Can Soybean Cultivars with Larger Seed Size Produce More Protein, Lipids, and Seed Yield? A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
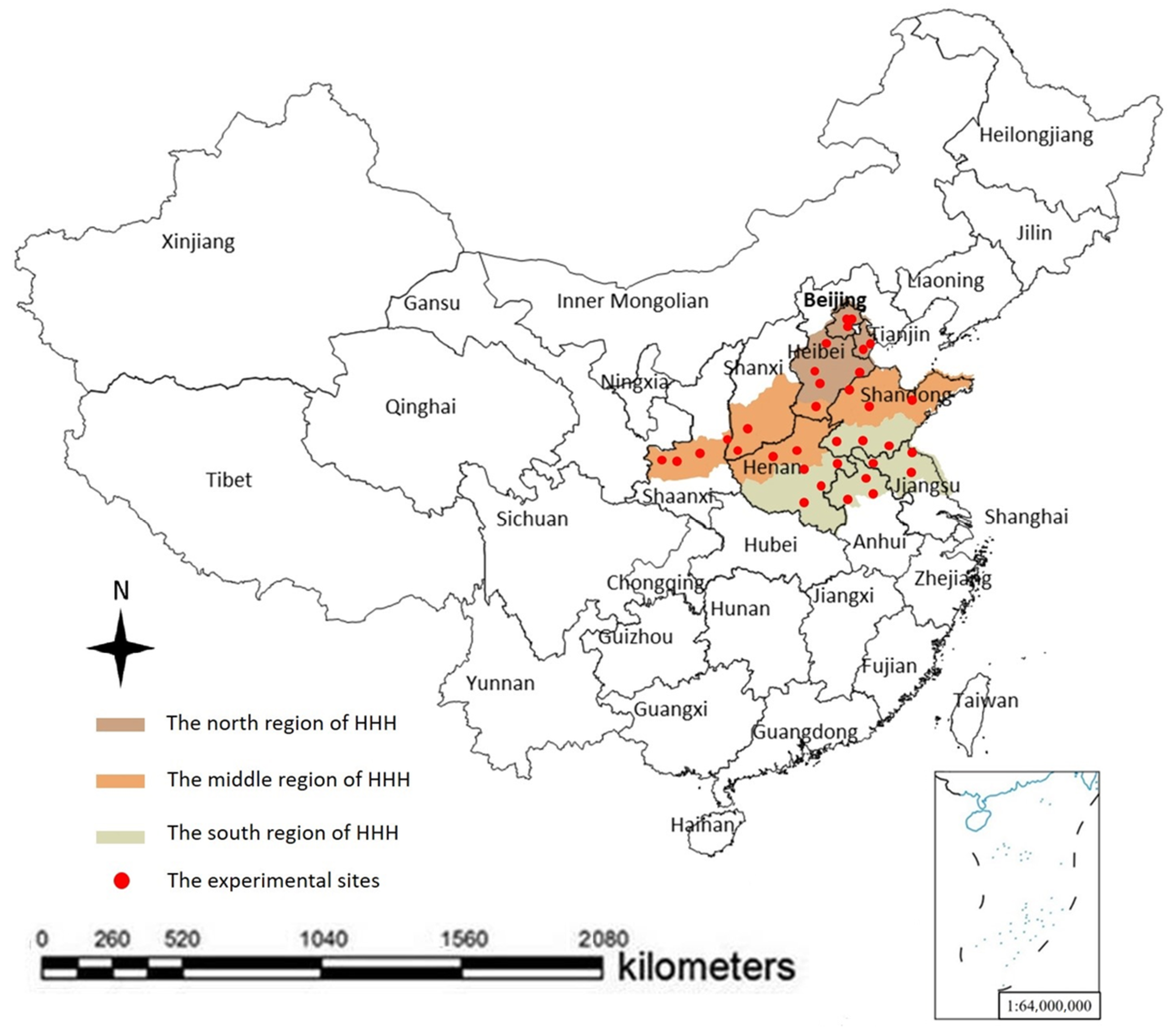
2.1. Study Area
2.2. Field Experiment
2.3. Data Collection
2.4. Statistical Analyses
3. Results
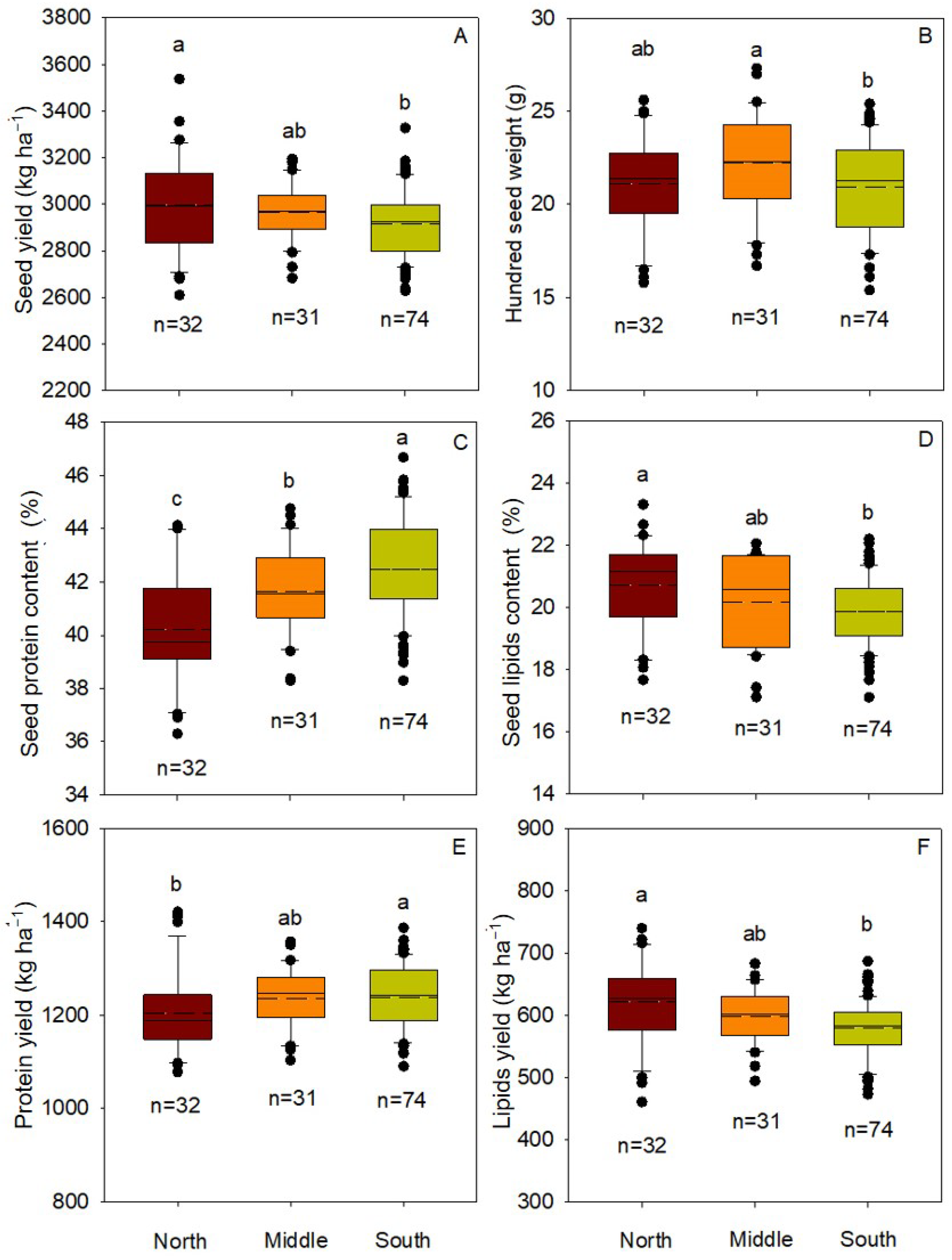
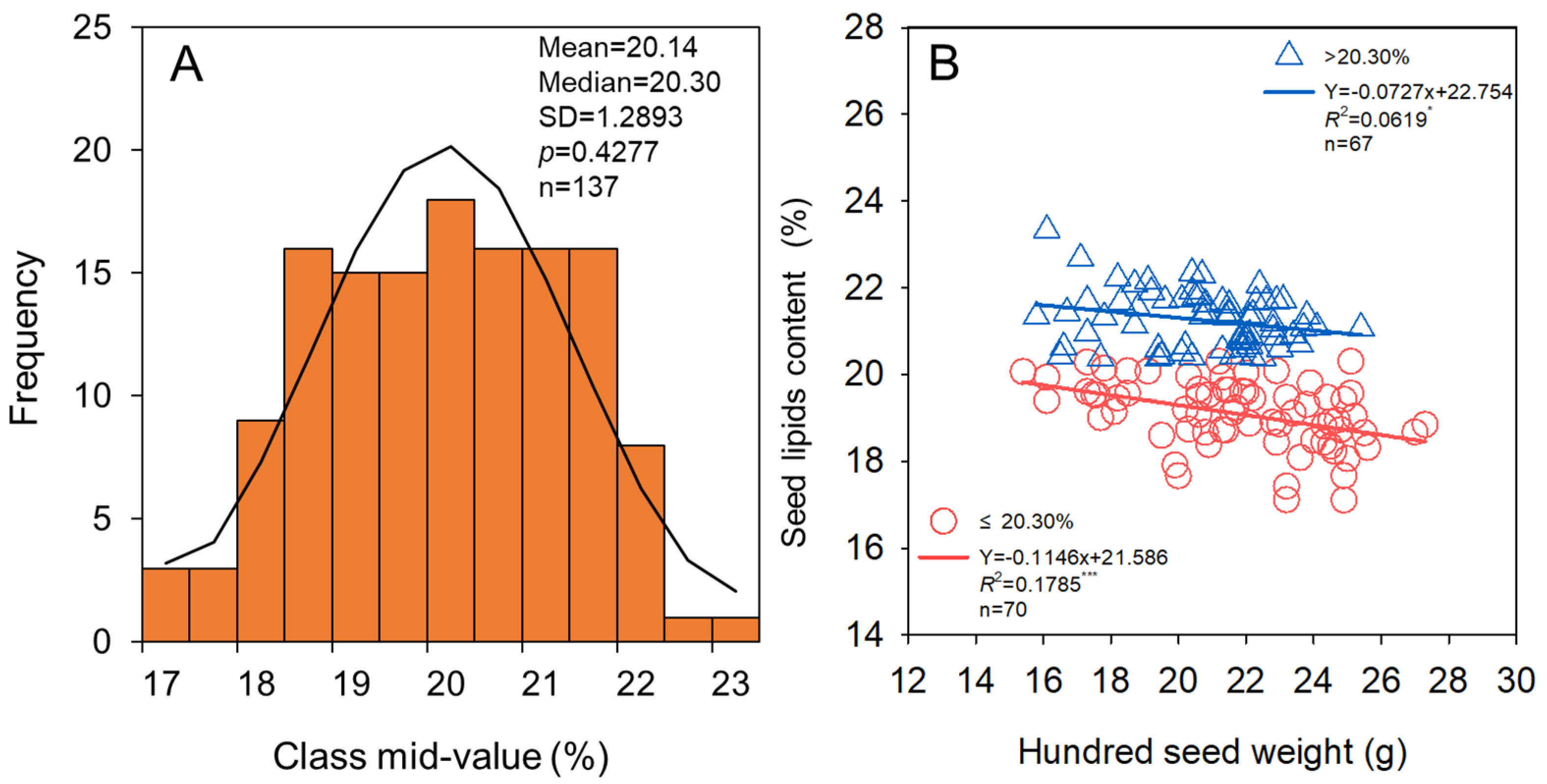
3.1. Seed Yield, Hundred Seed Weight, and Protein and Lipids Content of Seed
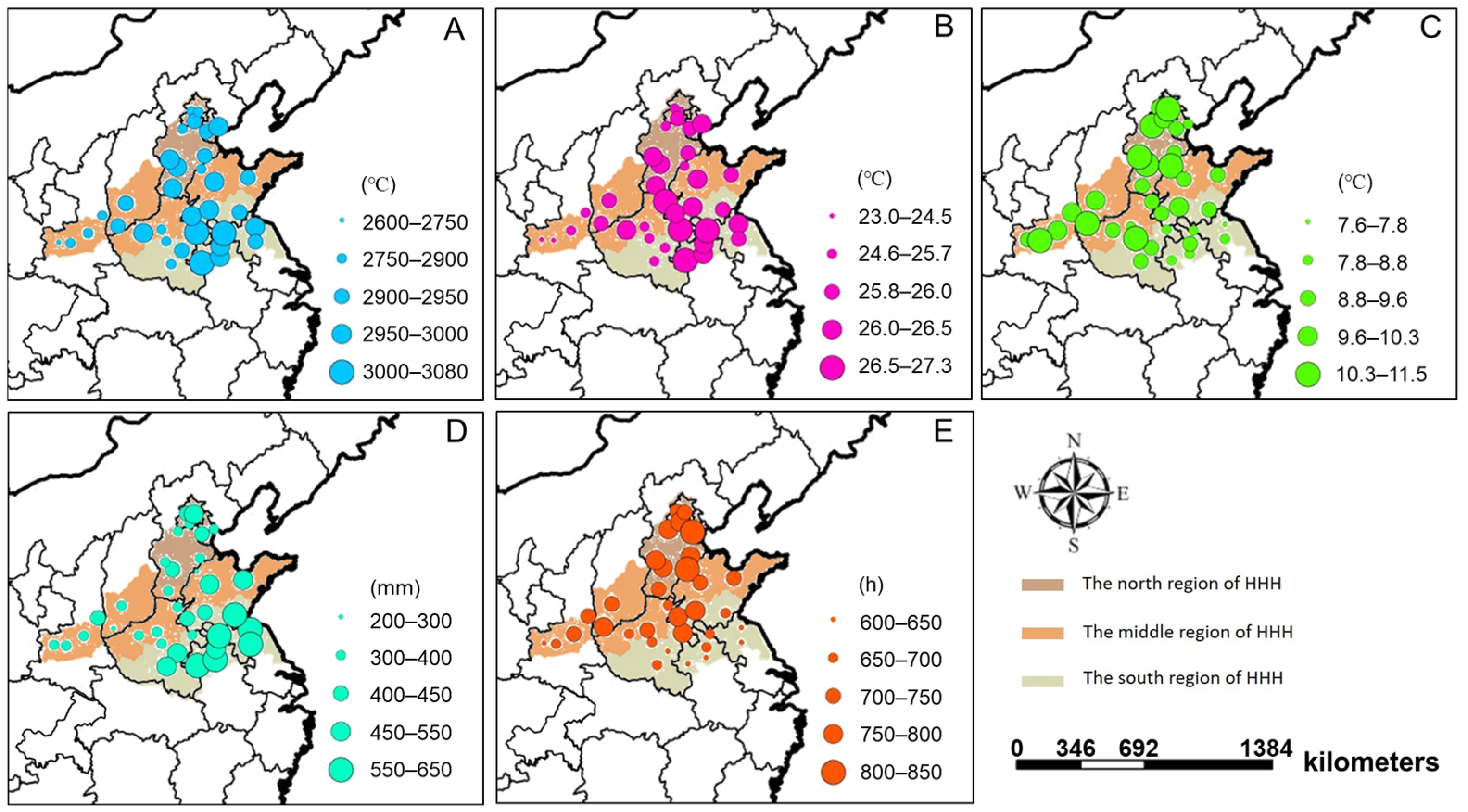
3.2. Differences of Meteorological Factors and Their Relationship with Seed Yield
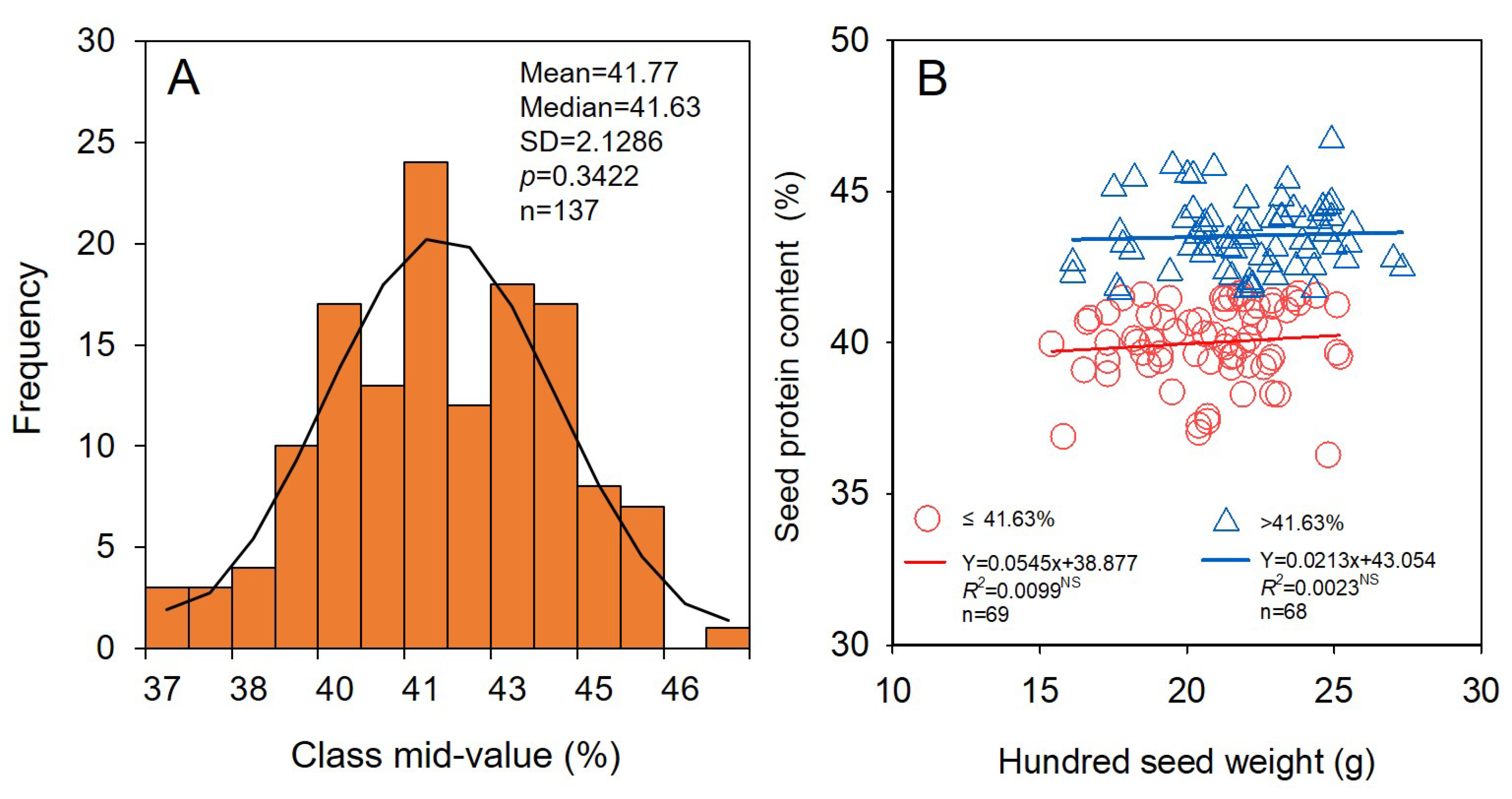
3.3. Relationship of Seed Yield Stability and Properties of Seed Quality with Seed Size
4. Discussion
4.1. Spatial Characteristics of Soybean Production and Their Relationship with Climatic Factors in HHH
4.2. Soybean Cultivars with Smaller Seed Size Have Stronger Seed Yield Stability
4.3. Soybean Cultivars with Larger Seed Size Can Not Produce More Protein and Lipids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HHH | Huang-Huai-Hai region |
| DTR | diurnal temperature range |
| YSI | seed yield stability index |
References
- Mauser, W.; Klepper, G.; Zabel, F.; Delzeit, R.; Hank, T.; Putzenlechner, B.; Calzadilla, A. Global biomass production potentials exceed expected future demand without the need for cropland expansion. Nat. Commun. 2015, 6, 8946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oslaj, M.; Mursec, B.; Vindis, P. Biogas production from maize hybrids. Biomass Bioenergy 2010, 34, 1538–1545. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Hur, M.; Lee, J.Y.; Zhou, W.; Song, Z.; Ransom, N.; Demirkale, C.Y.; Nettleton, D.; Westgate, M.; Arendsee, Z.; et al. A systems biology approach toward understanding seed composition in soybean. BMC Genom. 2015, 16, S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Yang, R.; Wu, T.; Wu, C.; Sun, S.; Zhang, S.; Jiang, B.; Tian, S.; Liu, X.; Han, T. Analyzing the effects of climate factors on soybean protein, oil contents, and composition by extensive and high-density sampling in China. J. Agric. Food Chem. 2016, 64, 4121–4130. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT-Agriculture Database. 2021. Available online: http://www.fao.org (accessed on 1 January 2022).
