Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Wholemeal Flours
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Amylose, Total Starch, Resistant Starch and β-Glucan Content
2.3. Quantification of Total (TOT-AXs) and Water-Extractable (WE-AXs) Arabinoxylans
2.4. Phytochemical Profiling of Wholemeal Flours
2.4.1. Chemicals
2.4.2. Extraction of Phenolic Acids and Flavonoids and HPLC Analyses
2.4.3. Extraction of Isoprenoids and HPLC Analysis
2.5. Determination of Antioxidant Capacity of Extracts
2.6. Determination of Total Antioxidant Capacity of Wholemeal Flours
2.7. Metabolomics Profiling through 1H NMR Spectroscopy
2.7.1. Chemicals
2.7.2. Extraction Procedure for 1H NMR Measurements
2.7.3. Statistical Analysis
3. Results
3.1. Wholemeal Flours Nutrition Facts
3.2. Starch and Dietary Fibres Composition
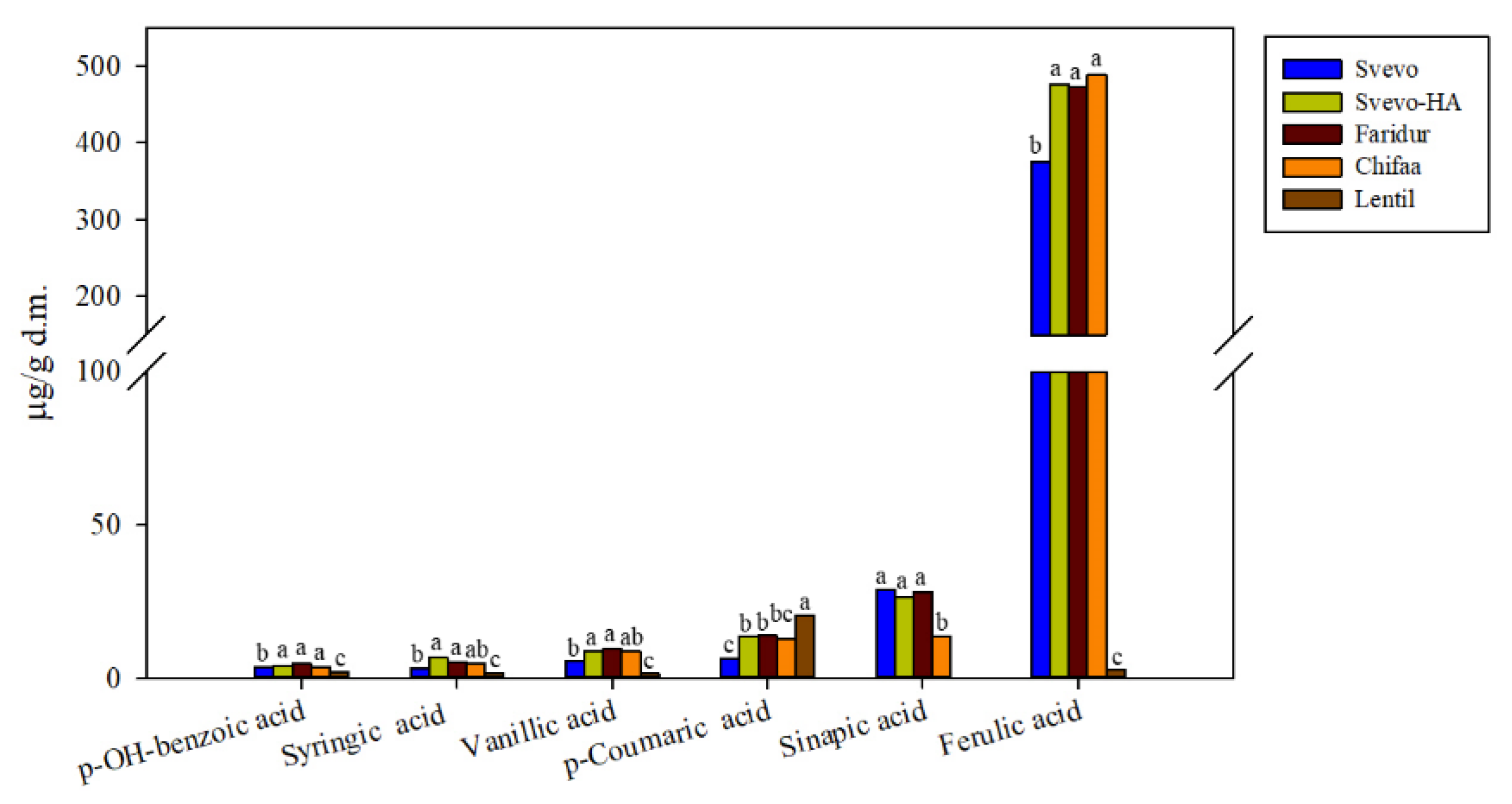
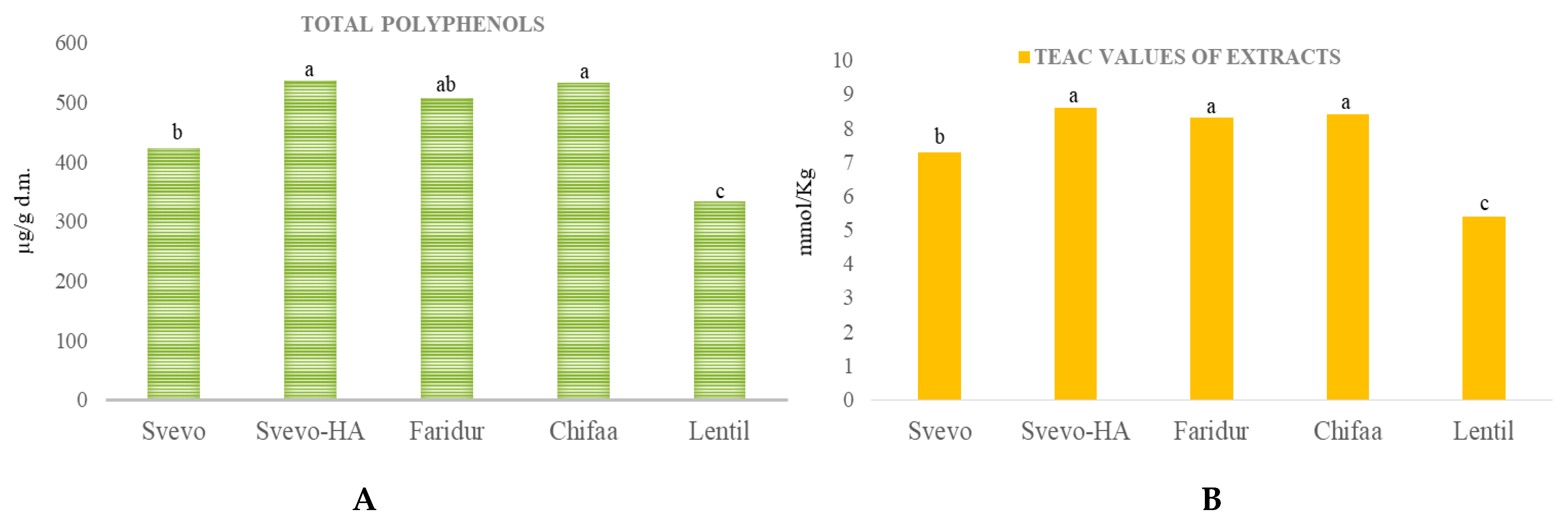
3.3. Phytochemical Profiling
3.3.1. Phenolic Acids and Flavonoids
3.3.2. Isoprenoids (Tocochromanols and Carotenoids)
3.4. Antioxidant Capacity of Extracts and Wholemeal Flours
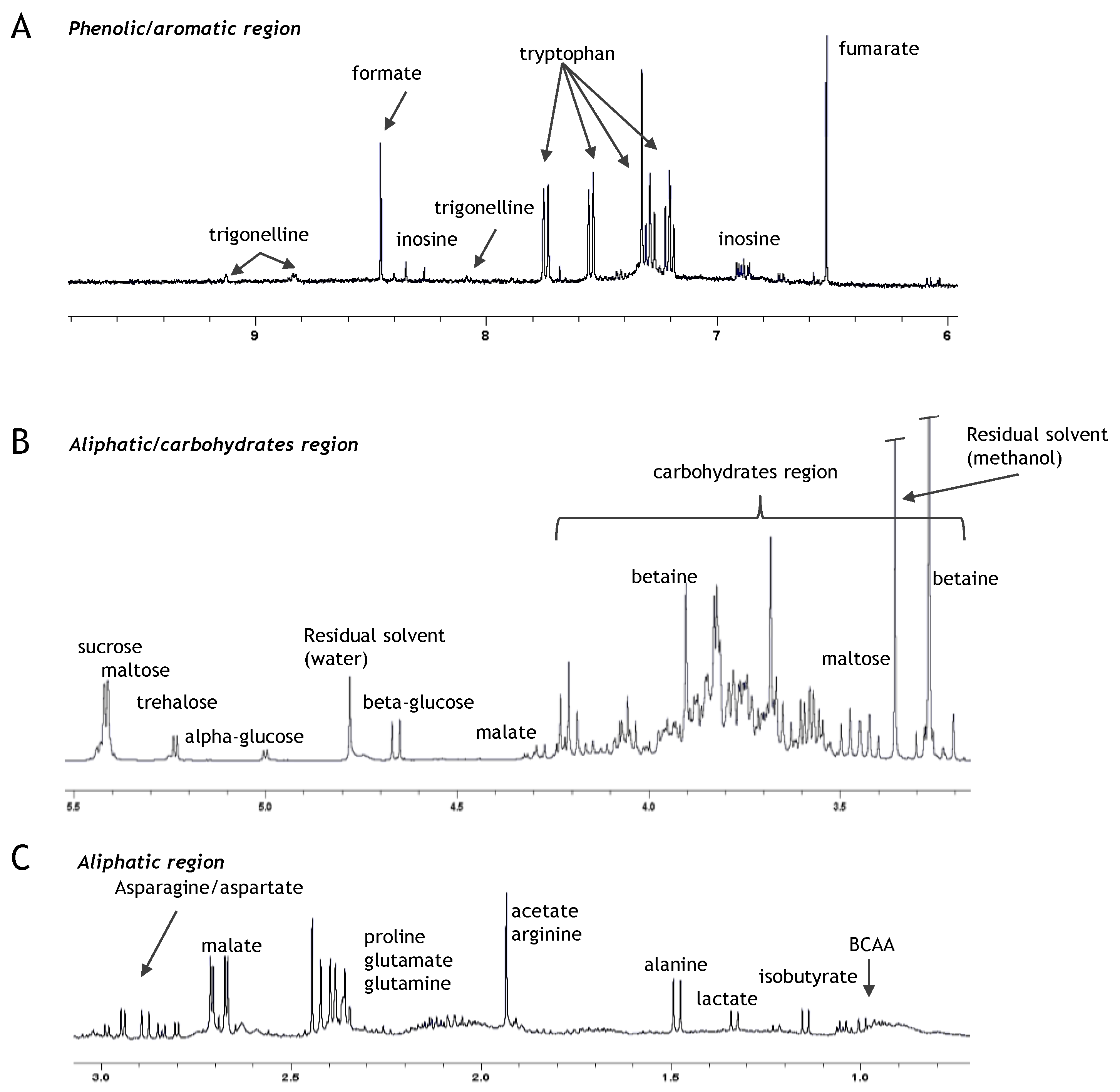
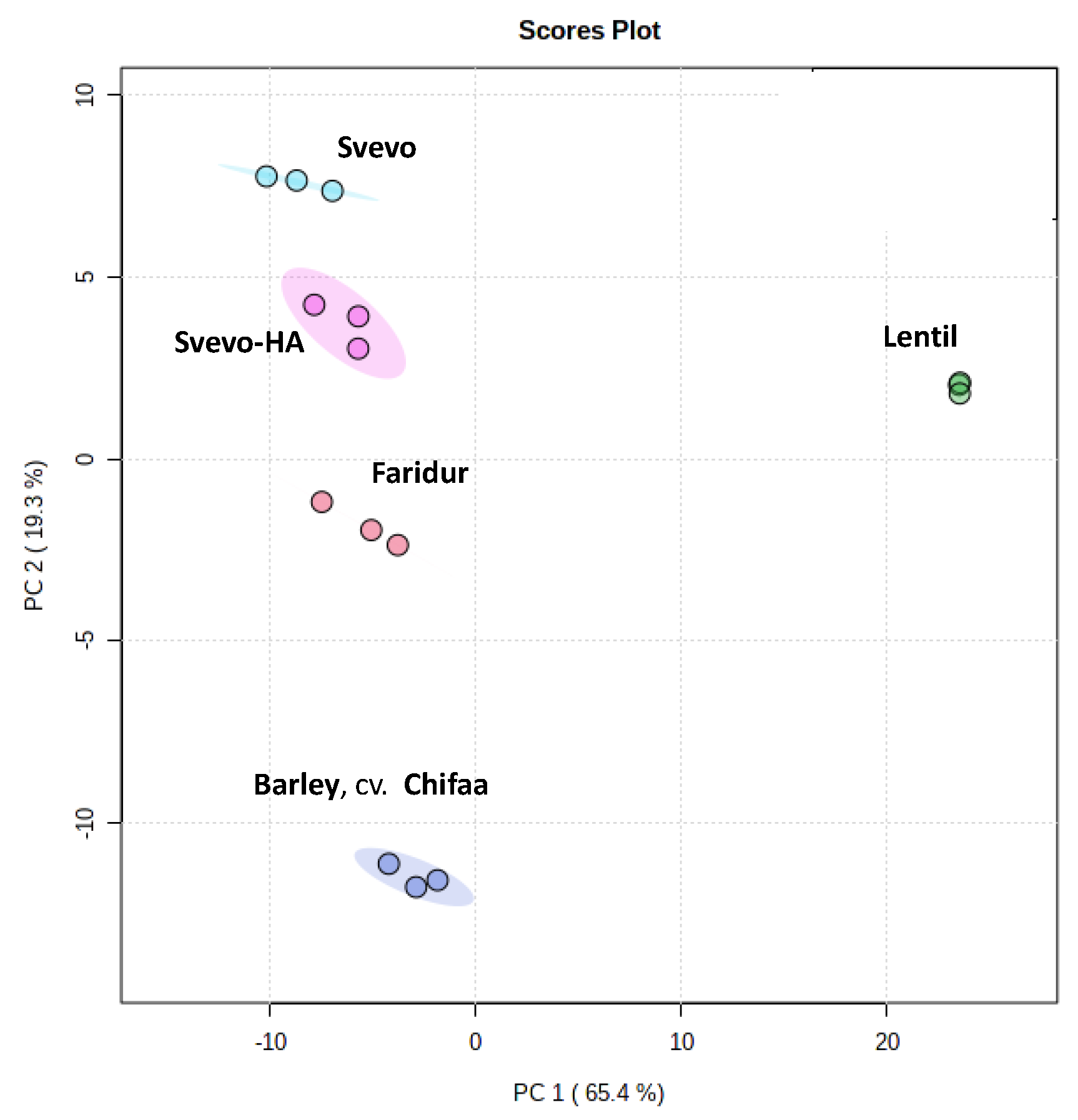
3.5. Untargeted Metabolomic Profiling through 1H NMR Spectroscopy
4. Discussion
4.1. Starch and Dietary Fibres Composition
4.2. Phytochemical Profiles
4.3. Antioxidant Capacities of Phytochemicals Extracts and Wholemeal Flours
4.4. Untargeted Metabolomic Profiling through 1H NMR Spectroscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nutrition Facts | Svevo | Svevo-HA | Faridur | Chifaa | Lentil |
|---|---|---|---|---|---|
| Energy (kcal/100 g) | 328.0 | 337.0 | 331.0 | 324.0 | 304.0 |
| Carbohydrate (%) | 56.04 | 49.50 | 55.00 | 53.80 | 35.10 |
| Protein (%) | 15.81 | 19.93 | 16.30 | 15.19 | 25.67 |
| Total fat (%) | 1.81 | 3.76 | 2.58 | 2.39 | 1.51 |
| Total dietary fibre (%) | 11.40 | 12.70 | 11.20 | 13.50 | 23.60 |
| Pentadecanoic Acid (%) | 0.85 | 0.73 | 0.72 | 0.81 | 2.24 |
| Palmitic Acid (%) | 15.46 | 14.87 | 16.63 | 18.77 | 11.10 |
