Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Materials and Methods
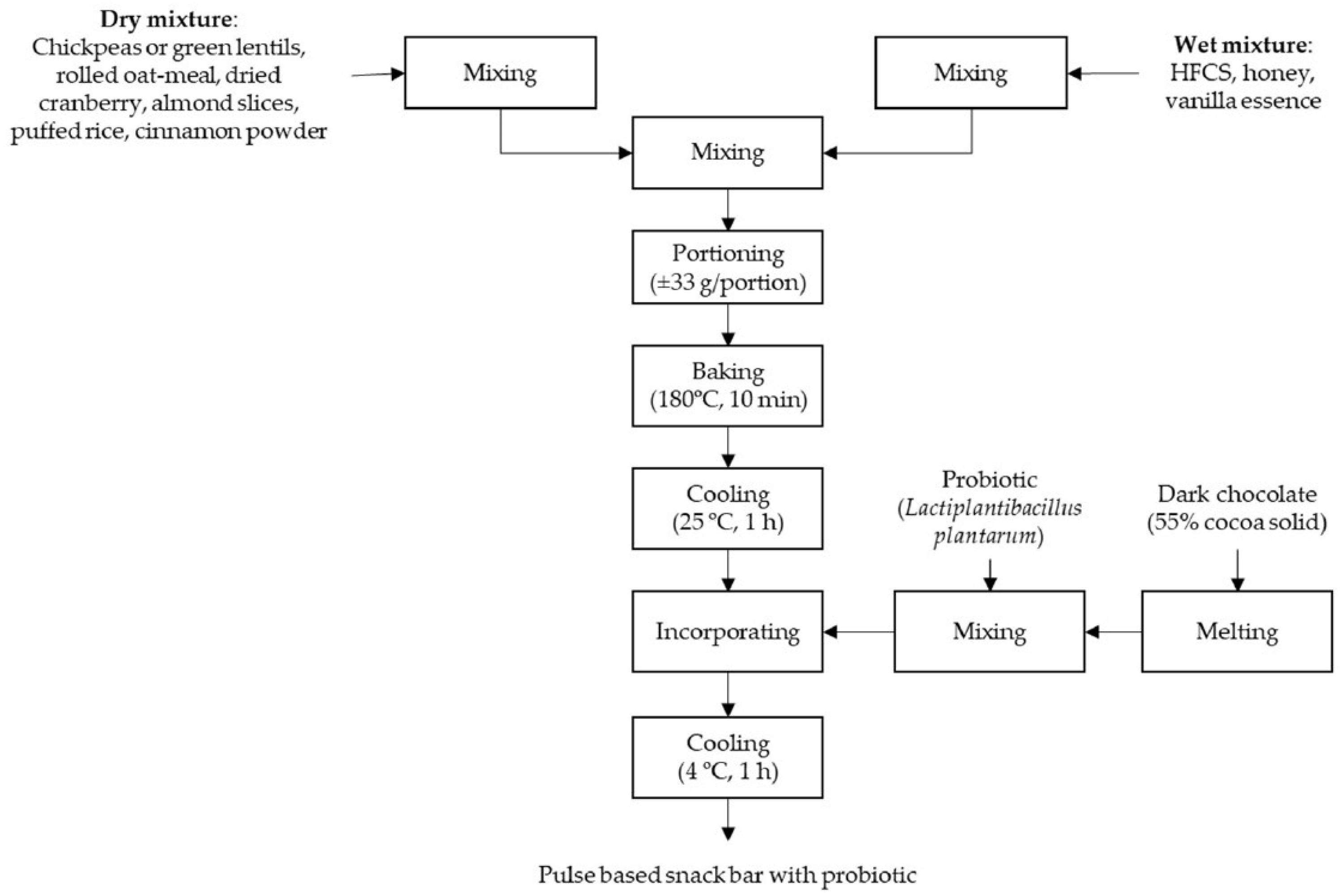
2.1. Preparation of Chickpea- and Green Lentil-Based Snack Bars with Probiotics
2.2. Preparation of Dried Chickpeas and Green Lentils
2.3. Preparation of Pulse-Based Snack Bars with Probiotics
2.4. Basic Composition Analysis
2.5. Total Phenolic Content Analysis