- National Bureau of Statistics of China. Annual Data. 2021. Available online: http://www.stats.gov.cn (accessed on 1 January 2022).
- Liu, Z.; Ying, H.; Chen, M.; Bai, J.; Xue, Y.; Yin, Y.; Batchelor, W.; Yang, Y.; Bai, Z.; Du, M.; et al. Optimization of China’s maize and soy production can ensure feed sufficiency at lower nitrogen and carbon footprints. Nat. Food 2021, 2, 426–433. [Google Scholar] [CrossRef]
- Xu, C.; Li, R.; Song, W.; Wu, T.; Sun, S.; Han, T.; Wu, C. High density and uniform plant distribution improve soybean yield by regulating population uniformity and canopy light interception. Agronomy 2021, 11, 1880. [Google Scholar] [CrossRef]
- Xu, C.; He, Y.; Sun, S.; Song, W.; Wu, T.; Han, T.; Wu, C. Analysis of soybean yield formation differences across different production regions in China. Agron. J. 2020, 112, 4195–4206. [Google Scholar] [CrossRef]
- Jin, J.; Liu, X.; Wang, G.; Mi, L.; Shen, Z.; Chen, X.; Herbert, S.J. Agronomic and physiological contributions to the yield improvement of soybean cultivars released from 1950 to 2006 in Northeast China. Field Crops Res. 2010, 115, 116–123. [Google Scholar] [CrossRef]
- Meena, B.; Biswas, A.; Singh, M.; Chaudhary, R.; Singh, A.; Das, H.; Patra, A. Long-term sustaining crop productivity and soil health in maize–chickpea system through integrated nutrient management practices in Vertisols of central India. Field Crops Res. 2019, 232, 62–76. [Google Scholar] [CrossRef]
- Carciochi, W.; Schwalbert, R.; Andrade, F.; Corassa, G.; Carter, P.; Gaspar, A.; Schmidt, J.; Ciampitti, I. Soybean seed yield response to plant density by yield environment in North America. Agron. J. 2019, 111, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Dardanelli, J.L.; Balzarini, M.; Martinez, M.J.; Cuniberti, M.; Resnik, S.; Ramunda, S.E.; Herrero, R.; Baigorri, H. Soybean maturity groups, environments, and their interaction define mega-environments for seed composition in Argentina. Crop Sci. 2006, 46, 1939–1947. [Google Scholar] [CrossRef]