| Stearic Acid (%) | 1.32 | 1.22 | 1.41 | 1.34 | 1.58 |
| Oleic Acid (%) | 20.68 | 20.48 | 18.20 | 19.28 | 22.10 |
| Linoleic Acid (%) | 56.12 | 58.00 | 57.81 | 53.20 | 47.65 |
| Alpha-Linolenic Acid (%) | 4.75 | 4.02 | 4.39 | 5.34 | 13.73 |
| Saturated fat (%) | 16.78 | 16.09 | 18.04 | 20.31 | 13.48 |
| Monounsaturated (%) | 22.35 | 21.89 | 19.76 | 21.15 | 25.18 |
| Polyunsaturated (%) | 60.87 | 62.02 | 62.2 | 58.54 | 61.38 |
| Saturated fat on 100 g | 0.30 | 0.61 | 0.47 | 0.49 | 0.20 |
| Moisture (%) | 12.66 | 11.75 | 12.98 | 11.34 | 11.03 |
| Sodium (mg/kg) | 27.0 | 25.0 | 35.0 | 66.0 | 21.1 |
| Ash (%) | 1.94 | 2.38 | 1.93 | 3.81 | 3.14 |
Appendix B
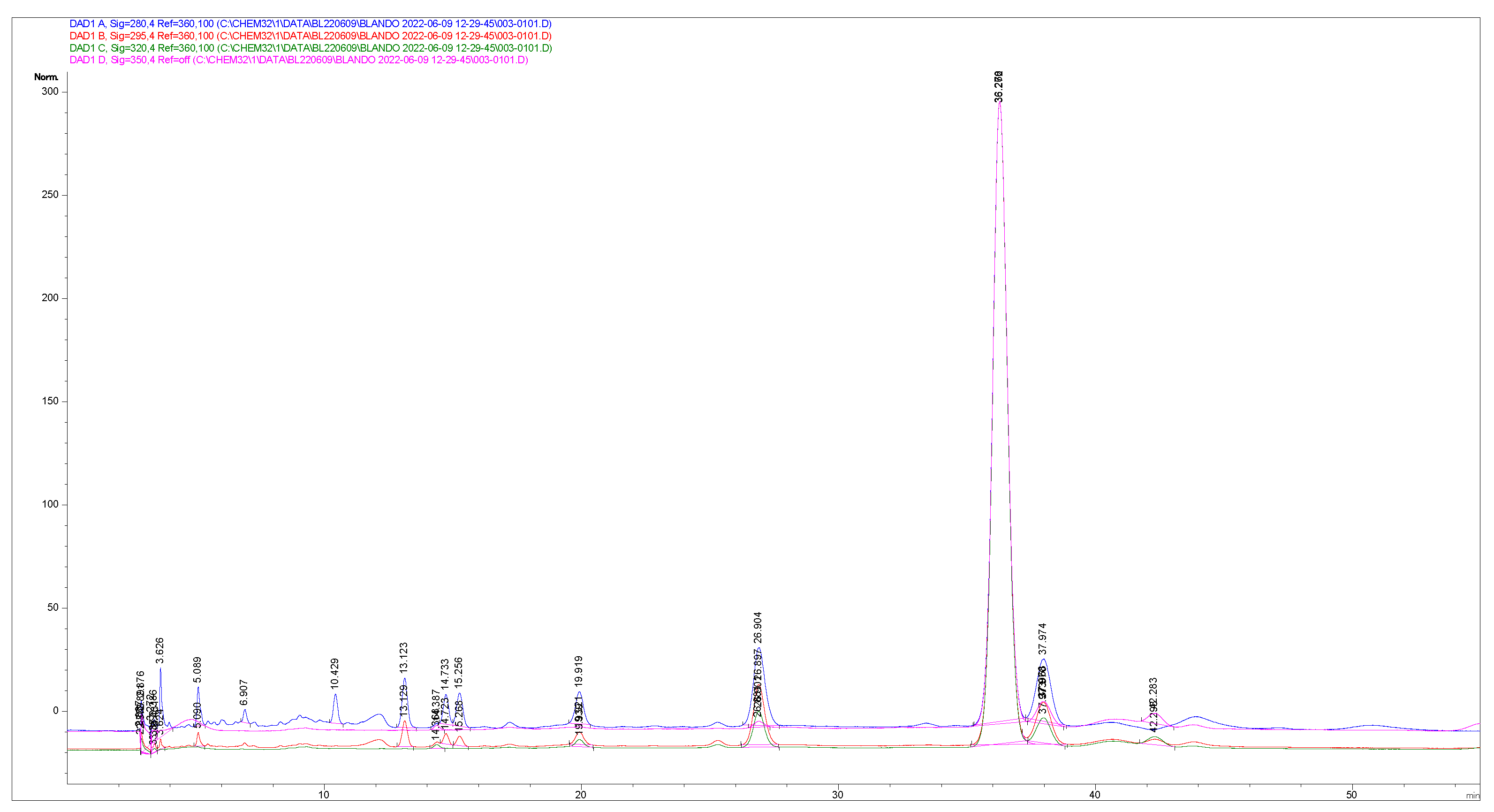
Appendix C
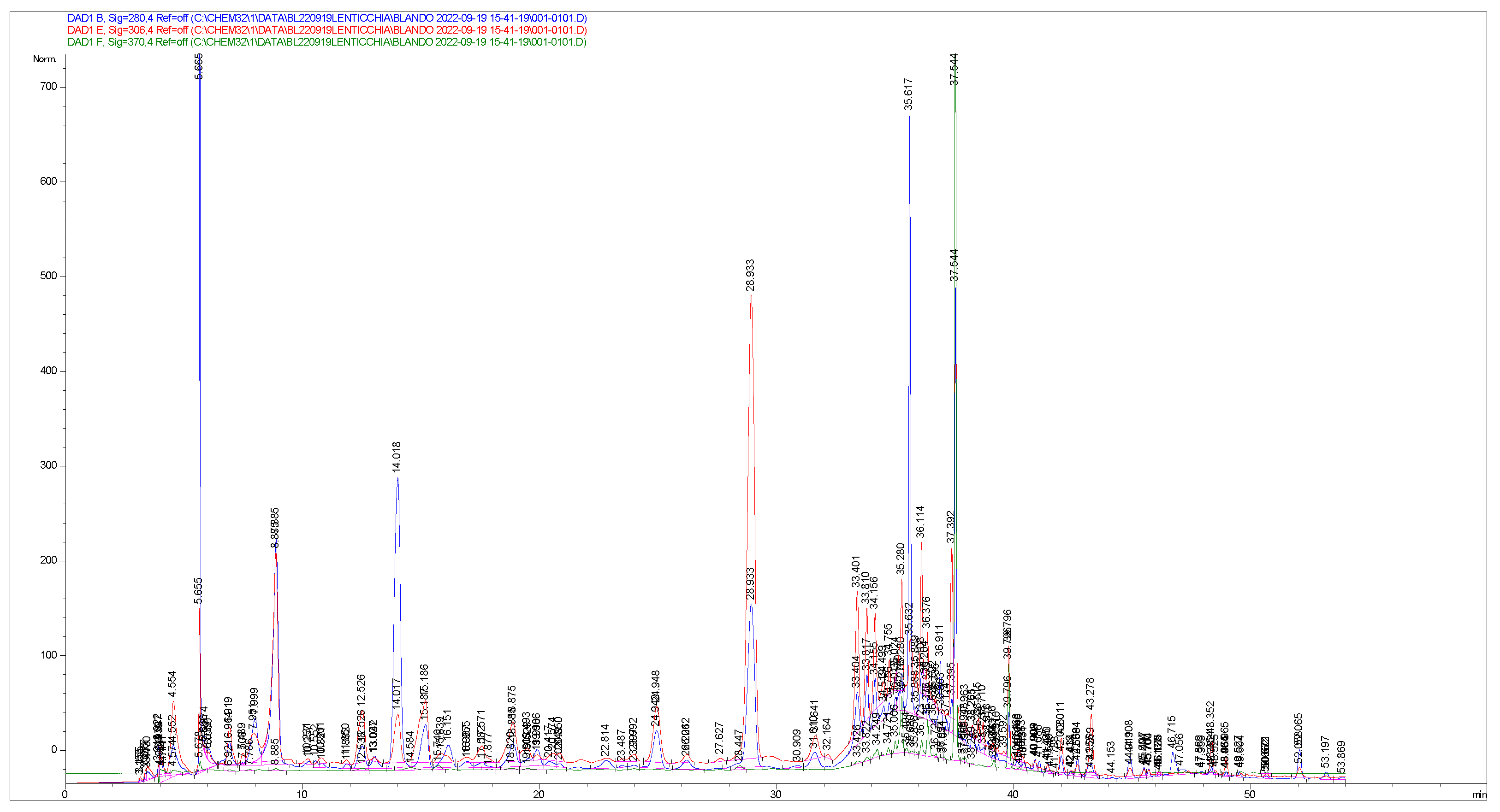
Appendix D

Appendix E

Appendix F
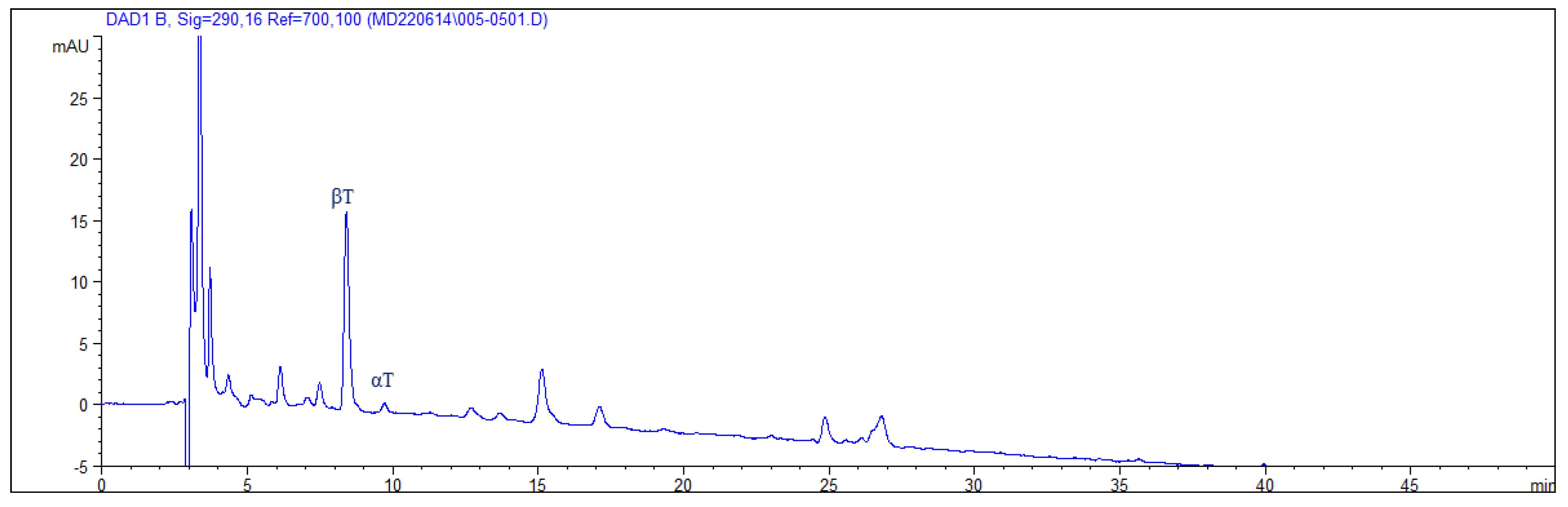
Appendix G

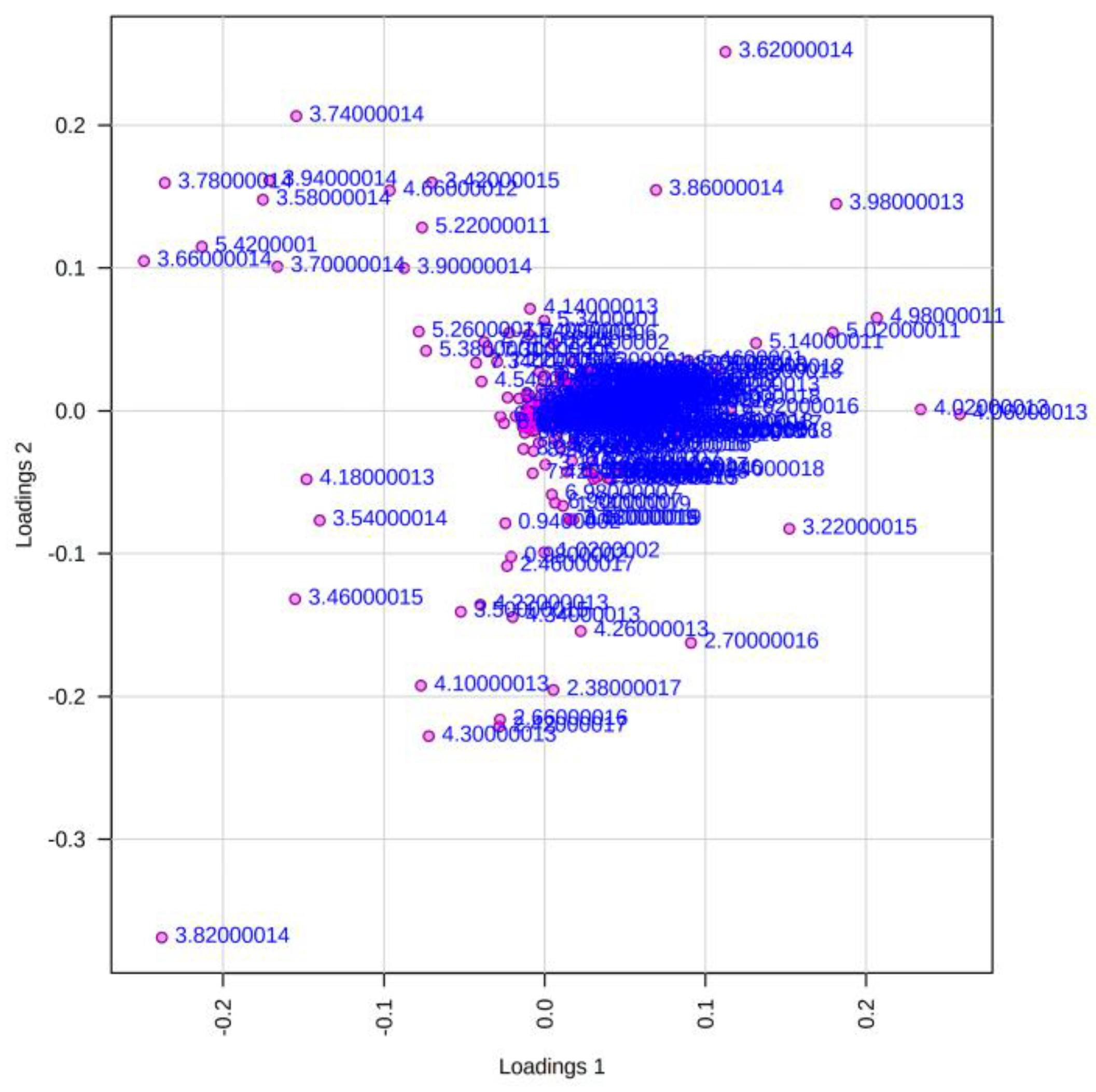
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Espinosa, N.; Laddomada, B.; Payne, T.; Huerta-Espino, J.; Govindan, V.; Ammar, K.; Ibba, M.I.; Pasqualone, A.; Guzman, C. Nutritional quality characterization of a set of durum wheat landraces from Iran and Mexico. LWT 2020, 124, 109198. [Google Scholar] [CrossRef]
- Kumari, P.V.; Sangeetha, N. Utilization of Cereals and Legumes in Traditional Food Products. Nutr. Food Sci. Int. J. 2017, 3, 555616. [Google Scholar] [CrossRef][Green Version]
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 28998. [Google Scholar] [CrossRef]
- Misra, A.; Khurana, L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008, 93, S930. [Google Scholar] [CrossRef]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef]
- Newman, R.K.; Newman, C.W. Barley Food Product Research and Development. In Barley for Food and Health: Science, Technology, and Products; Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 144–177. [Google Scholar]
