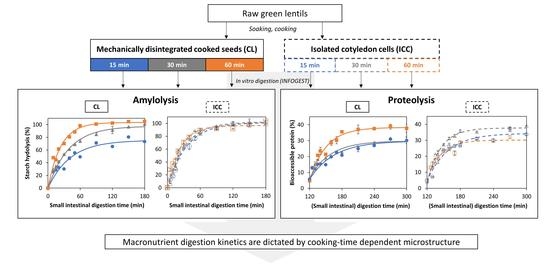
How Cooking Time Affects In Vitro Starch and Protein Digestibility of Whole Cooked Lentil Seeds versus Isolated Cotyledon Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
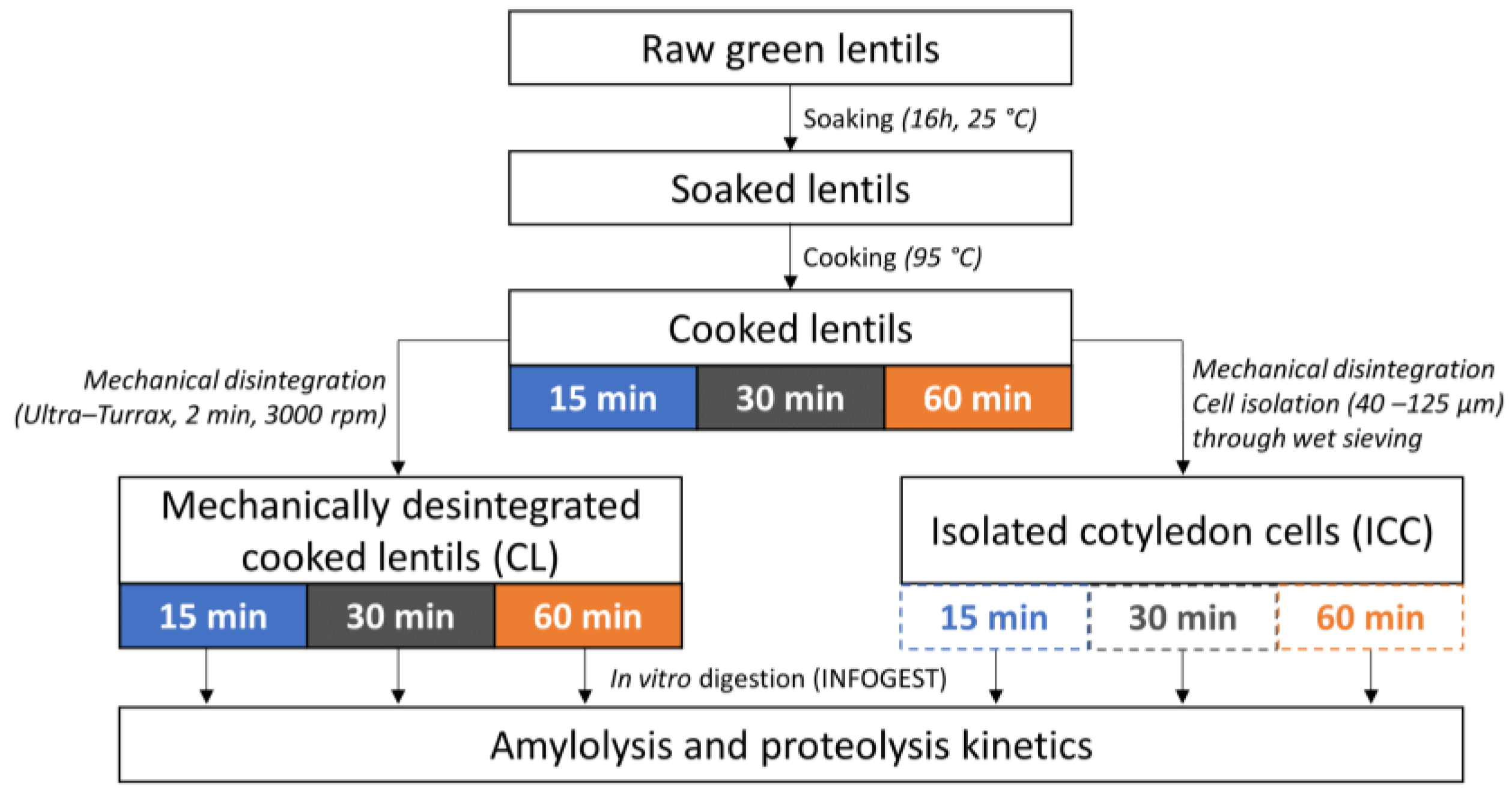
2.2. Thermal Processing of Lentil Seeds and Isolation of Cotyledon Cells
2.2.1. Whole Cooked Lentil Seeds (CL)
2.2.2. Isolated Cotyledon Cells (ICC)
2.3. Sample Characterization
2.3.1. Compositional Analysis
2.3.2. Microscopy
2.3.3. Particle Size Distribution
2.4. In Vitro Digestion: INFOGEST 2.0
2.4.1. Simulated Oral Phase
2.4.2. Simulated Gastric Phase
2.4.3. Simulated Small Intestinal Phase
2.5. Quantitative Evaluation of Macronutrient Digestion
2.5.1. Determination of Digested Starch
2.5.2. Determination of Readily Bioaccessible Protein
2.6. Kinetic Modeling and Statistical Analysis
3. Results and Discussion
3.1. Characterization of Cooked Lentils and Derived Isolated Cells
3.1.1. Microstructural Characterization
3.1.2. Compositional Characterization
3.2. Effect of Cooking Time on In Vitro Amylolysis
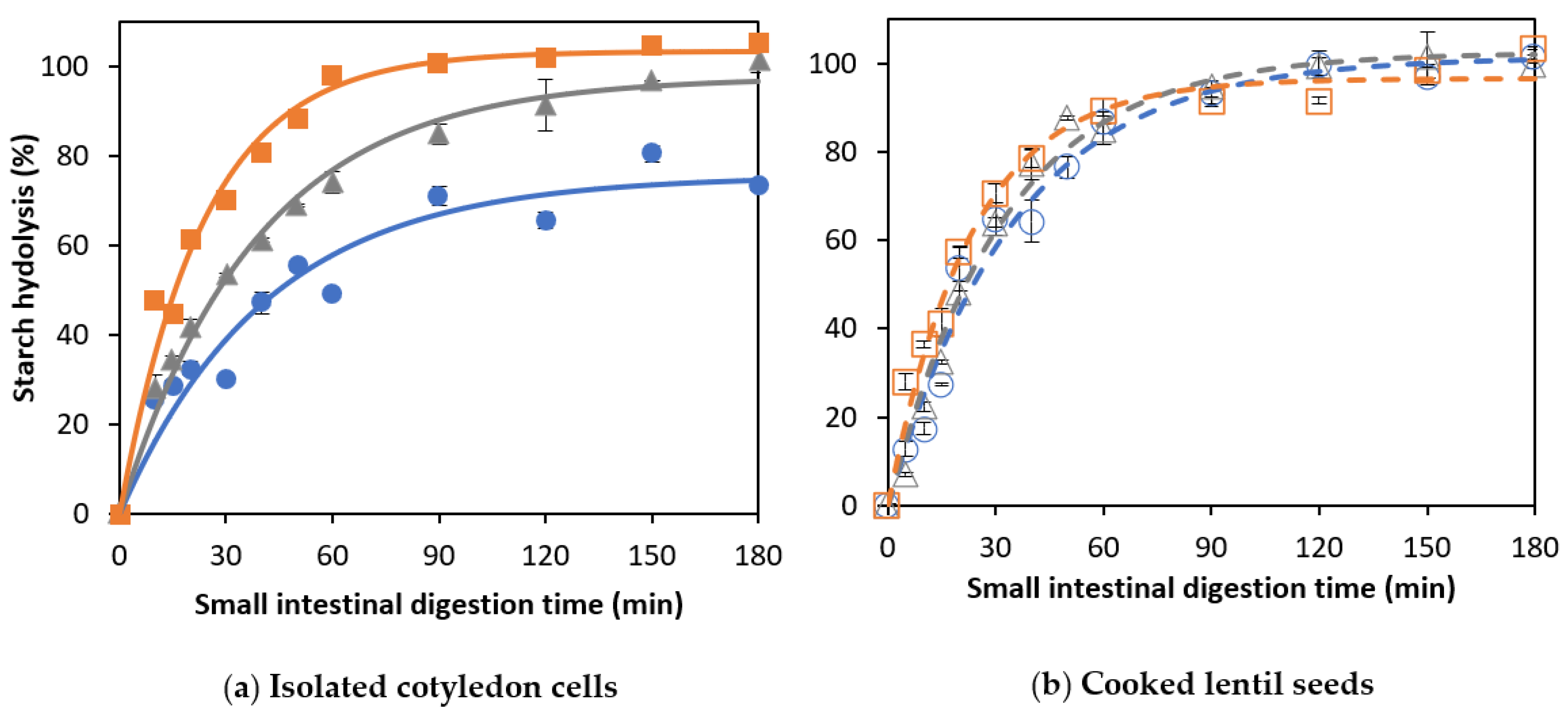
3.2.1. Amylolysis Kinetics of Isolated Lentil Cotyledon Cells (ICC)
3.2.2. Amylolysis Kinetics of Whole Cooked Lentil Seeds (CL)
3.3. Effect of Cooking Time on In Vitro Proteolysis
3.3.1. Proteolysis Kinetics of Isolated Lentil Cotyledon Cells (ICC)
3.3.2. Proteolysis Kinetics of Whole Cooked Lentil Seeds (CL)
3.4. Qualitative Evaluation of Amylolysis and Proteolysis Using Microscopic Observations
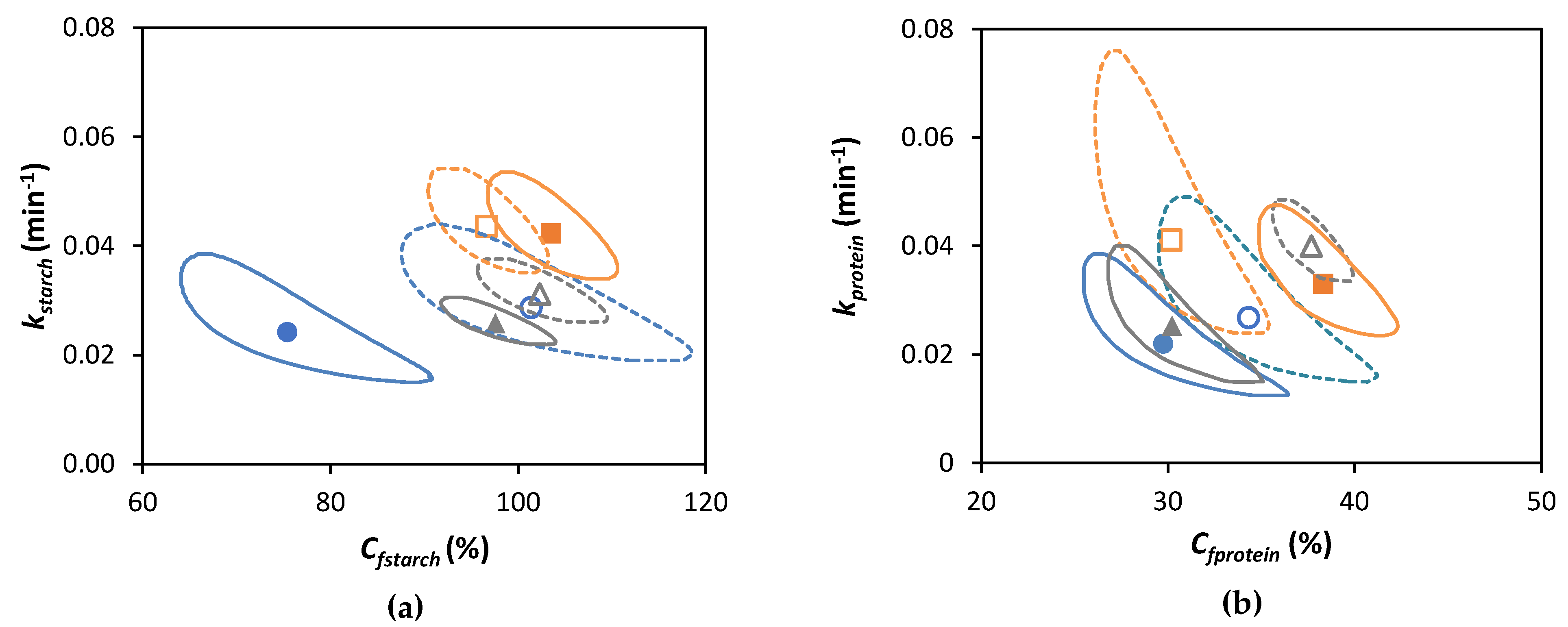
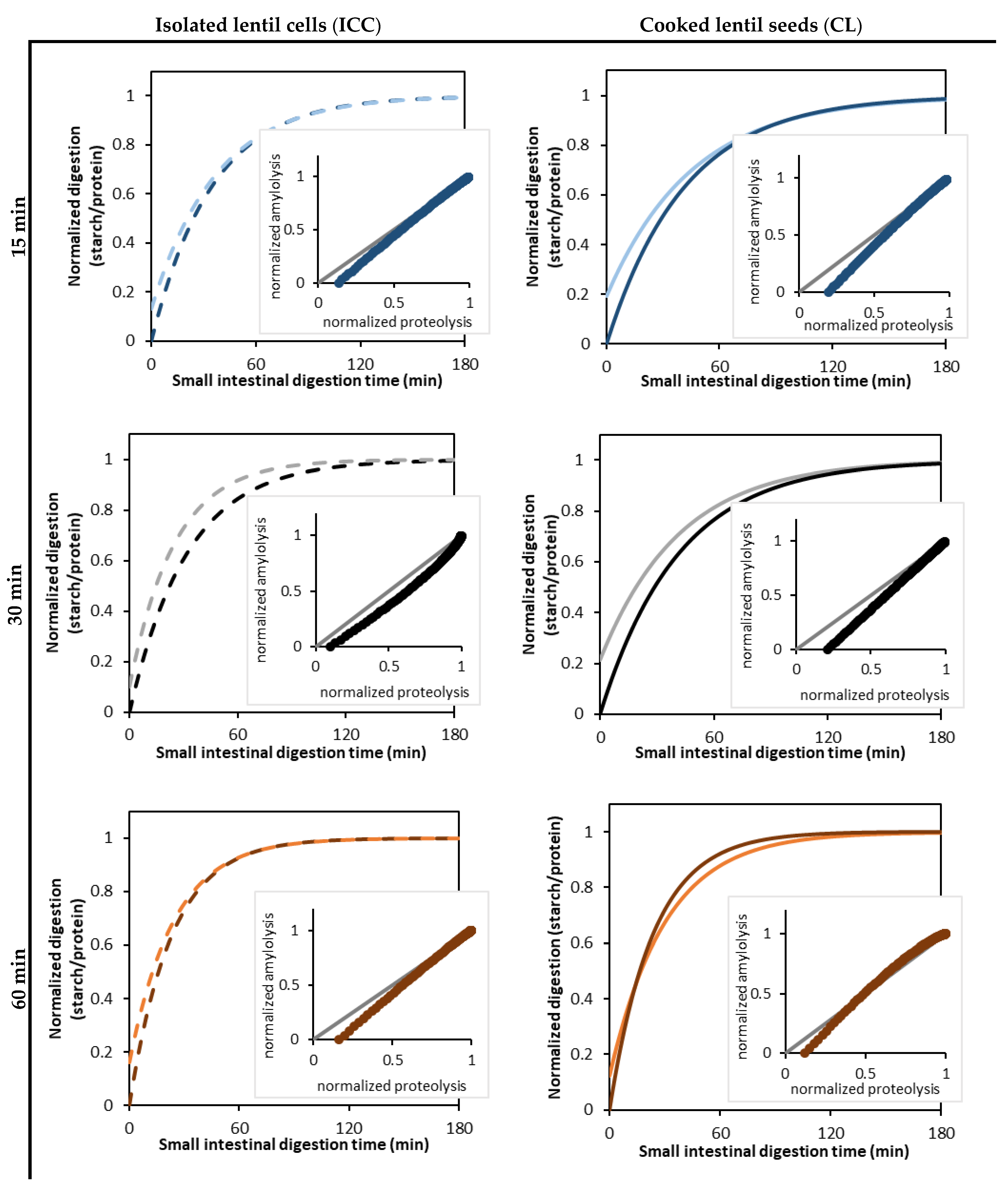
3.5. Linking Starch and Protein Digestion Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Lugo, I.; Parra, C.; Portilla, M.; Peña-Valdivia, C.B.; Moreno, E. Cotyledon thermal behavior and pectic solubility as related to cooking quality in common beans. Plant Foods Hum. Nutr. 1997, 50, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chigwedere, C.M.; Nkonkola, C.M.; Rai, S.; Kyomugasho, C.; Kermani, Z.J.; Pallares Pallares, A.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Cotyledon pectin molecular interconversions explain pectin solubilization during cooking of common beans (Phaseolus vulgaris). Food Res. Int. 2019, 116, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, D.M.; Kinyanjui, P.K.; Chigwedere, C.M.; Christiaens, S.; Makokha, A.O.; Sila, D.N.; Hendrickx, M.E. Mechanistic insight into common bean pectic polysaccharide changes during storage, soaking and thermal treatment in relation to the hard-to-cook defect. Food Res. Int. 2016, 81, 39–49. [Google Scholar] [CrossRef]
- Pallares Pallares, A.; Gwala, S.; Pälchen, K.; Duijsens, D.; Hendrickx, M.; Grauwet, T. Pulse seeds as promising and sustainable source of ingredients with naturally bioencapsulated nutrients: Literature review and outlook. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1524–1553. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moral, N.; Saha, S.; Pinto, A.M.; Bajka, B.H.; Edwards, H. In vitro protein bioaccessibility and human serum amino acid responses to white bread enriched with intact plant cells. Food Chem. 2022, 404, 134538. [Google Scholar] [CrossRef]