- Tagliapietr, E.; Zanon, A.; Streck, N.A.; Balest, D.; da Rosa, S.; Bexaira, K.; Richter, G.; Ribas, G.; da Silva, M. Biophysical and management factors causing yield gap in soybean in the subtropics of Brazil. Agron. J. 2021, 113, 1882–1894. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, W.; Yang, F.; Wang, X.; Yong, T.; Liu, J.; Pu, T.; Yan, Y.; Yang, W. Dynamic of recovery growth of intercropped soybean after maize harvest in maize–soybean relay strip intercropping system. Food Energy Secur. 2022, 11, e350. [Google Scholar] [CrossRef]
- Ren, C.; Bilyeu, K.D.; Beuselinck, P. Composition, vigor, and proteome of mature soybean seeds developed under high temperature. Crop Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Boote, K.J.; Pan, D.; Allen, L.H. Elevated temperature delays onset of reproductive growth and reduces seed growth rate of soybean. J. Agron. Crop Sci. 2010, 1, 19–32. [Google Scholar]
- Abdelghany, A.; Zhang, S.; Azam, M.; Shaibu, A.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Profiling of seed fatty acid composition in 1025 Chinese soybean accessions from diverse ecoregions. Crop J. 2020, 8, 635–644. [Google Scholar] [CrossRef]
- Hymowitz, T.; Newell, C.A. Taxonomy of the genus Glycine, domestication and uses of soybeans. Econ. Bot. 1981, 35, 272–288. [Google Scholar] [CrossRef]
- Guan, R.; Chang, R.; Li, Y.; Wang, L.; Liu, Z.; Qiu, L. Genetic diversity comparison between Chinese and Japanese soybeans (Glycine max (L.) Merr.) revealed by nuclear SSRs. Genet. Resour. Crop Evol. 2010, 57, 229–242. [Google Scholar] [CrossRef]
- Norsworthy, J.; Shipe, E. Effect of row spacing and soybean genotype on mainstem and branch yield. Agron. J. 2005, 97, 919–923. [Google Scholar] [CrossRef]
- Cox, W.; Cherney, J.; Shields, E. Soybean compensate at low seeding rates but not at high thinning rates. Agron. J. 2010, 102, 1238–1243. [Google Scholar] [CrossRef]