- Sestili, F.; Palombieri, S.; Botticella, E.; Mantovani, P.; Bovina, R.; Lafiandra, D. TILLING mutants of durum wheat result in a high amylose phenotype and provide information on alternative splicing mechanisms. Plant Sci. 2015, 233, 127–133. [Google Scholar] [CrossRef]
- Morris, C.F.; Simeone, M.C.; King, G.E.; Lafiandra, D. Transfer of soft kernel texture from Triticum aestivum to durum wheat, Triticum turgidum ssp. durum. Crop Sci. 2011, 51, 114–122. [Google Scholar] [CrossRef]
- Botticella, E.; Savatin, D.V.; Sestili, F. The Triple Jags of Dietary Fibres in Cereals: How Biotechnology Is Longing for High Fibre Grains. Front. Plant Sci. 2021, 1959. [Google Scholar]
- Bojarczuk, A.; Skąpska, S.; Khaneghah, A.M.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Bird, A.R.; Regina, A. High amylose wheat: A platform for delivering human health benefits. J. Cereal Sci. 2018, 82, 99–105. [Google Scholar] [CrossRef]
- Pasqualone, A.; Palombieri, S.; Koksel, H.; Summo, C.; De Vita, P.; Sestili, F. Milling performance and bread-making performance of the new soft kernel durum wheat variety Faridur. Int. J. Food Sci. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Woo, S.M.; Kwon, S.C.; Ko, S.G.; Cho, S.G. Barley grass extract causes apoptosis of cancer cells by increasing intracellular reactive oxygen species production. Biomed. Rep. 2017, 6, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant Activity of β-Glucan. ISRN Pharm. 2012, 2012, 125864. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pasqualone, A.; Laddomada, B.; Carluccio, M.A. Phenolic extracts from whole wheat biofortified bread dampen overwhelming 1 inflammatory response in human endothelial cells and monocytes: Major role of VCAM-1 and CXCL-10. Eur. J. Nutr. 2020, 59, 2603–2615. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Maffia, M.; Chieppa, M.; Laddomada, B.; Carluccio, M.A. Non-Celiac Gluten Sensitivity and Protective Role of Dietary Polyphenols. Nutrients 2022, 14, 2679. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Gumienna, M.; Jędrusek-Golińska, A.; Waszkowiak, K.; Szwengiel, M.A.; Gramza-Michałowska, A. Innovative Application of Phytochemicals from Fermented Legumes and Spices/Herbs Added in Extruded Snacks. Nutrients 2021, 13, 4538. [Google Scholar] [CrossRef]
