Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gross Composition Analysis
2.3. Protein Isolation
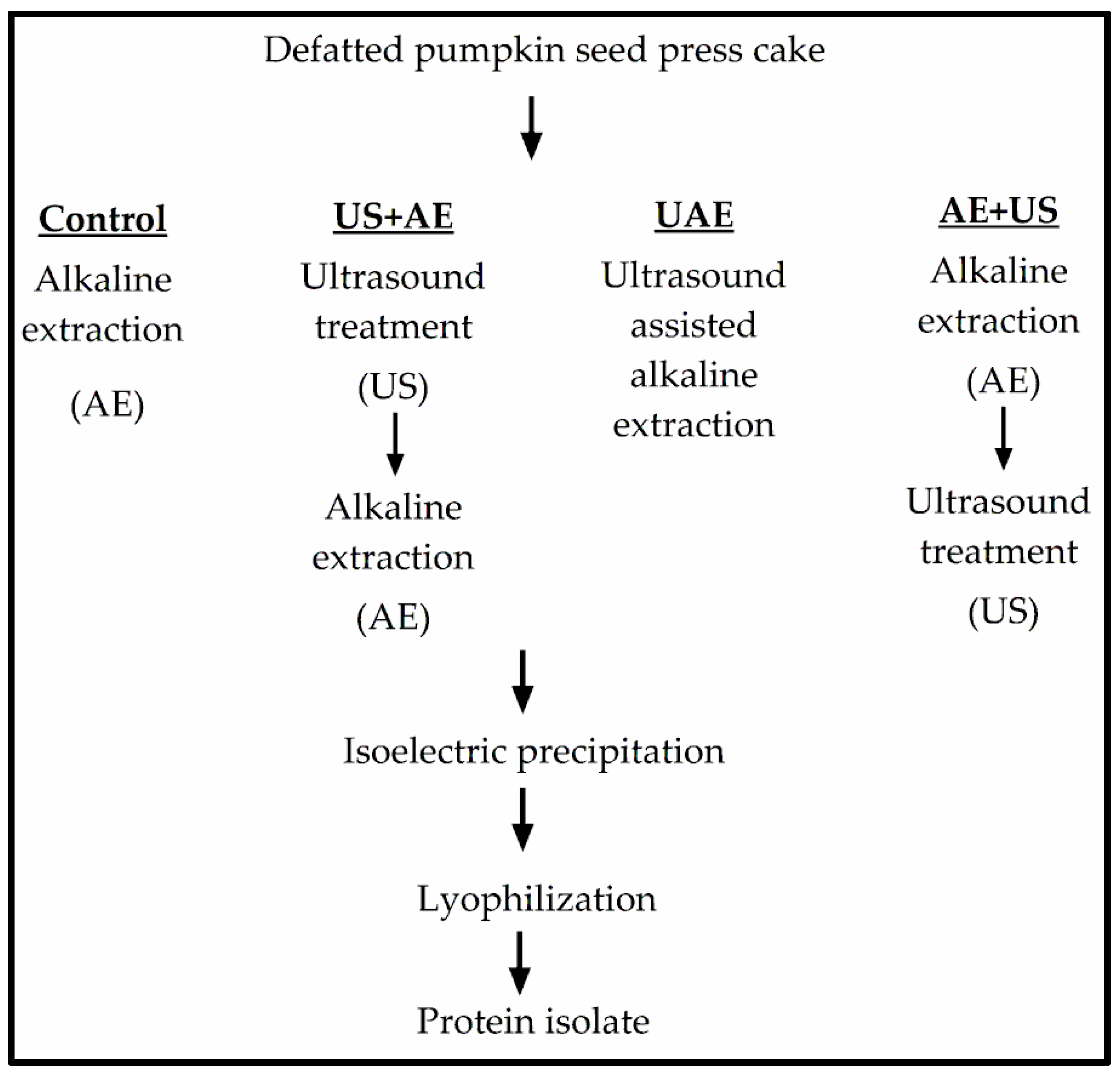
2.3.1. Control Procedure
2.3.2. Protein Extraction with Ultrasonic Support
- ultrasonic treatment followed by alkaline extraction (US+AE),
- concomitant ultrasonic treatment and alkaline extraction (UAE), and
- alkaline extraction followed by ultrasonic treatment (AE+US).