- Pälchen, K.; Bredie, W.L.P.; Duijsens, D.; Alfie, A.I.; Hendrickx, M.; Van Loey, A.; Raben, A.; Grauwet, T. Effect of processing and microstructural properties of chickpea-flours on in vitro digestion and appetite sensations. Food Res. Int. 2022, 157, 111245. [Google Scholar] [CrossRef]
- Bajka, B.H.; Pinto, A.M.; Ahn-Jarvis, J.; Ryden, P.; Perez-Moral, N.; van der Schoot, A.; Stocchi, C.; Bland, C.; Berry, S.E.; Ellis, P.R.; et al. The impact of replacing wheat flour with cellular legume powder on starch bioaccessibility, glycaemic response and bread roll quality: A double-blind randomised controlled trial in healthy participants. Food Hydrocoll. 2021, 114, 106565. [Google Scholar] [CrossRef]
- Singh, M.; Manickavasagan, A.; Shobana, S.; Mohan, V. Glycemic index of pulses and pulse-based products: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1567–1588. [Google Scholar] [CrossRef]
- Lal, M.K.; Singh, B.; Sharma, S.; Singh, M.P.; Kumar, A. Glycemic index of starchy crops and factors affecting its digestibility: A review. Trends Food Sci. Technol. 2021, 111, 741–755. [Google Scholar] [CrossRef]
- Henn, K.; Goddyn, H.; Bøye Olsen, S.; Bredie, W.L.P.; Olsen, S.B.; Bredie, W.L.P. Identifying behavioral and attitudinal barriers and drivers to promote consumption of pulses: A quantitative survey across five European countries. Food Qual. Prefer. 2021, 98, 104455. [Google Scholar] [CrossRef]
- Faris, M.A.I.E.; Takruri, H.R.; Issa, A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Med. J. Nutrition Metab. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; Van De Velde, F.; De Kok, P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.H.; Manthey, F.A.; Chang, S.K.C.; Hou, H.-J.; Yuan, S.H. Quality Characteristics of Spaghetti as Affected by Green and Yellow Pea, Lentil, and Chickpea Flours. J. Food Sci. 2005, 70, 371–376. [Google Scholar] [CrossRef]
- Duijsens, D.; Gwala, S.; Pallares, A.P.; Pälchen, K.; Hendrickx, M.; Grauwet, T. How postharvest variables in the pulse value chain affect nutrient digestibility and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1–30. [Google Scholar] [CrossRef]
- Sozer, N.; Holopainen-Mantila, U.; Poutanen, K. Traditional and new food uses of pulses. Cereal Chem. 2017, 94, 66–73. [Google Scholar] [CrossRef]
- Sissons, M. Development of Novel Pasta Products with Evidence Based Impacts on Health—A Review. Foods 2022, 11, 123. [Google Scholar]
- Duijsens, D.; Pälchen, K.; De Coster, A.; Verkempinck, S.H.E.; Hendrickx, M.E.; Grauwet, T. Effect of manufacturing conditions on in vitro starch and protein digestibility of (cellular) lentil-based ingredients. Food Res. Int. 2022, 158, 111546. [Google Scholar] [CrossRef]