- Fiocchetti, F.; Laddomada, B.; Roselli, M.; Crinò, P.; Lucretti, S. Fingerprinting of Italian lentil (Lens culinaris Medik.) landraces using fluorescence-based AFLP. Sci. Hort 2009, 12, 383–387. [Google Scholar] [CrossRef]
- Zhao, X.C.; Sharp, P.J. An improved 1-D SDS-PAGE method for the identification of three bread wheat «Waxy» proteins. J. Cereal. Sci. 1996, 23, 191–193. [Google Scholar] [CrossRef]
- Chrastil, J. Improved colorimetric determination of amylose in starches or flours. Carbohydr. Res. 1987, 159, 154–158. [Google Scholar] [CrossRef]
- Douglas, S.G. A rapid method for the determination of pentosans in wheat flour. Food Chem. 1981, 7, 139–145. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Taddei, F.; Nicoletti, I.; Gazza, L.; Corradini, D.; D’Egidio, M.G.; Martini, D. Use of bran fractions and debranned kernels for the development of pasta with high nutritional and healthy potential. Food Chem. 2017, 225, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Finnie, S.M.; Bettge, A.D.; Morris, C.F. Influence of cultivar and environment on water-soluble and water-insoluble arabinoxylans in soft wheat. Cereal. Chem. 2006, 83, 617–623. [Google Scholar] [CrossRef]
- Alzuwaid, N.T.; Fellows, C.M.; Laddomada, B.; Sisson, M. Impact of wheat bran particle size on the technological and phytochemical properties of durum wheat pasta. J. Cereal. Sci. 2020, 95, 103033. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Laddomada, B.; Durante, M.; Minervini, F.; Garbetta, A.; Cardinali, A.; D’Antuono, I.; Caretto, S.; Blanco, A.; Mita, G. Phytochemical composition and anti-inflammatory activity of extracts from the whole-meal flour of Italian durum wheat cultivars. Int. J. Mol. Sci. 2015, 16, 3512–3527. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Marrese, P.P.; Rizzi, V.; De Caroli, M.; Piro, G.; Fini, P.; Russo, G.L.; Mita, G. α-Cyclodextrin encapsulation of supercritical CO2 extracted oleoresins from different plant matrices: A stability study. Food Chem. 2016, 199, 684–693. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct measurement of the total antioxidant capacity of cereal products. J. Cereal. Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Fanizzi, F.P. 1H NMR metabolic profile of scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in Female gonads and somatic tissues: Preliminary results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Southam, A.D.; Hines, A.; Viant, M.R. High-throughput tissue extraction protocol for NMR-and MS-based metabolomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; Van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomic. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, 388–396. [Google Scholar] [CrossRef]
- Belahsen, R.; Rguibi, M. Population health and Mediterranean diet in southern Mediterranean countries. Public Health Nutr. 2006, 9, 1130–1135. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Garcia Molina, M.D.; Botticella, E.; Beleggia, R.; Palombieri, S.; De Vita, P.; Masci, S.; Lafiandra, D.; Sestili, F. Enrichment of provitamin A content in durum wheat grain by suppressing β-carotene hydroxylase 1 genes with a TILLING approach. Appl. Genet. 2021, 134, 4013–4024. [Google Scholar] [CrossRef]
- Slade, A.J.; McGuire, C.; Loeffler, D.; Mullenberg, J.; Skinner, W.; Fazio, G.; Holm, A.; Brandt, K.M.; Steine, M.N.; Goodstal, J.F.; et al. Development of high amylose wheat through TILLING. BMC Plant Biol. 2012, 12, 69. [Google Scholar] [CrossRef]
- Hazard, B.; Zhang, X.; Naemeh, M.; Dubcovsky, J. Registration of durum wheat germplasm lines with combined mutations in SBEIIa and SBEIIb genes conferring increased amylose and resistant starch. J. Plant Regist. 2014, 8, 334–338. [Google Scholar] [CrossRef]