2.4. Particle Size Distribution in Alkaline Extracts
2.5. Determination of Techno-Functional Properties
2.5.1. Protein Solubility
2.5.2. Water Binding Capacity
2.5.3. Foaming Properties
2.6. Different Scanning Calorimetry (DSC)
2.7. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis
2.8. Color Profile
2.9. Statistical Analysis
3. Results and Discussion
3.1. Composition of Pumpkin Press Cake
3.2. Optimization of the Protein Extraction Procedure
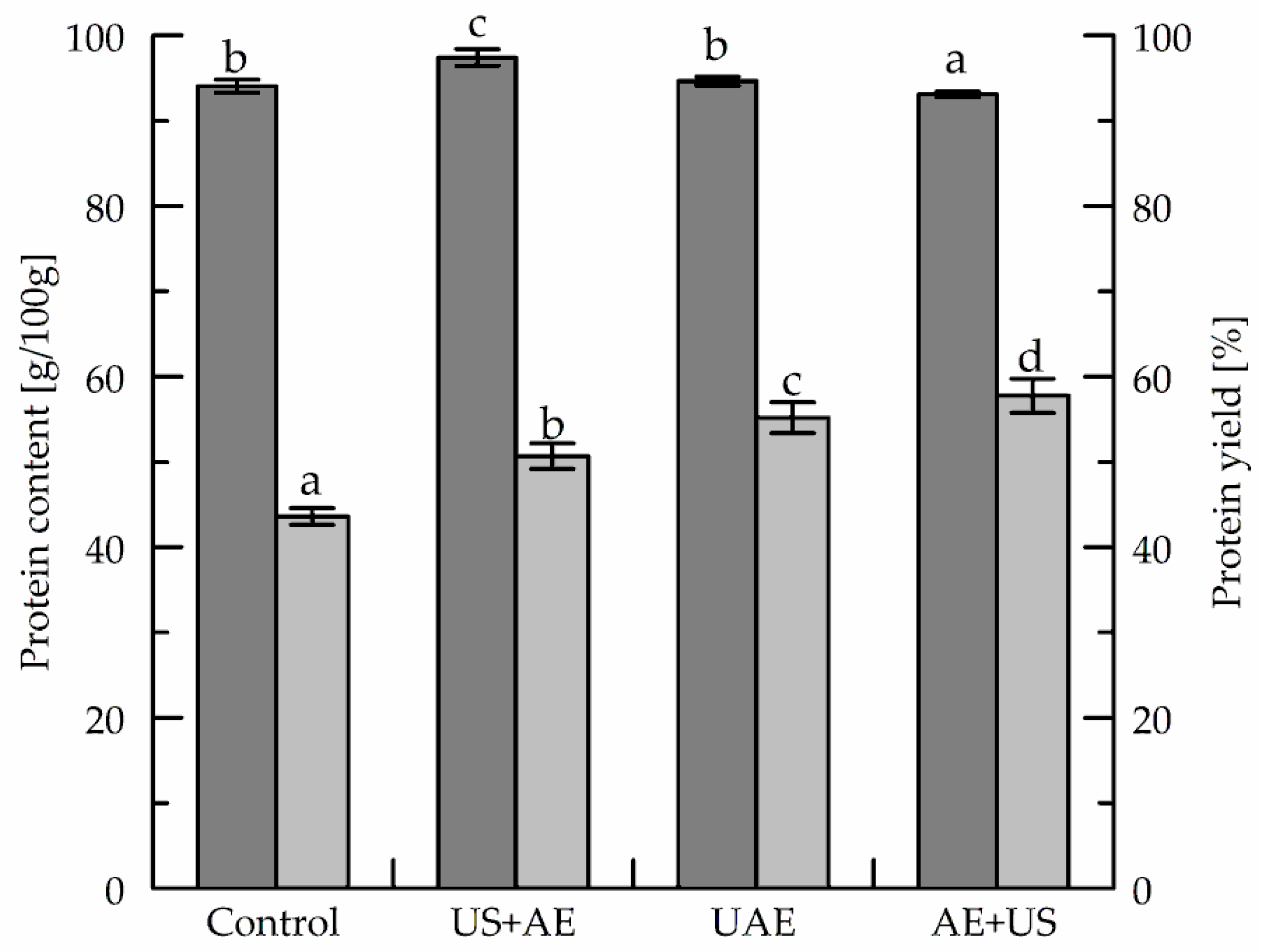
3.3. Effect of Ultrasonic Treatment on Protein Yield and Content
3.4. Particle Size Distribution of Alkaline Extracts
3.5. Protein Solubility
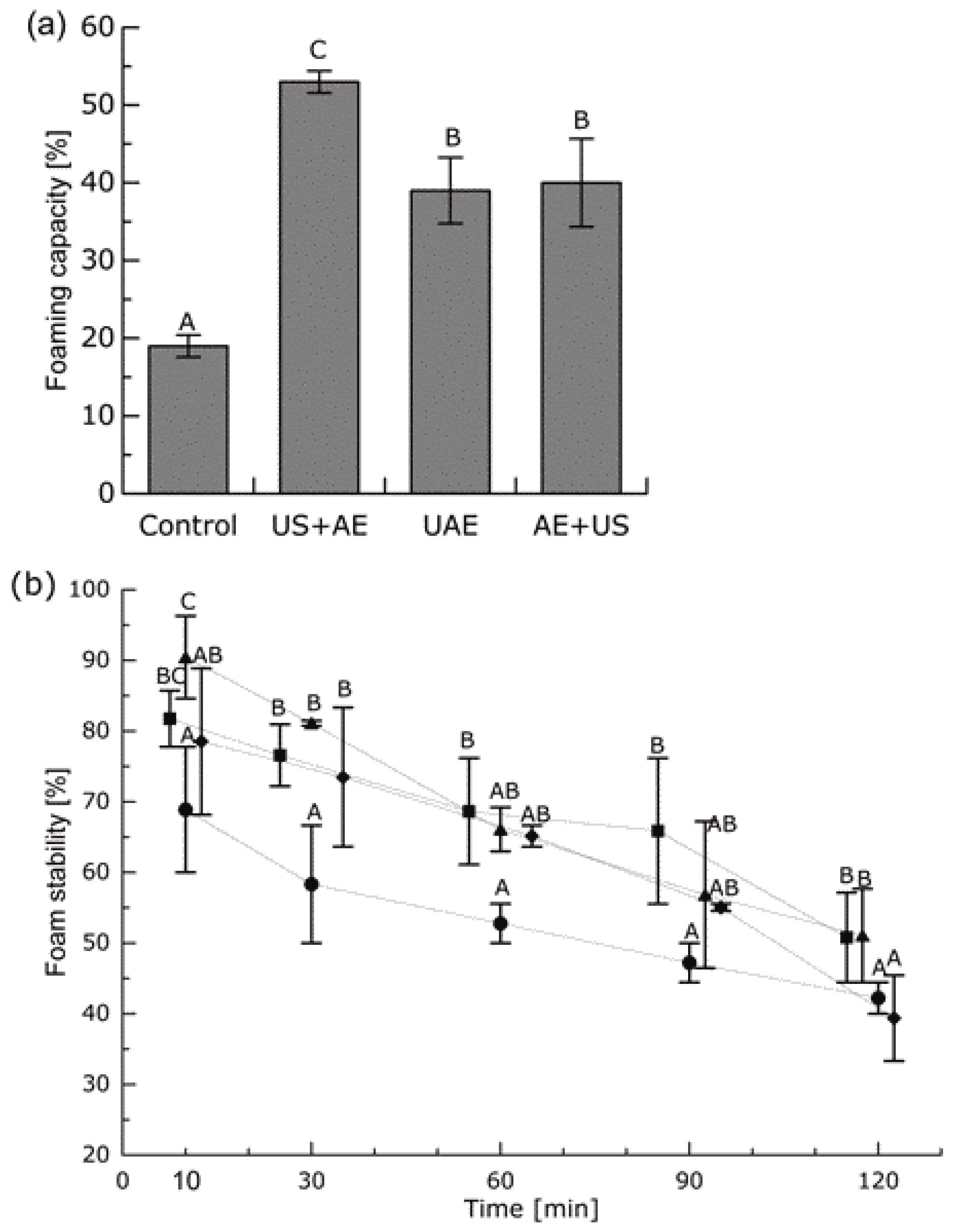
3.6. Water Binding and Foaming Capacity
3.7. Stability of the Proteins
3.8. Color Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: Effects in yield and functional properties of protein isolates. Food Bioprocess Technol. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Tomar, M.; Potkule, J.; Punia, S.; Dhakane, J.; Singh, S.; Kennedy, J.F. Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocoll. 2021, 123, 106986. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, A.B.; Zang, X.P.; Tan, L.; Xu, B.Y.; Chen, H.H.; Ma, W.H. Physicochemical, functional and emulsion properties of edible protein from avocado (Persea americana Mill.) oil processing by-products. Food Chem. 2019, 288, 146–153. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, X.; Zheng, Y.; Wang, D.; Deng, Y.; Zhao, Y. Impact of ultrasonication/shear emulsifying/microwave-assisted enzymatic extraction on rheological, structural, and functional properties of Akebia trifoliata (Thunb.) Koidz. seed protein isolates. Food Hydrocoll. 2021, 112, 106355. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Preliminary characterisation of wastes generated from the rapeseed and sunflower protein isolation process and their valorisation in delaying oil oxidation. Food Bioprocess Technol. 2021, 14, 1962–1971. [Google Scholar] [CrossRef]
- Vinayashree, S.; Vasu, P. Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chem. 2021, 340, 128177. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nations Statistics (FAOSTAT). 2020. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 14 May 2022).