- Edwards, C.H.; Ryden, P.; Pinto, A.M.; van der Schoot, A.; Stocchi, C.; Perez-Moral, N.; Butterworth, P.J.; Bajka, B.; Berry, S.E.; Hill, S.E.; et al. Chemical, physical and glycaemic characterisation of PulseON®: A novel legume cell-powder ingredient for use in the design of functional foods. J. Funct. Foods 2020, 68, 103918. [Google Scholar] [CrossRef]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. Effect of bean structure on microbiota utilization of plant nutrients: An in-vitro study using the simulator of the human intestinal microbial ecosystem (SHIME®). J. Funct. Foods 2020, 73, 104087. [Google Scholar] [CrossRef]
- Pallares Pallares, A.; Rousseau, S.; Chigwedere, C.M.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Temperature-pressure-time combinations for the generation of common bean microstructures with different starch susceptibilities to hydrolysis. Food Res. Int. 2018, 106, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Bhattarai, R.R.; Gorham, J.; Gidley, M.J. Intactness of cell wall structure controls the in vitro digestion of starch in legumes. Food Funct. 2016, 7, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.R.R.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. Digestion of isolated legume cells in a stomach-duodenum model: Three mechanisms limit starch and protein hydrolysis. Food Funct. 2017, 8, 2573–2582. [Google Scholar] [CrossRef]
- Berg, T.; Singh, J.; Hardacre, A.; Boland, M.J. The role of cotyledon cell structure during in vitro digestion of starch in navy beans. Carbohydr. Polym. 2012, 87, 1678–1688. [Google Scholar] [CrossRef]
- Edwards, C.H.; Warren, F.J.; Campbell, G.M.; Gaisford, S.; Royall, P.G.; Butterworth, P.J.; Ellis, P.R. A study of starch gelatinisation behaviour in hydrothermally-processed plant food tissues and implications for in vitro digestibility. Food Funct. 2015, 6, 3634–3641. [Google Scholar] [CrossRef] [Green Version]
- Junejo, S.A.; Ding, L.; Fu, X.; Xiong, W.; Zhang, B.; Huang, Q.; Ahmed, S.; Ding, L.; Fu, X.; Xiong, W.; et al. Pea cell wall integrity controls the starch and protein digestion properties in the INFOGEST in vitro simulation. Int. J. Biol. Macromol. 2021, 182, 1200–1207. [Google Scholar] [CrossRef]
- Li, P.; Dhital, S.; Fu, X.; Huang, Q.; Liu, R.; Zhang, B.; He, X. Starch digestion in intact pulse cotyledon cells depends on the extent of thermal treatment. Food Chem. 2020, 315, 126268. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, B.; Huang, Q.; Li, C.; Pletsch, E.A.; Fu, X. Variation in the rate and extent of starch digestion is not determined by the starch structural features of cooked whole pulses. Food Hydrocoll. 2018, 83, 340–347. [Google Scholar] [CrossRef]
- Pälchen, K.; Michels, D.; Duijsens, D.; Gwala, S.T.; Pallares Pallares, A.K.; Hendrickx, M.; Van Loey, A.; Grauwet, T. In vitro protein and starch digestion kinetics of individual chickpea cells: From static to more complex in vitro digestion approaches. Food Funct. 2021, 12, 18–22. [Google Scholar] [CrossRef]