- Arp, C.G.; Correa, M.J.; Ferrero, C. Resistant starches: A smart alternative for the development of functional bread and other starch-based foods. Food Hydrocoll. 2021, 121, 106949. [Google Scholar] [CrossRef]
- Cimini, A.; Poliziani, A.; Antonelli, G.; Sestili, F.; Lafiandra, D.; Moresi, M. Characterization of fresh pasta made of common and high-amylose wheat flour mixtures. Foods 2022, 11, 2510. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Sestili, F.; Vitale, M.; Botticella, E.; Giacco, R.; Griffo, E.; Riccardi, G. Metabolic response to amylose-rich wheat-based rusks in overweight individuals. Eur. J. Clin. Nutr. 2018, 72, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Sissons, M.; Sestili, F.; Botticella, E.; Masci, S.; Lafiandra, D. Can manipulation of durum wheat amylose content reduce the glycaemic index of spaghetti? Foods 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Qi, L.; Fahey, G.C.; Klurfeld, D.M. Consumption of cereal fibre, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucan from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.F.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. β-Glucan in promoting health: Prevention against mutation and cancer. Mutat. Res. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef]
- Laddomada, B.; Durante, M.; Mangini, G.; D’Amico, L.; Lenucci, M.S.; Simeone, R.; Piarulli, L.; Mita, G.; Blanco, A. Genetic var-iation for phenolic acids concentration and composition in a tetraploid wheat (Triticum turgidum L.) collection. Genet. Resour. Crop Evol. 2017, 64, 587–597. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of Drought, Heat and Their Interaction on the Growth, Yield and Photosynthetic Function of Lentil (Lens culinaris Medikus) Genotypes Varying in Heat and Drought Sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin and the macular pigment. Arch Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics provides valuable insight for the study of durum wheat: A review. J. Agric. Food Chem. 2019, 67, 3069–3308. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.; Vassiliadis, S.; Maharjan, P.; Brand, J.; Panozzo, J. NMR based metabolomic analysis of health promoting phytochemicals in lentils. Metabolites 2019, 9, 168. [Google Scholar] [CrossRef]