2.6. Antioxidant Activity Analysis
2.7. Evaluation of the Lactiplantibacillus plantarum Content in Samples
2.8. Sensory Analysis
2.8.1. Fresh Pulse Snack Bar Sensory and Hedonic Evaluations
2.8.2. Stored Pulse Snack Bar Hedonic Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Basic Composition Analysis
3.2. Total Phenolic Compound and Antioxidant Analysis
3.3. Evaluation of the Lactiplantibacillus plantarum Content in Samples
3.4. Sensory Analysis
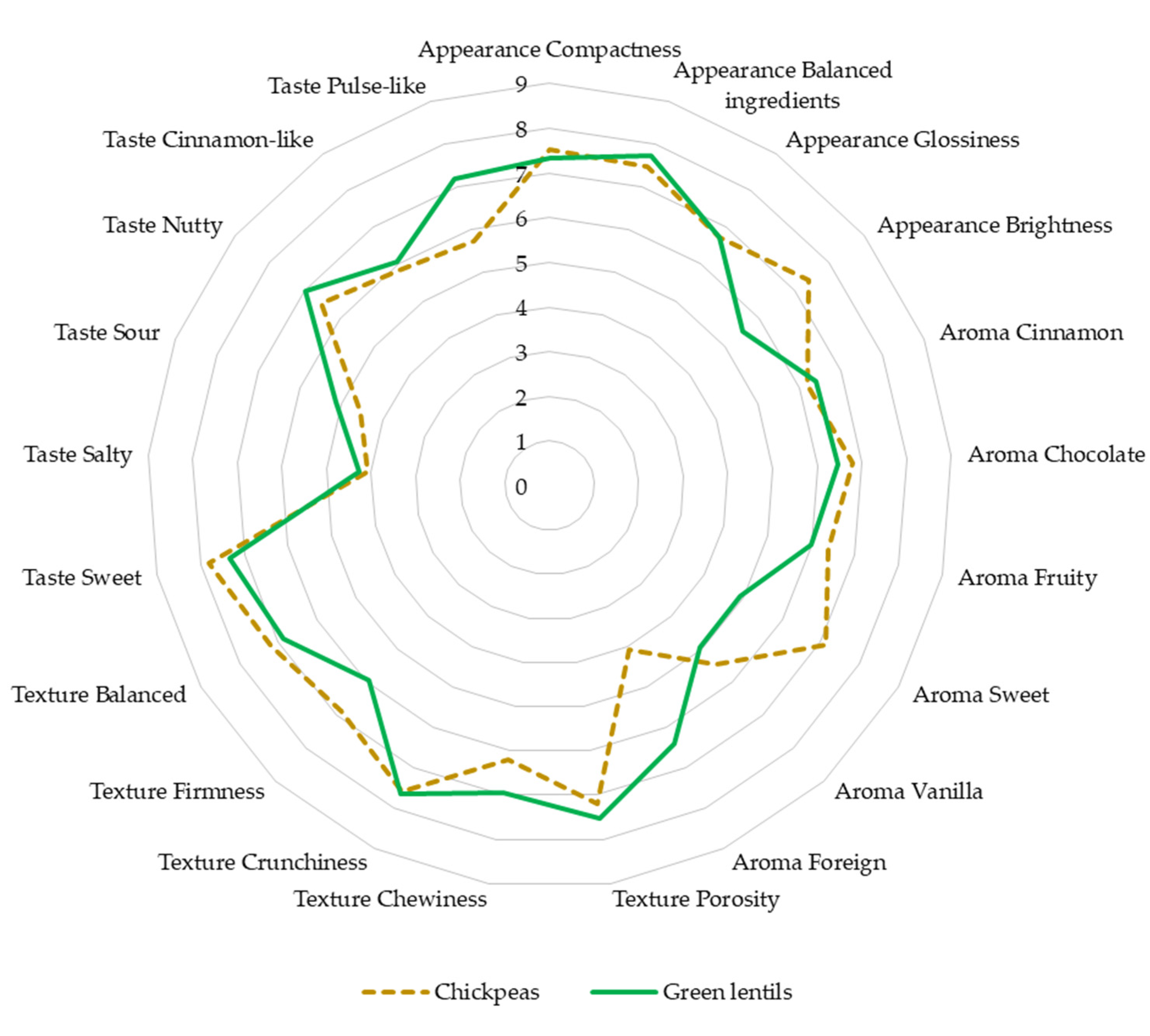
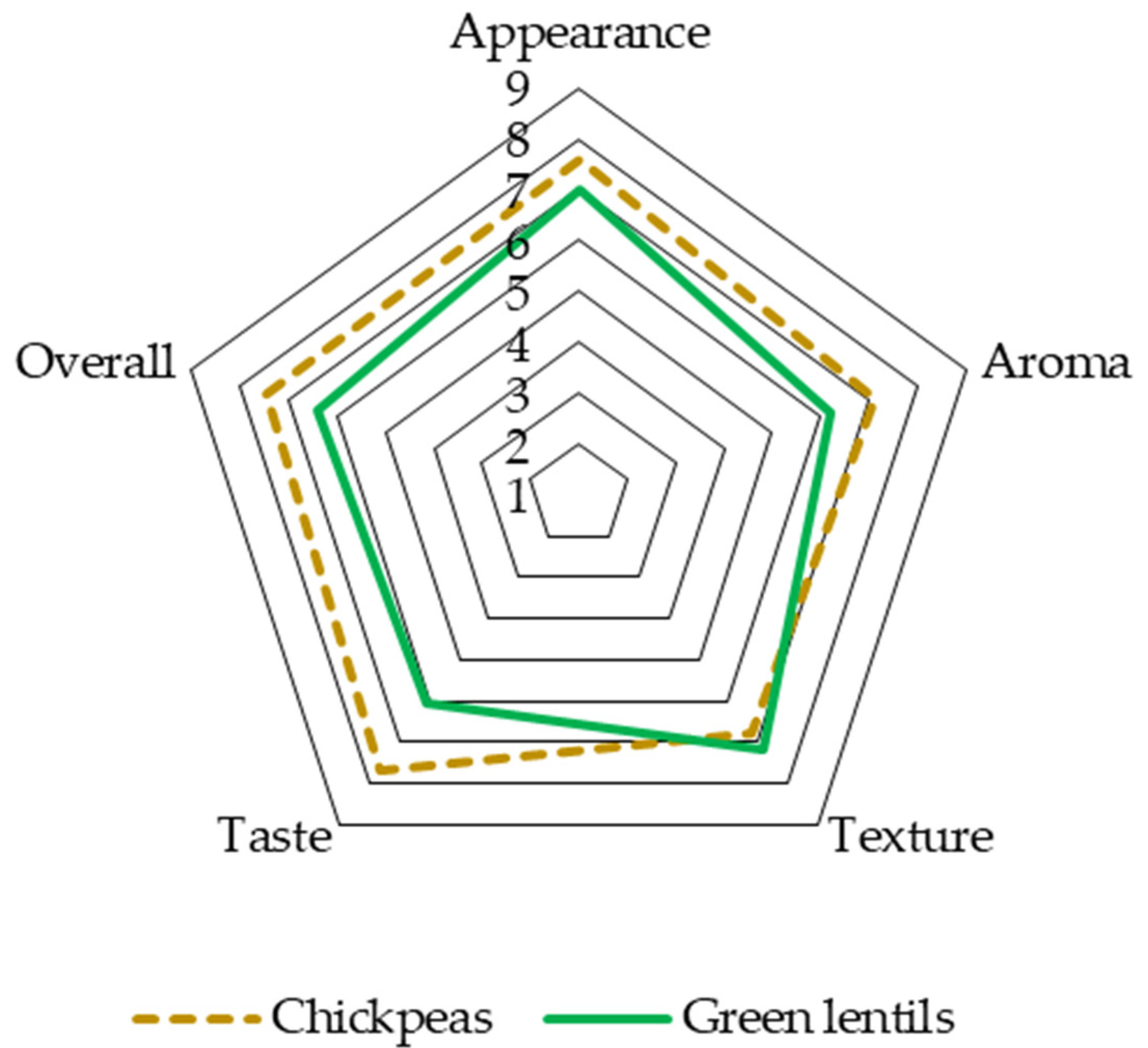
3.4.1. Sensory Analysis of Fresh Pulse Snack Bars
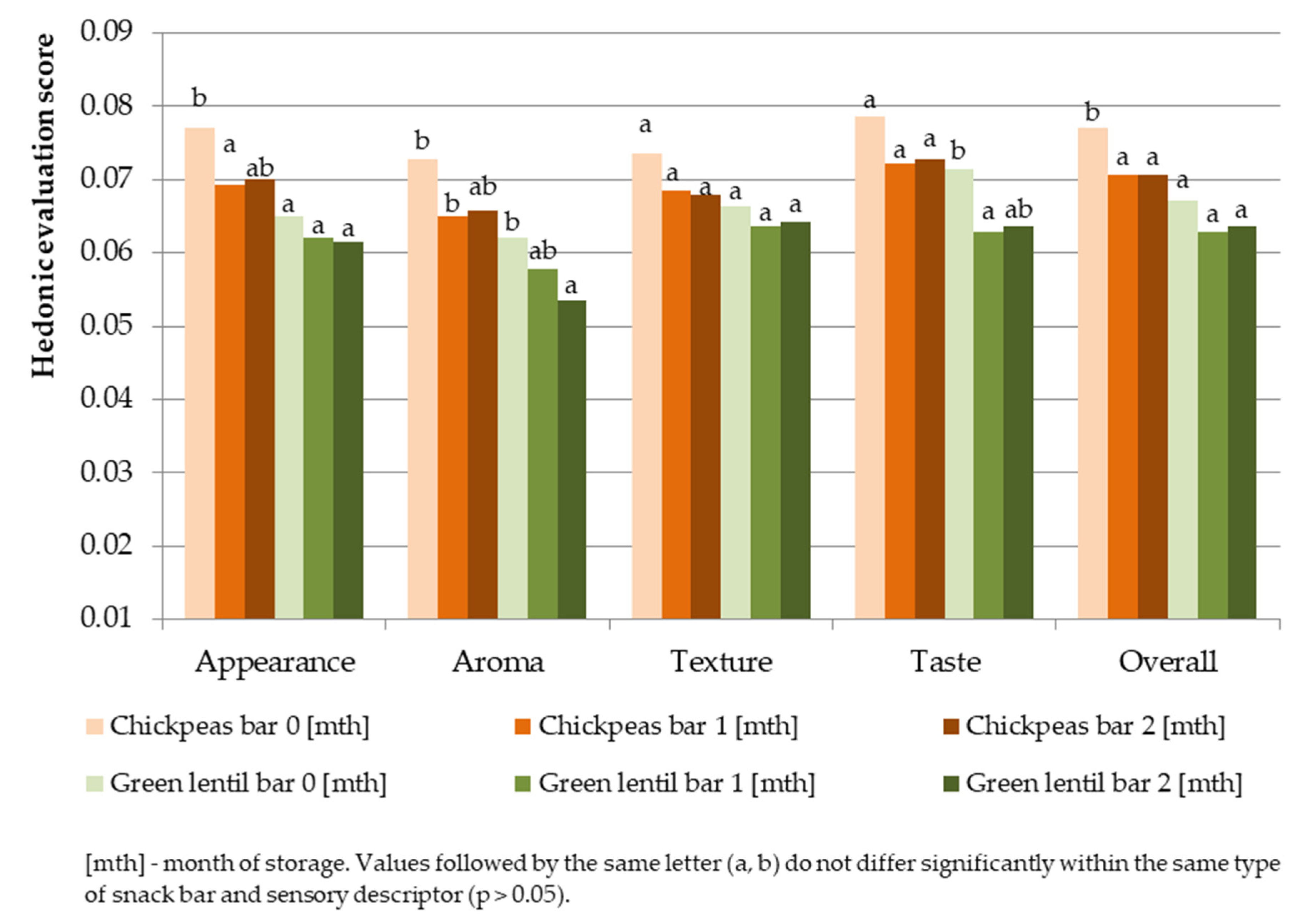
3.4.2. Hedonic Evaluation of Pulse Snack Bars during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Considine, M.J.; Siddique, K.H.M.; Foyer, C.H. Nature’s Pulse Power: Legumes, Food Security and Climate Change. J. Exp. Bot. 2017, 68, 1815–1818. [Google Scholar] [CrossRef] [PubMed]
- Rawal, V.; Navarro, D.K. The Global Economy of Pulses; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Crops: Chickpeas and Lentils. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 November 2021).