- Li, H.T.; Chen, S.Q.; Bui, A.T.; Xu, B.; Dhital, S. Natural ‘capsule’ in food plants: Cell wall porosity controls starch digestion and fermentation. Food Hydrocoll. 2021, 117, 106657. [Google Scholar] [CrossRef]
- Pälchen, K.; Van Den Wouwer, B.; Duijsens, D.; Hendrickx, M.E.; Van Loey, A.; Grauwet, T. Utilizing Hydrothermal Processing to Align Structure and In Vitro Digestion Kinetics between Three Different Pulse Types. Foods 2022, 11, 206. [Google Scholar] [CrossRef]
- Gwala, S.; Pallares Pallares, A.; Pälchen, K.; Hendrickx, M.; Grauwet, T. In vitro starch and protein digestion kinetics of cooked Bambara groundnuts depend on processing intensity and hardness sorting. Food Res. Int. 2020, 137, 109512. [Google Scholar] [CrossRef]
- Pallares Pallares, A.; Alvarez Miranda, B.; Truong, N.Q.A.; Kyomugasho, C.; Chigwedere, C.M.; Hendrickx, M.; Grauwet, T. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food Funct. 2018, 9, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Aguirre Montesdeoca, V.; Capuano, E. A mechanistic model to study the effect of the cell wall on starch digestion in intact cotyledon cells. Carbohydr. Polym. 2021, 253, 117351. [Google Scholar] [CrossRef] [PubMed]
- Khrisanapant, P.; Leong, S.Y.; Kebede, B.; Oey, I. Effects of hydrothermal processing duration on the texture, starch and protein in vitro digestibility of cowpeas, chickpeas and kidney beans. Foods 2021, 10, 1415. [Google Scholar] [CrossRef] [PubMed]
- Gwala, S.; Wainana, I.; Pallares Pallares, A.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Texture and interlinked post-process microstructures determine the in vitro starch digestibility of Bambara groundnuts with distinct hard-to-cook levels. Food Res. Int. 2019, 120, 1–11. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Kyomugasho, C.; Kamau, P.G.; Aravindakshan, S.; Hendrickx, M.E. Evaluation of storage stability of low moisture whole common beans and their fractions through the use of state diagrams. Food Res. Int. 2021, 140, 109794. [Google Scholar] [CrossRef]
- Wainaina, I.; Wafula, E.; Sila, D.; Kyomugasho, C.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Thermal treatment of common beans (Phaseolus vulgaris L.): Factors determining cooking time and its consequences for sensory and nutritional quality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3690–3718. [Google Scholar] [CrossRef] [PubMed]
- Jood, S.; Bishnoi, S.; Sharma, A. Chemical analysis and physico-chemical properties of chickpea and lentil cultivars. Nahrung–Food 1998, 42, 71–74. [Google Scholar] [CrossRef]
- Sharif, H.R.; Zhong, F.; Anjum, F.M.; Khan, M.I.; Sharif, M.K.; Khan, M.A.; Haider, J.; Shah, F.-H. Effect of soaking and microwave pretreatments on nutritional profile and cooking quality of different lentil cultivars. Pakistan J. Food Sci. 2014, 24, 186–194. [Google Scholar]
- Singh, K.B.; Erskine, W.; Robertson, L.D.; And, H.N.; Williams, P.C. Influence of pretreatment on cooking quality parameters of dry food legumes. J. Sci. Food Agric. 1988, 44, 135–142. [Google Scholar] [CrossRef]