- Ilavenil, S.; Kim, D.H.; Jeon, Y.-I.; Arasu, M.V.; Vijayakumar, M.V.; Prabhu, P.N.; Srigopalram, S.; Choi, K.C. Trigonelline protects the cardiocyte from hydrogen peroxide induced apoptosis in H9c2 cells. Asian Pac. J. Trop. Med. 2015, 8, 263–268. [Google Scholar] [CrossRef]
| Genotype | Total Starch (%) | RS (%) | Amylose (%) | TOT-AX (%) | We-AX (%) | β-Glucan (%) |
|---|---|---|---|---|---|---|
| Svevo | 59.5 ± 0.1 a | 0.2 ± 0.03 c | 34.0 ± 0.9 b | 3.4 ± 0.30 b | 0.45 ± 0.1 | 0.49 ± 0.01 c |
| Svevo-HA | 57.0 ± 0.9 a | 6.9 ± 0.20 a | 58.7 ± 1.1 a | 4.7 ± 0.30 a | 0.44 ± 0.2 | 1.4 ± 0.01 b |
| Faridur | 60.1 ± 0.3 a | 0.38 ± 0.01 c | 33.0 ± 0.8 b | 3.5 ± 0.30 b | 0.44 ± 0.5 | 0.48 ± 0.03 c |
| Chifaa | 44.5 ± 1.0 b | 0.33 ± 0.03 c | 26.0 ± 0.8 c | 3.3 ± 0.20 b | 0.44 ± 0.4 | 7.05 ± 0.15 a |
| Lentil | 42.9 ± 0.8 b | 2.12 ± 0.10 b | 25.2 ± 1.8 c | 2.5 ± 0.30 c | nd | nd |
| Flavonoids | µg/g d.m. |
|---|---|
| Procyanidin B2 | 12.68 ± 1.2 |
| Procyanidin B3 | 3.13 ± 0.2 |
| (+)-Catechin | 187.9 ± 2.5 |
| (−)-Epicatechin | 3.57 ± 0.3 |
| Luteolin | 0.06 ± 0.00 |
| Kaempferol-7-O-neohesperidoside | 96.83 ± 0.8 |
| Whole-Meals | β-Tocotrienol | α-Tocotrienol | β-Tocopherol | α-Tocopherol | Lutein | Zeaxanthin | TEAC |
|---|---|---|---|---|---|---|---|
| Svevo | 9.96 ± 1.12 b | 1.04 ± 0.17 b | nd | nd | 0.94 ± 0.13 d | 0.13 ± 0.01 c | 0.039 ± 0.037 d |
| Svevo-HA | 13.54 ± 2.24 ab | 1.06 ± 0.12 b | nd | nd | 1.39 ± 0.09 b | 0.16 ± 0-01 c | 0.119 ± 0.091 c |
| Faridur | 16.63 ± 1.14 a | 2.41 ± 0.14 a | nd | 1.77 ± 0.19 a | 1.33 ± 0.08 bd | 0.12 ± 0.01 c | 0.106 ± 0.084 c |
| Chifaa | nd | nd | 24.19 ± 2.23 a | 1.60 ± 0.12 a | 2.12 ± 0.30 c | 0.93 ± 0.10 b | 0.164 ± 0.095 b |
| Lentil | nd | nd | 19.86 ± 2.01 a | 1.32 ± 0.21 a | 3.12 ± 0.10 a | 1.29 ± 0.08 a | 0.147 ± 0.115 a |