- Wallace, T.C.; Murray, R.; Zelman, K.M. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Becerra-Tomás, N.; Bulló, M.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E.; Arós, F.; Schroder, H.; Fitó, M.; et al. Legume Consumption and Risk of All-Cause, Cardiovascular, and Cancer Mortality in the PREDIMED Study. Clin. Nutr. 2019, 38, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Merga, B.; Haji, J. Economic Importance of Chickpea: Production, Value, and World Trade. Cogent Food Agric. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Arnold, M.; Gramza-Michałowska, A. Pulse Probiotic Superfood as Iron Status Improvement Agent in Active Women—A Review. Molecules 2021, 26, 2121. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, V.; Erkinbaev, C. Lentils. In Pulses; Manickavasagan, A., Thirunathan, P., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Erkan, S.B.; Gürler, H.N.; Bilgin, D.G.; Germec, M.; Turhan, I. Production and Characterization of Tempehs from Different Sources of Legume by Rhizopus oligosporus. LWT 2020, 119, 108880. [Google Scholar] [CrossRef]
- Shariati-Ievari, S.; Ryland, D.; Edel, A.; Nicholson, T.; Suh, M.; Aliani, M. Sensory and Physicochemical Studies of Thermally Micronized Chickpea (Cicer arietinum) and Green Lentil (Lens culinaris) Flours as Binders in Low-Fat Beef Burgers. J. Food Sci. 2016, 81, S1230–S1242. [Google Scholar] [CrossRef]
- Aylangan, A.; Ic, E.; Ozyardimci, B. Investigation of Gamma Irradiation and Storage Period Effects on the Nutritional and Sensory Quality of Chickpeas, Kidney Beans and Green Lentils. Food Control 2017, 80, 428–434. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural Antioxidants of Plant Origin, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- de Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; De La Rosa, L.A.; Geraldi, M.V.; Maróstica, M.R.; Shahidi, F.; Schwember, A.R. Is Chickpea a Potential Substitute for Soybean? Phenolic Bioactives and Potential Health Benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Hermenean, A. Natural Antioxidants in Anemia Treatment. Int. J. Mol. Sci. 2021, 22, 1883. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Robredo, S.; Troszyńska, A.; Kosińska, A.; Pegg, R.B. Free Radical-Scavenging Capacity, Antioxidant Activity, and Phenolic Composition of Green Lentil (Lens culinaris). Food Chem. 2010, 121, 705–711. [Google Scholar] [CrossRef]
- Ahmmed, T.; Rahman, A.; Salma, U.; Akter, Z.; Ansary, M.M.U.; Khalil, M.I.; Karim, N.; Bari, L. Nutritional, Phytochemicals and Antioxidant Properties of Some Popular Pulse Varieties of Bangladesh. J. Agric. Chem. Environ. 2020, 9, 343–368. [Google Scholar] [CrossRef]
- Arruda, H.S.; Neri-Numa, I.A.; Kido, L.A.; Maróstica Júnior, M.R.; Pastore, G.M. Recent Advances and Possibilities for the Use of Plant Phenolic Compounds to Manage Ageing-Related Diseases. J. Funct. Foods 2020, 75, 104203. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the Human Gut Microbiota by Phenolics and Phenolic Fiber-Rich Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef]
- Abdelhaleim, A.F.; Amer, A.Y.; Soliman, J.S.A. Association of Zinc Deficiency with Iron Deficiency Anemia and Its Symptoms: Results from a Case-Control Study. Cureus 2019, 11, e3811. [Google Scholar] [CrossRef]