- De Almeida Costa, G.E.; Da Silva Queiroz-Monici, K.; Pissini Machado Reis, S.M.; De Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.D.L.P.; Ramírez-Miranda, M.; Díaz-Ramírez, M.; Alamilla-Beltran, L.; Calderón-Domínguez, G. Microstructural characterisation and glycemic index evaluation of pita bread enriched with chia mucilage. Food Hydrocoll. 2017, 69, 141–149. [Google Scholar] [CrossRef]
- Noordraven, L.E.C.; Bernaerts, T.; Mommens, L.; Hendrickx, M.E.; Van Loey, A.M. Impact of cell intactness and starch state on the thickening potential of chickpea flours in water-flour systems. LWT 2021, 146, 111409. [Google Scholar] [CrossRef]
- Arntfield, S.D.; Murray, E.D.; Ismond, M.A.H.; Murray, E.D. Use of intrinsic fluorescence to follow the denaturation of Vicilin, a storage protein from Vicia Faba. Int. J. Pept. Protein Res. 1987, 29, 9–20. [Google Scholar] [CrossRef]
- Pallares Pallares, A. Influence of distinct process-induced (micro)structures on the in vitro starch digestibility of common beans: A kinetic approach. 2019. Available online: https://lirias.kuleuven.be/2836436?limo=0 (accessed on 8 October 2019).
- Pallares Pallares, A.; Loosveldt, B.; Karimi, S.N.; Hendrickx, M.; Grauwet, T. Effect of process-induced common bean hardness on structural properties of in vivo generated boluses and consequences for in vitro starch digestion kinetics. Br. J. Nutr. 2019, 122, 388–399. [Google Scholar] [CrossRef]
- Freitas, D.; Le Feunteun, S.; Panouillé, M.; Souchon, I. The important role of salivary α-amylase in the gastric digestion of wheat bread starch. Food Funct. 2018, 9, 200–208. [Google Scholar] [CrossRef]
- Miller, L.G. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Englyst, H.N.; Hudson, G.J. Colorimetric method for routine measurement of dietary fibre as non-starch polysaccharides. A comparison with gas-liquid chromatography. Food Chem. 1987, 24, 63–76. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Zahir, M.A.; Fogliano, V.; Capuano, E. Food matrix and processing modulate: In vitro protein digestibility in soybeans. Food Funct. 2018, 9, 6326–6336. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. The effect of cell wall encapsulation on macronutrients digestion: A case study in kidney beans. Food Chem. 2019, 286, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Zahir, M.A.; Fogliano, V.; Capuano, E. Effect of soybean processing on cell wall porosity and protein digestibility. Food Funct. 2019, 11, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.E.J. Intestinal absorption of protein hydrolysis products: A review. J. Anim. Sci. 1990, 68, 3011–3022. [Google Scholar] [CrossRef] [Green Version]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Carrillo, C.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Duijsens, D.; Pälchen, K.; Guevara-Zambrano, J.M.; Verkempinck, S.H.E.H.E.; Infantes-Garcia, M.R.; Hendrickx, M.E.E.; Van Loey, A.; Grauwet, T.; Duijsens, D.P.K. Strategic choices for in vitro food digestion methodologies enabling food digestion design. Trends Food Sci. Technol. 2022, 126, 61–72. [Google Scholar] [CrossRef]
