 ± 0.6 d C | 39.67 ± 0.09 c B | 41.78 ± 0.29 b A |
| Ga-NPsE (600 ppm) | 1.01 ± 0.03 a G | 12.30 ± 0.02 c F | 14.43 ± 0.13 c E | 18.45 ± 0.32 d D | 28.13 ± 0.17 e C | 32.09 ± 0.22 d B | 38.65 ± 0.11d A |
| Ga-NPsE (1000 ppm) | 1.03 ± 0.02 a G | 5.24 ± 0.04 a F | 10.16 ± 0.07 d E | 13.32 ± 0.01 e D | 17.75 ± 0.05 f C | 18.81 ± 0.46 f B | 20.14 ± 0.16 e A |
| Ga-LPE (600 ppm) | 1.12 ± 0.04 a G | 16.44 ± 0.06 b F | 28.54 ± 0.11a E | 35.33 ± 0.05 b D | 37.87 ± 0.28 c C | 39.34 ± 0.03 c B | 46.43 ± 0.12 c A |
| BHT (200 ppm) | 1.11 ± 0.01 a G | 3.12 ± 0.16 d F | 4.16 ± 0.10 e E | 10.52 ± 0.14 f D | 13.66 ± 0.26 g C | 16.54 ± 0.12 g B | 20.22 ± 0.18 f A |
| α-Tocopherol (200 ppm) | 1.02 ± 0.01 a G | 15.14 ± 0.31c F | 28.11 ± 0.07 a E | 40.43 ± 0.15 a D | 57.16 ± 0.18 b C | 65.36 ± 0.43 b B | 70.62 ± 0.07 b A |
| p-Anisidine value (mg kg−1) | |||||||
| Control | 4.20 ± 0.04 a G | 6.52 ± 0.25 a F | 11.65 ± 0.15 a E | 14.38 ± 0.31a D | 16.55 ± 0.42 a C | 19.12 ± 0.11 a B | 25.26 ± 0.04 a A |
| Ga-NPsE (200 ppm) | 4.12 ± 0.10 a G | 5.04 ± 0.12 b F | 7.31 ± 0.30 c E | 9.96 ± 0.05 d D | 11.44 ± 0.05 c C | 14.22 ± 0.12 d B | 16.65 ± 0.02 d A |
| Ga-NPsE (600 ppm) | 4.03 ± 0.03 a G | 4.80 ± 0.22 c F | 6.93 ± 0.09 c E | 7.46 ± 0.82 e D | 9.12 ± 0.21 d C | 10.12 ± 0.06 e B | 12.81 ± 0.31 e A |
| Ga-NPsE (1000 ppm) | 4.11 ± 0.23 a G | 4.24 ± 0.04 c F | 5.19 ± 0.11 d E | 6.15 ± 0.04 f D | 7.75 ± 0.23 e C | 8.83 ± 0.04 f B | 10.02 ± 0.12 f A |
| Ga-LPE (600 ppm) | 4.31 ± 0.05 a G | 5.49 ± 0.20 b F | 7.75 ± 0.57 c E | 12.04 ± 0.03 c D | 15.27 ± 0.06 b C | 16.11 ± 0.17 c B | 17.78 ± 0.28 c A |
| BHT (200 ppm) | 3.98 ± 0.21 a G | 4.33 ± 0.01 c F | 4.19 ± 0.07 e E | 5.02 ± 0.13 g D | 6.68 ± 0.09 f C | 8.84 ± 0.12 f B | 9.72 ± 0.09 g A |
| α-Tocopherol (200 ppm) | 4.02 ± 0.09 a G | 5.56 ± 0.21 b F | 8.67± 0.02 b E | 13.43 ± 0.12 b D | 16.91 ± 0.01 a C | 17.93 ± 0.30 b B | 19.54 ± 0.23 b A |
| Sample | Storage Period (day) | ||||||
|---|---|---|---|---|---|---|---|
| Time Zero | 4 | 8 | 12 | 16 | 20 | 24 | |
| Control | 6.61 ± 0.31 a G | 46.97 ± 0.71 a F | 61.95 ± 0.73 b E | 95.47 ± 0.29 a D | 172.55 ± 0.98 a C | 359.33 ± 0.67 a A | 357.58 ± 1.17 a B |
| Ga-NPsE (200 ppm) | 6.36 ± 0.55 a G | 40.26 ± 0.82 b F | 55.94± 0.08 c E | 74.36 ± 0.23 c D | 85.77 ± 0.75 d C | 95.46 ± 0.53 c B | 101.34 ± 0.7 d A |
| Ga-NPsE (600 ppm) | 6.05 ± 0.40 a G | 29.4 ± 0.45 e F | 35.79 ± 0.51 d E | 44.36 ± 1.2 d D | 65.37 ± 0.69 e C | 74.31 ± 0.54 e B | 90.11 ± 1.3 e A |