- Cox, W.; Cherney, J. Growth and yield responses of soybean to row spacing and seeding rate. Agron. J. 2011, 103, 123–128. [Google Scholar] [CrossRef]
- Dai, J.; Kong, X.; Zhong, D.; Li, W.; Dong, H. Technologies and theoretical basis of light and simplified cotton cultivation in China. Field Crops Res. 2017, 214, 142–148. [Google Scholar] [CrossRef]
- Kong, X.; Li, X.; Lu, H.; Li, Z.; Xu, S.; Li, W.; Zhang, Y.; Zhang, H.; Dong, H. Monoseeding improves stand establishment through regulation of apical hook formation and hypocotyl elongation in cotton. Field Crops Res. 2018, 222, 50–58. [Google Scholar] [CrossRef]
- Austin, R.B.; Edrich, J.A.; Ford, M.A.; Blackwell, R.D. The fate of the dry matter, carbohydrates and 14C lost from the leaves and stems of wheat during grain filling. Ann. Bot. 1977, 41, 1309–1321. [Google Scholar] [CrossRef]
- Yang, J.; Peng, S.; Visperas, R.; Sanico, A.; Zhu, Q.; Gu, S. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant Growth Regul. 2000, 30, 261–270. [Google Scholar] [CrossRef]
- Lee, H.; Choi, M.; Hwang, W.; Jeong, J.; Yang, S.; Lee, C. Occurrence of rice preharvest sprouting varies greatly depending on past weather conditions during grain filling. Field Crops Res. 2021, 264, 108087. [Google Scholar] [CrossRef]
- Lv, X.; Ding, Y.; Long, M.; Liang, W.; Gu, X.; Liu, Y.; Wen, X. Effect of foliar application of various nitrogen forms on starch accumulation and grain filling of wheat (Triticum aestivum L.) under drought stress. Front. Plant Sci. 2021, 12, 645379. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Wu, C.; Han, T. Comparison of soybean variety trial systems and procedures in the USA and China. Online. Crop Manag. 2010, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Huang, S.; Tian, B.; Ren, J.; Meng, Q.; Wang, P. Manipulating planting density and nitrogen fertilizer application to improve yield and reduce environmental impact in Chinese maize production. Front. Plant Sci. 2017, 8, 1234. [Google Scholar] [CrossRef]