- Edwards, C.H.; Warren, F.J.; Milligan, P.J.; Butterworth, P.J.; Ellis, P.R. A novel method for classifying starch digestion by modelling the amylolysis of plant foods using first-order enzyme kinetic principles. Food Funct. 2014, 5, 2751–2758. [Google Scholar] [CrossRef]
- Do, D.T.; Singh, J.; Oey, I.; Singh, H. Modulating effect of cotyledon cell microstructure on in vitro digestion of starch in legumes. Food Hydrocoll. 2019, 96, 112–122. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Dueñas, M.; Sarmento, T.; Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of cooking and germination on phenolic composition and dietary fibre fractions in dark beans (Phaseolus vulgaris L.) and lentils (Lens culinaris L.). LWT—Food Sci. Technol. 2016, 66, 72–78. [Google Scholar] [CrossRef]
- Bhatty, R.S. Cooking Quality of Lentils: The Role of Structure and Composition of Cell Walls. J. Agric. Food Chem. 1990, 38, 376–383. [Google Scholar] [CrossRef]
- Reyes-Moreno, C.; Paredes-López, O.; Gonzalez, E. Hard to cook phenomenon in common beans—A review. Crit. Rev. Food Sci. Nutr. 1993, 33, 227–286. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyaru, R.; Shrestha, A.K.; Arcot, J. Effect of various processing techniques on digestibility of starch in Red kidney bean (Phaseolus vulgaris) and two varieties of peas (Pisum sativum). Food Res. Int. 2009, 42, 956–962. [Google Scholar] [CrossRef]
- Desphande, S.S.; Salunkhe, D.K. Interactions of tannic acid and catechin with legume starches. J. Food Sci. 1982, 47, 2080–2081. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008, 110, 1–13. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Montoya, C.A.; Lallés, J.-P.; Beebe, S.; Montagne, L.; Souffrant, W.B.; Leterme, P. Influence of the Phaseolus vulgaris phaseolin level of incorporation, type and thermal treatment on gut characteristics in rats. Br. J. Nutr. 2006, 95, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Drulyte, D.; Orlien, V. The effect of processing on digestion of legume proteins. Foods 2019, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Human α-amylase and starch digestion: An interesting marriage. Starch/Staerke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.; Melvaer, K.L.; Hensten-Pettersen, A. Some properties of salivary amylase: A survey of the literature and some observations. J. Dent. Res. 1972, 51, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fan, Y.; Wang, X.; Xia, M.; Cai, Y. In vitro and in vivo digestibility of pea and chickpea powder prepared by cooking and drying treatment. Int. J. Food Prop. 2020, 23, 1187–1199. [Google Scholar] [CrossRef]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Sitrin, M.D. Digestion and Absorption of Carbohydrates and Proteins—The gastrointestinal system. In ACSM’s Advanced Exercise Physiology: Second Edition; 2011; pp. 348–362. ISBN 9781451161823. [Google Scholar]