| Ga-NPsE (1000 ppm) | 6.16 ± 0.24 a G | 14.72 ± 0.39 f F | 25.51 ± 0.9 e E | 32.8 ± 0.60 e D | 43.25 ± 0.19 f C | 52.45 ± 0.34 f B | 60.31 ± 1.1f A |
| Ga-LPE (600 ppm) | 6.35 ± 0.32 a G | 37.91 ± 0.37 c F | 64.39± 0.33 a E | 80.61 ± 0.96 b D | 87.18 ± 0.39 c C | 92.9 ± 0.36 d B | 109.51 ± 0.98 c A |
| BHT (200 ppm) | 6.21 ± 0.29 a G | 10.58 ± 0.93 g F | 12.51 ± 0.11 f E | 26.06 ± 0.41f D | 34 ± 0.92 g C | 41.92 ± 0.43 g B | 50.15 ± 0.87 g A |
| α-Tocopherol (200 ppm) | 6.06 ± 0.62 a G | 35.85 ± 0.81d F | 64.88± 0.97 a E | 94.29 ± 0.12 a D | 131.23 ± 0.88 b C | 148.66 ± 0.77 b B | 160.78 ± 0.96 b A |
| Sample | Storage Period (day) | ||||||
|---|---|---|---|---|---|---|---|
| Time Zero | 4 | 8 | 12 | 16 | 20 | 24 | |
| Conjugated dienes value (ε1%1cm λ233) | |||||||
| Control | 2.50 ± 0.03 a G | 6.17 ± 0.04 a F | 9.19 ± 0.10 a E | 11.07 ± 0.2 a D | 13.65 ± 0.11 a C | 15.22 ± 0.67 a B | 19.22 ± 0.01 a A |
| Ga-NPsE (200 ppm) | 2.46 ± 0.06 a G | 4.11 ± 0.2 b F | 5.34± 0.06 c E | 7.01 ± 0.9 d D | 8.08 ± 0.03 d C | 9.00 ± 0.33 d B | 12.11± 0.98 d A |
| Ga-NPsE (600 ppm) | 2.45 ± 0.04 a G | 4.04 ± 0.04 b F | 5.00 ± 0.05 c E | 5.06± 0.02 e D | 5.37 ± 0.06 e C | 6.13 ± 0.51 e B | 8.21 ± 0.8 e A |
| Ga-NPsE (1000 ppm) | 2.45 ± 0.09a G | 3.15 ± 0.03 c F | 3.50 ± 0.1d E | 4.10 ± 0.40 f D | 4.65 ± 0.19 f C | 5.05 ± 0.3 f B | 6.71 ± 0.01 f A |
| Ga-LPE (600 ppm) | 2.47 ± 0.09 a G | 4.33 ± 0.82 b F | 5.82± 0.08 c E | 8.18 ± 0.02 c D | 9.79 ±0.06 c C | 10.14 ± 0.05 c B | 13.55 ± 0.7 c A |
| BHT (200 ppm) | 2.41 ± 0.07 a G | 2.98 ± 0.14 d F | 3.53 ± 0.21 d E | 3.91 ± 0.04 g D | 4.61 ± 0.12 f C | 5.0 ± 0.21 f B | 6.15 ± 0.07 f A |
| α-Tocopherol (200 ppm) | 2.46 ± 0.07 a G | 4.29 ± 0.17 b F | 7.46± 0.27 b E | 9.21 ± 0.22 b D | 10.00 ± 0.23 b C | 12.56 ± 0.30 b B | 16.00 ± 0.09 b A |
| Conjugated trienes value (ε1%1cm λ268) | |||||||
| Control | 0.54 ± 0.03 a G | 1.55 ± 0.01 a F | 1.89 ± 0.10 a E | 2.09 ± 0.02 a D | 2.60 ± 0.01 a C | 3.00 ± 0.01 a B | 4.12 ± 0.02 a A |
| Ga-NPsE (200 ppm) | 0.53± 0.04 a G | 1.00 ± 0.2 a F | 1.22± 0.04 c E | 1.61 ± 0.9 b D | 1.78 ± 0.03 b C | 2.54 ± 0.03 b B | 3.42 ± 0.09 b A |
| Ga-NPsE (600 ppm) | 0.52 ± 0.03 a G | 0.94 ± 0.03 b F | 1.01 ± 0.05 c E | 1.39± 0.1b D | 1.62 ± 0.04 c C | 2.22 ± 0.02 c B | 3.33 ± 0.01b A |
| Ga-NPsE (1000 ppm) | 0.52 ± 0.03a G | 0.81 ± 0.01b F | 0.91 ± 0.01d E | 1.20 ± 0.04 b D | 1.42 ± 0.01 c C | 2.15 ± 0.03 c B | 3.11 ± 0.07 c A |