- Wang, J.; Wang, E.L.; Yang, X.G.; Zhang, F.S.; Yin, H. Increased yield potential of wheat-maize cropping system in the North China Plain by climate change adaptation. Clim. Change 2012, 113, 825–840. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Gao, H.; Qiu, L.; Chang, R.; Chen, S.; He, C. Molecular and geographic evolutionary support for the essential role of GIGANTEAa in soybean domestication of flowering time. BMC Evol. Biol. 2016, 16, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.P.; Wang, L.W.; Chen, L.; Wu, T.T.; Liu, L.P.; Sun, S.; Wu, C.X.; Yao, W.W.; Jiang, B.J.; Yuan, S.; et al. Mutagenesis of GmFT2a and GmFT5a mediated by CRISPR/Cas9 contributes for expanding the regional adaptability of soybean. Plant Biotechnol. J. 2020, 18, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.W.; Yang, R.P.; Yang, X.S.; Sun, S.; Mentreddy, S.R.; Jiang, B.J.; Wu, T.T.; Tian, S.Y.; Sapey, E.; Wu, C.X.; et al. Spatial differences in soybean bioactive components across China and their influence by weather factors. Crop J. 2018, 6, 659–668. [Google Scholar] [CrossRef]
- Challinor, A.J.; Koehler, A.K.; Ramirez-Villegas, J.; Whitfield, S.; Das, B. Current warming will reduce yields unless maize breeding and seed systems adapt immediately. Nat. Clim. Chang. 2016, 6, 954–958. [Google Scholar] [CrossRef]
- Boote, K.J.; Prasad, V.; Allen, L.H.; Singh, P.; Jones, J.W. Modeling sensitivity of grain yield to elevated temperature in the DSSAT crop models for peanut, soybean, dry bean, chickpea, sorghum, and millet. Eur. J. Agron. 2018, 100, 99–109. [Google Scholar] [CrossRef]
- Isoda, A.; Mori, M.; Matsumoto, S.; Li, Z.; Wang, P. High yielding performance of soybean in northern Xinjiang, China. Plant Prod. Sci. 2006, 9, 401–407. [Google Scholar] [CrossRef]
- Peraudeau, S.; Lafarge, T.; Roques, S.; Quiñones, C.O.; Clement-Vidal, A.; Ouwerkerk, P.B.F.; Van Rie, J.; Fabre, D.; Jagadish, K.S.V.; Dingkuhn, M. Effect of carbohydrates and night temperature on night respiration in rice. J. Exp. Bot. 2015, 66, 3931–3944. [Google Scholar] [CrossRef] [Green Version]