- Boye, J.; Zare, F.; Pletch, A.; Giménez, M.A.; González, R.J.; Wagner, J.; Torres, R.; Lobo, M.O.; Samman, N.C. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Feher, J. Digestion and Absorption of the Macronutrients. Quant. Hum. Physiol. 2012, 731–743. [Google Scholar] [CrossRef]
- Bora, P. Anti-Nutritional Factors in Foods and their Effects. J. Acad. Ind. Res. 2014, 3, 285–290. [Google Scholar]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Rutherfurd, S.M.; Muñoz, L.S.; Peters, M.; Montoya, C.A. The impact of heating and soaking on the in vitro enzymatic hydrolysis of protein varies in different species of tropical legumes. Food Chem. 2016, 194, 377–382. [Google Scholar] [CrossRef]
- Salazar-Villanea, S.; Hendriks, W.H.; Bruininx, E.M.A.M.; Gruppen, H.; Van Der Poel, A.F.B. Protein structural changes during processing of vegetable feed ingredients used in swine diets: Implications for nutritional value. Nutr. Res. Rev. 2016, 29, 126–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, A.; Afseth, N.K.; Böcker, U.; Knutsen, S.H.; Kirkhus, B.; Mæhre, H.K.; Ballance, S.; Wubshet, S.G. Improved estimation of in vitro protein digestibility of different foods using size exclusion chromatography. Food Chem. 2021, 358, 129830. [Google Scholar] [CrossRef] [PubMed]
- Byars, J.A.; Singh, M.; Kenar, J.A.; Felker, F.C.; Winkler-Moser, J.K. Effect of particle size and processing method on starch and protein digestibility of navy bean flour. Cereal Chem. 2021, 98, 829–839. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. In vitro protein digestibility and physico-chemical properties of flours and protein concentrates from two varieties of lentil (Lens culinaris). Food Funct. 2013, 4, 310–321. [Google Scholar] [CrossRef]
- Orlien, V.; Aalaei, K.; Poojary, M.M.; Nielsen, D.S.; Ahrné, L.; Carrascal, J.R. Effect of processing on in vitro digestibility (IVPD) of food proteins. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–50. [Google Scholar] [CrossRef]
- Chen, Y.; Capuano, E.; Stieger, M. Chew on it: Influence of oral processing behaviour on in vitro protein digestion of chicken and soy-based vegetarian chicken. Br. J. Nutr. 2020, 126, 1408–1419. [Google Scholar] [CrossRef]
- Wang, T.L.; Bogracheva, T.Y.; Hedley, C.L. Starch: As simple as A, B, C. J. Exp. Bot. 1998, 49, 481–502. [Google Scholar] [CrossRef] [Green Version]
- Gwala, S.; Kyomugasho, C.; Wainaina, I.; Rousseau, S.; Hendrickx, M.; Grauwet, T. Ageing, dehulling and cooking of Bambara groundnuts: Consequences for mineral retention and: In vitro bioaccessibility. Food Funct. 2020, 11, 2509–2521. [Google Scholar] [CrossRef]
- Rousseau, S.; Celus, M.; Duijsens, D.; Gwala, S.; Hendrickx, M.; Grauwet, T. The impact of postharvest storage and cooking time on mineral bioaccessibility in common beans. Food Funct. 2020, 11, 7584–7595. [Google Scholar] [CrossRef]



| Moisture (g/100 g) | Starch (g/100 g DM) | Protein (g/100 g DM) | Starch-to-Protein Ratio | |
|---|---|---|---|---|