| Wholemeal Sample | mmol Trolox/Kg |
|---|---|
| Svevo | 27.8 ± 3.5 a |
| Svevo HA | 33.3 ± 1.1 a |
| Faridur | 29.7 ± 2.2 a |
| Chifaa | 43.5 ± 0.3 b |
| Lentil | 140.4 ± 10.9 c |
| TEAC μmol/g Experimental | TEAC μmol/g Calculated | |
|---|---|---|
| Svevo | 0.05 ± 0.02 b | 0.055 ± 0.006 d |
| Svevo HA | 0.07 ± 0.02 b | 0.073 ± 0.011 d |
| Faridur | 0.13 ± 0.02 a | 0.102 ± 0.007 c |
| Chifaa | 0.16 ± 0.09 a | 0.114 ± 0.011 b |
| Lentil | 0.14 ± 0.07 ba | 0.131 ± 0.012 a |
| TEAC μmol/g Experimental | TEAC μmol/g Calculated | |
|---|---|---|
| Svevo | 7.3 ± 0.4 c | 8.3 ± 0.4 b |
| Svevo HA | 8.3 ± 0.1 b | 10.2 ± 0.4 a |
| Faridur | 8.6 ± 0.2 a | 10.5 ± 0.5 a |
| Chifaa | 8.4 ± 0.2 a | 10.5 ± 0.3 a |
| Lentil | 5.4 ± 0.2 d | 2.6 ± 1.5 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, G.; Del Coco, L.; Milano, F.; Durante, M.; Palombieri, S.; Sestili, F.; Visioni, A.; Jilal, A.; Fanizzi, F.P.; Laddomada, B. Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Wholemeal Flours. Foods 2022, 11, 4070. https://doi.org/10.3390/foods11244070
Romano G, Del Coco L, Milano F, Durante M, Palombieri S, Sestili F, Visioni A, Jilal A, Fanizzi FP, Laddomada B. Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Wholemeal Flours. Foods. 2022; 11(24):4070. https://doi.org/10.3390/foods11244070
Chicago/Turabian StyleRomano, Giuseppe, Laura Del Coco, Francesco Milano, Miriana Durante, Samuela Palombieri, Francesco Sestili, Andrea Visioni, Abderrazek Jilal, Francesco Paolo Fanizzi, and Barbara Laddomada. 2022. "Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Wholemeal Flours" Foods 11, no. 24: 4070. https://doi.org/10.3390/foods11244070
APA StyleRomano, G., Del Coco, L., Milano, F., Durante, M., Palombieri, S., Sestili, F., Visioni, A., Jilal, A., Fanizzi, F. P., & Laddomada, B. (2022). Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Wholemeal Flours. Foods, 11(24), 4070. https://doi.org/10.3390/foods11244070