- Sunoj, V.; Shroyer, K.; Jagadish, S.; Prasad, P. Diurnal temperature amplitude alters physiological and growth response of maize (Zea mays L.) during the vegetative stage. Environ. Exp. Bot. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Hou, P.; Liu, G.; Liu, W.; Wang, Y.; Zhao, R.; Ming, B.; Xie, R.; Wang, K.; et al. Improving maize grain yield by matching maize growth and solar radiation. Sci. Rep.-UK 2019, 9, 3635. [Google Scholar] [CrossRef] [Green Version]
- Hou, P.; Liu, Y.; Liu, W.; Yang, H.; Xie, R.; Wang, K.; Ming, B.; Liu, G.; Xue, J.; Wang, Y.; et al. Quantifying maize grain yield losses caused by climate change based on extensive field data across China. Resour. Conserv. Recy. 2021, 174, 105811. [Google Scholar] [CrossRef]
- He, L.; Jin, N.; Yu, Q. Impacts of climate change and crop management practices on soybean phenology changes in China. Sci. Total Environ. 2020, 707, 135638. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, H.; Chen, X.; Zhang, C.; Ma, W.; Huang, C.; Zhang, W.; Mi, G.; Miao, Y.; Li, X. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef]
- Asseng, S.; Cammarano, D.; Basso, B.; Chung, U.; Alderman, P.D.; Sonder, K.; Reynolds, M.; Lobell, D.B. Hot spots of wheat yield decline with rising temperatures. Glob. Change Biol. 2017, 23, 2464–2472. [Google Scholar] [CrossRef]
- McDougall, R.; Kristiansen, P.; Rader, R. Small-scale urban agriculture results in high yields but requires judicious management of inputs to achieve sustainability. Proc. Natl. Acad. Sci. USA 2019, 116, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Ittersun, M.V.; Cassman, K.G.; Grassini, P.; Wolf, J.; Tittonell, P.; Hochman, Z. Yield gap analysis with local to global relevance-A review. Field Crops Res. 2013, 143, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Cassman, K.G.; Field, C.B. Crop yield gaps: Their importance, magnitudes, and causes. Annu. Rev. Env. Resour. 2009, 34, 179–204. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.B.; Zhang, M.; Zhang, Z.F.; Liang, S.; Fan, L.; Yang, X.; Yuan, Y.Q.; Pan, Y.; Zhou, G.A.; Liu, S.L.; et al. Natural allelic variation of GmST05 controlling seed size and quality in soybean. Plant Biotechnol. J. 2022, 20, 1807–1818. [Google Scholar] [CrossRef]