| Raw lentil | 9.4 ± 0.4 d | 48.3 ± 1.0 b | 22.3 ± 0.4 a,b | 2.17 |
| Isolated cotyledon cells (ICC) | ||||
| 15 min | 73.0 ± 0.1 b | 62.2 ± 1.0 a | 20.6 ± 0.1 c,d | 3.02 |
| 30 min | 73.1 ± 0.2 b | 60.8 ± 1.5 a | 19.8 ± 0.7 d | 3.06 |
| 60 min | 76.1 ± 0.1 a | 62.6 ± 1.7 a | 21.2 ± 0.4 b,c,d | 2.96 |
| Cooked lentil seeds (CL) | ||||
| 15 min | 68.4 ± 0.7 c | 44.7 ± 2.2 c | 21.7 ± 0.1 a,b,c | 2.06 |
| 30 min | 70.1 ± 0.1 c | 45.9 ± 1.6 b,c | 22.7 ± 0.1 a | 2.02 |
| 60 min | 75.9 ± 1.0 a | 46.9 ± 0.8 b,c | 22.3 ± 0.2 a,b | 2.10 |
| Cooking Time | vinitial (%/min) | k (/min) | Cf (%) | C120 (%) | R2adj |
|---|---|---|---|---|---|
| Isolated cotyledon cells (ICC) | |||||
| 15 min | 2.9 ± 0.3 b | 0.029 ± 0.003 c | 101.4 ± 3.2 a | 98.2 ± 2.4 a,b | 0.99 |
| 30 min | 3.2 ± 0.2 b | 0.031 ± 0.002 b,c | 102.3 ± 2.5 a | 99.8 ± 2.0 a,b | 0.99 |
| 60 min | 4.2 ± 0.3 a | 0.044 ± 0.003 a | 92.8 ± 2.1 a | 92.3 ± 2.0 b | 0.99 |
| Cooked lentil seeds (CL) | |||||
| 15 min | 1.8 ± 0.3 d | 0.024 ± 0.003 c | 75.4 ± 4.3 b | 71.3 ± 2.9 c | 0.99 |
| 30 min | 2.5 ± 0.2 c | 0.026 ± 0.001 c | 97.6 ± 2.1 a | 93.2 ± 1.4 b | 0.99 |
| 60 min | 4.3 ± 0.4 a | 0.042 ± 0.003 a,b | 103.5 ± 2.39 a | 102.8 ± 2.2 a | 0.99 |
| Cooking Time | vinitial (%/min) | k (/min) | Cf (%) | R2adj |
|---|---|---|---|---|
| Isolated cotyledon cells (ICC) | ||||
| 15 min | 0.798 ± 0.177 c | 0.027 ± 0.005 a | 34.32 ± 1.82 a,b | 0.99 |
| 30 min | 1.356 ± 0.186 a | 0.040 ± 0.003 a | 37.68 ± 0.74 a | 0.99 |
| 60 min | 1.083 ± 0.162 b | 0.041 ± 0.006 a | 30.15 ± 1.25 b | 0.99 |
| Cooked lentil seeds (CL) | ||||
| 15 min | 0.528 + 0.101 d | 0.022 ± 0.004 a | 29.74 ± 1.67 b | 0.99 |
| 30 min | 0.752 + 0.144 c | 0.025 ± 0.004 a | 30.21 ± 1.36 b | 0.99 |
| 60 min | 1.109 + 0.137 b | 0.033 ± 0.004 a | 38.31 ± 1.24 a | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duijsens, D.; Verkempinck, S.H.E.; De Coster, A.; Pälchen, K.; Hendrickx, M.; Grauwet, T. How Cooking Time Affects In Vitro Starch and Protein Digestibility of Whole Cooked Lentil Seeds versus Isolated Cotyledon Cells. Foods 2023, 12, 525. https://doi.org/10.3390/foods12030525
Duijsens D, Verkempinck SHE, De Coster A, Pälchen K, Hendrickx M, Grauwet T. How Cooking Time Affects In Vitro Starch and Protein Digestibility of Whole Cooked Lentil Seeds versus Isolated Cotyledon Cells. Foods. 2023; 12(3):525. https://doi.org/10.3390/foods12030525
Chicago/Turabian StyleDuijsens, Dorine, Sarah H. E. Verkempinck, Audrey De Coster, Katharina Pälchen, Marc Hendrickx, and Tara Grauwet. 2023. "How Cooking Time Affects In Vitro Starch and Protein Digestibility of Whole Cooked Lentil Seeds versus Isolated Cotyledon Cells" Foods 12, no. 3: 525. https://doi.org/10.3390/foods12030525
APA StyleDuijsens, D., Verkempinck, S. H. E., De Coster, A., Pälchen, K., Hendrickx, M., & Grauwet, T. (2023). How Cooking Time Affects In Vitro Starch and Protein Digestibility of Whole Cooked Lentil Seeds versus Isolated Cotyledon Cells. Foods, 12(3), 525. https://doi.org/10.3390/foods12030525