- European Commission, eAmbrosia, the EU Geographical Indications Register. 2022. Available online: https://ec.europa.eu/info/food-farming-fisheries/food-safety-and-quality/certification/quality-labels/geographical-indications-register/ (accessed on 15 November 2022).
- Wang, H.; Chen, K.; Cheng, J.; Jiang, L.; Yu, D.; Dai, Y.; Wang, L. Ultrasound-assisted three phase partitioning for simultaneous extraction of oil, protein and polysaccharide from pumpkin seeds. LWT-Food Sci. Technol. 2021, 151, 112200. [Google Scholar] [CrossRef]
- Quanhong, L.; Caili, F. Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem. 2005, 92, 701–706. [Google Scholar] [CrossRef]
- The American Heart Association (AHA). 2018. Available online: https://www.heart.org/ (accessed on 1 December 2022).
- Bučko, S.; Katona, J.; Popović, L.; Vaštag, Ž.; Petrović, L. Functional properties of pumpkin (Cucurbita pepo) seed protein isolate and hydrolysate. J. Serb. Chem. Soc. 2016, 81, 35–46. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Bernardi, S.; Lupatini-Menegotto, A.L.; Kalschne, D.L.; Moraes Flores, É.L.; Bittencourt, P.R.S.; Colla, E.; Canan, C. Ultrasound: A suitable technology to improve the extraction and techno-functional properties of vegetable food proteins. Plant Foods Hum. Nutr. 2021, 76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Proc. Eng. 2018, 41, 12799. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Proc. Proc. Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Karki, B. Use of High-Power Ultrasound During Soy Protein Production and Study of Its Effect on Functional Properties of Soy Protein Isolate. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2009. [Google Scholar]
- Silventoinen, P.; Sozer, N. Impact of ultrasound treatment and pH-shifting on physicochemical properties of protein-enriched barley fraction and barley protein isolate. Foods 2020, 9, 1055. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT-Food Sci. Technol. 2020, 127, 109348. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Tu, Z.C.; Xiao, H.; Wang, H.; Huang, X.Q.; Liu, G.X.; Lin, D.R. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Proc. 2014, 92, 30–37. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhang, Z.; Wang, Y.; Mintah, B.K.; Dabbour, M.; Jiang, H.; He, R.; Ma, H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrasonics Sonochem. 2020, 69, 105240. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Mintah, B.; Xiang, J.; Ma, H. Changes in functionalities, conformational characteristics and antioxidative capacities of sunflower protein by controlled enzymolysis and ultrasonication action. Ultrasonics Sonochem. 2019, 58, 104625. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrasonics Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef]
- Li, K.; Ma, H.; Li, S.; Zhang, C.; Dai, C. Effect of ultrasound on alkali extraction protein from rice dreg flour. J. Food Proc. Eng. 2017, 40, 12377. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Zhang, W.; Xu, W.; Hu, Z. Effects and mechanism of dilute acid soaking with ultrasound pretreatment on rice bran protein extraction. J. Cereal Sci. 2019, 87, 318–324. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, J.; Wang, S.; Manyande, A.; Wang, J. Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata Duch.)-seed protein isolates. LWT-Food Sci. Technol. 2022, 155, 112952. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Gonzalez-Perez, S. Physico-Chemical and Functional Properties of Sunflower Proteins. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2003. [Google Scholar]
- Chen, J.Y.; Piva, M.; Labuza, T.P. Evaluation of water binding capacity (WBC) of food fiber sources. J. Food Sci. 1984, 49, 59–63. [Google Scholar] [CrossRef]