- Huang, M.; Fang, S.L.; Cao, F.B.; Chen, J.; Shan, S.L.; Liu, Y.; Lei, T.; Tian, A.L.; Tao, Z.; Zou, Y.B. Early sowing increases grain yield of machine-transplanted late-season rice under single-seed sowing. Field Crops Res. 2020, 253, 107832. [Google Scholar] [CrossRef]
- Kelly, S.J.; Cano, M.G.; Fanello, D.D.; Tambussi, E.A.; Guiamet, J.J. Extended photoperiods after flowering increase the rate of dry matter production and nitrogen assimilation in mid maturing soybean cultivars. Field Crops Res. 2020, 265, 108104. [Google Scholar] [CrossRef]
- Singh, R.J.; Ghosh, B.N.; Sharma, N.K.; Patra, S.; Dadhwal, K.S.; Meena, V.S.; Deshwal, J.S.; Mishra, P.K. Effect of seven years of nutrient supplementation through organic and inorganic sources on productivity soil and water conservation, and soil fertility changes of maize-wheat rotation in north-western Indian Himalayas. Agric. Ecosyst. Environ. 2017, 249, 177–186. [Google Scholar] [CrossRef]
- Kambhampati, S.; Aznar-Moreno, J.A.; Bailey, S.R.; Arp, J.J.; Chu, K.L.; Bilyeu, K.D.; Durrett, T.P.; Allen, D.K. Temporal changes in metabolism late in seed development affect biomass composition. Plant Physiol. 2021, 186, 874–890. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinis, S.; Gaska, J.M.; Pedersen, P.; Conley, S.P. Effect of seed mass and emergence delay on soybean yield and quality. Agron. J. 2015, 107, 181–186. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “green revolution” for soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Liu, S.L.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.Q.; et al. Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef]