| Ga-LPE (600 ppm) | 0.53 ± 0.07 a G | 1.12 ± 0.02a F | 1.14± 0.03 c E | 1.88 ± 0.02 a D | 2.15 ±0.03 b C | 2.73 ± 0.02 b B | 3.54 ± 0.05 b A |
| BHT (200 ppm) | 0.51 ± 0.06 a G | 0.62 ± 0.01 c F | 0.87 ± 0.02 d E | 1.11 ± 0.04 c D | 1.33 ± 0.01c C | 1.98 ± 0.02 d B | 3.00 ± 0.05 d A |
| α-Tocopherol (200 ppm) | 0.53 ± 0.09 a G | 1.23 ± 0.01b F | 1.43± 0.07 b E | 1.98 ± 0.04 a D | 2.26 ± 0.01 b C | 2.86 ± 0.03 a B | 3.7 ± 0.17 b A |
| IP (h) | PF | AA | |
|---|---|---|---|
| Control | 7.6 ± 0.11 g | - | - |
| Ga-NPsE (200 ppm) | 15.4 ± 0.15d | 2.03± 0.12 d | 0.44 ± 0.09 c |
| Ga-NPsE (600 ppm) | 18.7 ± 0.30 c | 2.46± 0.31 c | 0.63 ± 0.1b |
| Ga-NPsE (1000 ppm) | 22.2 ± 0.24 b | 2.92± 0.15 b | 0.82 ± 0.08 a |
| Ga-LPE (600 ppm) | 12.0 ± 0.2 e | 1.57± 0.03 e | 0.27 ± 0.21 d |
| BHT (200 ppm) | 25.3 ± 0.21 a | 3.33± 0.09 a | 1 ± 0.11a |
| α-Tocopherol (200 ppm) | 9.2 ± 0.32f | 1.21 ± 0.15 f | 0.9 ± 0.12 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelli, N.; Mekawi, E.; Ebrahim Abdel-Alim, M.; Salim, N.S.; El-Nagar, M.; Al-Dalain, S.Y.; Adlan Abdalla, R.; Nagarajan, G.; Fadhal, E.; Ibrahim, R.I.H.; et al. QTRAP LC/MS/MS of Garlic Nanoparticles and Improving Sunflower Oil Stabilization during Accelerated Shelf Life Storage. Foods 2022, 11, 3962. https://doi.org/10.3390/foods11243962
Abdelli N, Mekawi E, Ebrahim Abdel-Alim M, Salim NS, El-Nagar M, Al-Dalain SY, Adlan Abdalla R, Nagarajan G, Fadhal E, Ibrahim RIH, et al. QTRAP LC/MS/MS of Garlic Nanoparticles and Improving Sunflower Oil Stabilization during Accelerated Shelf Life Storage. Foods. 2022; 11(24):3962. https://doi.org/10.3390/foods11243962
Chicago/Turabian StyleAbdelli, Nouara, Enas Mekawi, Mohammed Ebrahim Abdel-Alim, Nesreen Saad Salim, Mahran El-Nagar, Sati Y. Al-Dalain, Ridab Adlan Abdalla, Ganesan Nagarajan, Emad Fadhal, Rashid I. H. Ibrahim, and et al. 2022. "QTRAP LC/MS/MS of Garlic Nanoparticles and Improving Sunflower Oil Stabilization during Accelerated Shelf Life Storage" Foods 11, no. 24: 3962. https://doi.org/10.3390/foods11243962
APA StyleAbdelli, N., Mekawi, E., Ebrahim Abdel-Alim, M., Salim, N. S., El-Nagar, M., Al-Dalain, S. Y., Adlan Abdalla, R., Nagarajan, G., Fadhal, E., Ibrahim, R. I. H., Afkar, E., & Morsy, M. K. (2022). QTRAP LC/MS/MS of Garlic Nanoparticles and Improving Sunflower Oil Stabilization during Accelerated Shelf Life Storage. Foods, 11(24), 3962. https://doi.org/10.3390/foods11243962