- Moure, A.; Sineiro, J.; Domínguez, H. Extraction and functionality of membrane-concentrated protein from defatted Rosa rubiginosa seeds. Food Chem. 2001, 74, 327–339. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Rohm, H.; Jaros, D. Colour of hard cheese. Z. Lebensm.-Unters. Forsch. 1996, 203, 241–244. [Google Scholar] [CrossRef]
- Bučko, S.; Katona, J.; Popović, L.; Vaštag, Ž.; Petrović, L.; Vučinić–Vasić, M. Investigation on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. LWT-Food Sci. Technol. 2015, 64, 609–615. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Oilseed proteins–Properties and application as a food ingredient. Trends Food Sci. Technol. 2020, 106, 160–170. [Google Scholar] [CrossRef]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Kozak, M. Oilseed cake flour composition, functional properties and antioxidant potential as effects of sieving and species differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef]
- Sá, A.G.A.; da Silva, D.C.; Pacheco, M.T.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Oilseed by-products as plant-based protein sources: Amino acid profile and digestibility. Future Foods 2021, 3, 100023. [Google Scholar] [CrossRef]
- Lovatto, N.D.M.; Loureiro, B.B.; Bender, A.B.B.; Loureiro, C.B.; Goulart, F.R.; Speroni, C.S.; Silva, L.P.D. Phosphorylated protein concentrates pumpkin seed (Cucurbita moschata): Optimization by response surface methodology and nutritional characterization. Ciência Rural 2020, 50, 20190093. [Google Scholar] [CrossRef]
- Tu, G.L.; Bui, T.H.N.; Tran, T.T.; Ton, N.M.N.; Le, V.V.M. Comparison of enzymatic and ultrasonic extraction of albumin from defatted pumpkin (Cucurbita pepo) seed powder. Food Technol. Biotechnol. 2015, 53, 479–487. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.B.; Chen, B.; Rao, J. Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef]
- Ivanova, P.; Chalova, V.; Koleva, L.; Pishtiyski, I.; Perifanova-Nemska, M. Optimization of protein extraction from sunflower meal produced in Bulgaria. Bulgarian J. Agric. Sci. 2012, 18, 153–160. [Google Scholar]
- Ghodsvali, A.; Khodaparast, M.H.; Vosoughi, M.; Diosady, L.L. Preparation of canola protein materials using membrane technology and evaluation of meals functional properties. Food Res. Int. 2005, 38, 223–231. [Google Scholar] [CrossRef]
- Hadidi, M.; Khaksar, F.B.; Pagan, J.; Ibarz, A. Application of Ultrasound-Ultrafiltration-Assisted alkaline isoelectric precipitation (UUAAIP) technique for producing alfalfa protein isolate for human consumption: Optimization, comparison, physicochemical, and functional properties. Food Res. Int. 2019, 130, 108907. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Bao, T.; Zheng, X.; Chen, W.; Wang, J. A recyclable protein resource derived from cauliflower by-products: Potential biological activities of protein hydrolysates. Food Chem. 2017, 221, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Yanjun, S.; Jianhang, C.; Shuwen, Z.; Hongjuan, L.; Jing, L.; Lu, L.; Jiaping, L. Effect of power ultrasound pretreatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014, 124, 11. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Y.; Zhu, X.; Xiong, H.; Shi, S.; Hu, J.; Sun, W. Physicochemical and functional properties of the protein isolate and major fractions prepared from Akebia trifoliata var. australis seed. Food Chem. 2012, 133, 923–929. [Google Scholar] [CrossRef]