- Song, J.F.; Liu, C.Q.; Li, D.J.; Gu, Z.X. Evaluation of sugar, free amino acid, and organic acid compositions of different varieties of vegetable soybean (Glycine max L. Merr). Ind. Crop. Prod. 2013, 50, 743–749. [Google Scholar] [CrossRef]
- Assefa, Y.; Purcell, L.C.; Salmeron, M.; Naeve, S.; Casteel, S.N.; Kovács, P.; Archontoulis, S.; Licht, M.; Below, F.; Kandel, H.; et al. Assessing Variation in US Soybean Seed Composition (Protein and Oil). Front. Plant Sci. 2019, 10, 298. [Google Scholar] [CrossRef]
- Nakasathien, S.; Israel, D.W.; Wilson, R.F.; Kwanyuen, P. Regulation of seed protein concentration in soybean by supra-optimal nitrogen supply. Crop Sci. 2000, 40, 1277–1284. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.; Babka, H.L.; Graef, G.L.; Staswick, P.E.; Lee, D.J.; Cregan, P.B.; Shoemaker, R.C.; Specht, J.E. The seed protein, oil, and yield QTL on soybean linkage Group I. Crop Sci. 2003, 43, 1053–1067. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.D.; Fritschi, F.B.; Heatherly, L.G. Large applications of fertilizer N at planting affects seed protein and oil concentration and yield in the early soybean production system. Field Crops Res. 2006, 99, 67–74. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Westgate, M.E. Meta-analysis of environmental effects on soybean seed composition. Field Crops Res. 2009, 110, 147–156. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Wu, T.; Yuan, S.; Sun, S.; Han, T.; Song, W.; Wu, C. Can Soybean Cultivars with Larger Seed Size Produce More Protein, Lipids, and Seed Yield? A Meta-Analysis. Foods 2022, 11, 4059. https://doi.org/10.3390/foods11244059
Xu C, Wu T, Yuan S, Sun S, Han T, Song W, Wu C. Can Soybean Cultivars with Larger Seed Size Produce More Protein, Lipids, and Seed Yield? A Meta-Analysis. Foods. 2022; 11(24):4059. https://doi.org/10.3390/foods11244059
Chicago/Turabian StyleXu, Cailong, Tingting Wu, Shan Yuan, Shi Sun, Tianfu Han, Wenwen Song, and Cunxiang Wu. 2022. "Can Soybean Cultivars with Larger Seed Size Produce More Protein, Lipids, and Seed Yield? A Meta-Analysis" Foods 11, no. 24: 4059. https://doi.org/10.3390/foods11244059