- Chavan, U.D.; McKenzie, D.B.; Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrasonics Sonochem. 2017, 56, 960–967. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R. Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Morales, R.; Martínez, K.D.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrasonics Sonochem. 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pouramin, S. Optimization of extraction and deamidation of edible protein from evening primrose (Oenothera biennis L.) oil processing by-products and its effect on structural and techno-functional properties. Food Chem. 2021, 334, 127613. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Coldea, T.E.; Zhao, H. Modification of structural and functional characteristics of brewer’s spent grain protein by ultrasound assisted extraction. LWT-Food Sci. Technol. 2021, 139, 110582. [Google Scholar] [CrossRef]
- Sharma, G.M.; Su, M.; Joshi, A.U.; Roux, K.H.; Sathe, S.K. Functional properties of select edible oilseed proteins. J. Agric. Food Chem. 2010, 58, 5457–5464. [Google Scholar] [CrossRef] [PubMed]
- Karki, B.; Lamsal, B.P.; Grewell, D.; Pometto, A.L., III; Van Leeuwen, J.; Khanal, S.K.; Jung, S. Functional properties of soy protein isolates produced from ultrasonicated defatted soy flakes. J. Am. Oil Chem. Soc. 2009, 86, 1021–1028. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrasonics Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, J.; Li-Chan, E.C.; Zhu, L.; Zhang, F.; Xu, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocollois 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Chittapalo, T.; Noomhorm, A. Ultrasonic assisted alkali extraction of protein from defatted rice bran and properties of the protein concentrates. Int. J. Food Sci. Technol. 2009, 44, 1843–1849. [Google Scholar] [CrossRef]
| Component (g/100 g) | Untreated Press Cake | De-Oiled Press Cake |
|---|---|---|
| Moisture | 4.73 ± 0.32 a | 4.84 ± 0.19 a |
| Fat | 13.38 ± 0.12 a | 0.77 ± 0.14 b |
| Protein | 60.24 ± 0.05 a | 68.68 ± 0.13 b |
| Total dietary fibre | 13.13 ± 0.71 a | 17.39 ± 0.54 b |
| Ash | 8.02 ± 0.06 a | 9.11 ± 0.07 b |
| Treatment | d10 [µm] | d50 [µm] | d90 [µm] |
|---|---|---|---|
| Control | 4.82 ± 0.44 b | 72.12 ± 14.58 b | 376.68 ± 38.32 c |
| US+AE | 3.49 ± 0.20 a | 31.50 ± 2.51 a | 252.96 ± 24.85 b |
| UAE | 3.38 ± 0.25 a | 27.59 ± 4.02 a | 245.83 ± 34.60 b |
| AE+US | 8.05 ± 0.72 c | 36.25 ± 5.71 a | 179.93 ± 13.24 a |
| Treatment | Solubility [%] | WBC [g/g dm] | |||
|---|---|---|---|---|---|
| pH 3 | pH 5 | pH 7 | pH 9 | ||
| Control | 35.73 ± 1.42 a | 2.42 ± 0.06 a | 15.17 ± 0.20 a | 37.82 ± 1.07 a | 3.95 ± 0.04 b |
| US+AE | 37.33 ± 0.41 b | 3.83 ± 0.42 b | 18.72 ± 0.13 b | 39.88 ± 0.30 b | 3.81 ± 0.08 b |
| UAE | 38.00 ± 0.89 bc | 6.59 ± 0.99 d | 18.80 ± 0.52 b | 46.87 ± 0.17 c | 3.61 ± 0.21 a |
| AE+US | 38.84 ± 0.21 c | 4.90 ± 0.05 c | 23.07 ± 0.06 c | 46.93 ±0.61 c | 3.66 ± 0.15 a |
| Control | US+AE | UAE | AE+US | |
|---|---|---|---|---|
| Tden (°C) | 99.05 ± 4.15 c | 85.70 ± 2.29 a | 94.20 ± 2.84 b | 92.44 ± 3.58 b |
| ∆H (J/g) | 308.6 ± 9.6 a | 371.0 ± 21.6 b | 362.2 ± 10.5 b | 360.8 ± 2.6 b |
| Control | US+AE | UAE | AE+US | |
|---|---|---|---|---|
| ∆E* | 2.02 ± 1.26 | 1.84 ± 0.34 | 2.72 ±0.14 | |
| L* | 71.20 ± 0.48 b | 72.26 ± 1.76 c | 69.64 ± 0.10 a | 68.78 ± 0.17 a |
| hab | 78.70 ± 0.10 a | 80.33 ± 1.19 b | 81.05 ± 0.33 bc | 81.52 ± 0.08 c |
| C* | 21.66 ± 0.38 b | 20.57 ± 1.04 a | 22.08 ± 0.51 b | 21.29 ± 0.97 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sert, D.; Rohm, H.; Struck, S. Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality. Foods 2022, 11, 4029. https://doi.org/10.3390/foods11244029
Sert D, Rohm H, Struck S. Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality. Foods. 2022; 11(24):4029. https://doi.org/10.3390/foods11244029
Chicago/Turabian StyleSert, Deniz, Harald Rohm, and Susanne Struck. 2022. "Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality" Foods 11, no. 24: 4029. https://doi.org/10.3390/foods11244029
APA StyleSert, D., Rohm, H., & Struck, S. (2022). Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality. Foods, 11(24), 4029. https://doi.org/10.3390/foods11244029