- World Health Organizatoin (WHO). Global Nutrition Targets 2025: Anaemia Policy Brief; WHO/NMH/NHD/14.4; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Szczebyło, A.; Rejman, K.; Halicka, E.; Laskowski, W. Towards More Sustainable Diets—Attitudes, Opportunities and Barriers to Fostering Pulse Consumption in Polish Cities. Nutrients 2020, 12, 1589. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2013, 52, 676–684. [Google Scholar] [CrossRef]
- Alaunyte, I.; Stojceska, V.; Plunkett, A. Iron and the Female Athlete: A Review of Dietary Treatment Methods for Improving Iron Status and Exercise Performance. J. Int. Soc. Sports Nutr. 2015, 12, 38. [Google Scholar] [CrossRef]
- Sumengen, M.; Dincer, S.; Kaya, A. Production and Characterization of Phytase from Lactobacillus plantarum. Food Biotechnol. 2013, 27, 105–118. [Google Scholar] [CrossRef]
- Skrypnik, K.; Bogdański, P.; Schmidt, M.; Suliburska, J. The Effect of Multispecies Probiotic Supplementation on Iron Status in Rats. Biol. Trace Elem. Res. 2019, 192, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Priyodip, P.; Prakash, P.Y.; Balaji, S. Phytases of Probiotic Bacteria: Characteristics and Beneficial Aspects. Indian J. Microbiol. 2017, 57, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Tymczyszyn, E.; Mobili, P.; Gomez-Zavaglia, A. Prebiotics as protectants of lactic acid bacteria. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Academic Press: London, UK, 2015; pp. 155–164. [Google Scholar]
- Arnold, M.; Rajagukguk, Y.V.; Gramza-Michałowska, A. Characterization of Dadih: Traditional Fermented Buffalo Milk of Minangkabau. Beverages 2021, 7, 60. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Arnold, M. Tempoyak: Fermented Durian Paste of Malay Ethnic and Its Functional Properties. Int. J. Gastron. Food Sci. 2021, 23, 100297. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Medina, E.; Sánchez, B.; Benítez-Cabello, A.; Arroyo-López, F.N. Role of Lactic Acid Bacteria in Fermented Vegetables. Grasas Aceites 2020, 71, e358. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ahmad, A.; Ateeq, H.; Tufail, T.; Hussain, M. Survival and Storage Stability of Encapsulated Probiotic under Simulated Digestion Conditions and on Dried Apple Snacks. Food Sci. Nutr. 2020, 8, 5392–5401. [Google Scholar] [CrossRef]
- Bampi, G.B.; Backes, G.T.; Cansian, R.L.; de Matos, F.E.; Ansolin, I.M.A.; Poleto, B.C.; Corezzolla, L.R.; Favaro-Trindade, C.S. Spray Chilling Microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis Subsp. Lactis and Its Use in the Preparation of Savory Probiotic Cereal Bars. Food Bioprocess Technol. 2016, 9, 1422–1428. [Google Scholar] [CrossRef]
- Lalicic-Petronijevic, J.; Popov-Raljić, J.; Obradović, D.; Radulović, Z.; Paunović, D.; Petrušić, M.; Pezo, L. Viability of Probiotic Strains Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019 and Their Impact on Sensory and Rheological Properties of Milk and Dark Chocolates during Storage for 180 Days. J. Funct. Foods 2015, 15, 541–550. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Złotek, U.; Kapusta, I.; Kordowska-Wiater, M.; Baraniak, B. Effect of Cold Storage on the Potentially Bioaccessible Isoflavones and Antioxidant Activities of Soybean Sprouts Enriched with Lactobacillus plantarum 299v. LWT 2020, 118, 108820. [Google Scholar] [CrossRef]
- PN-EN ISO 3947. Starches, Native or Modified—Determination of Total Fat Content; International Organization for Standardization (ISO): Geneva, Switzerland, 2001. [Google Scholar]
- Fujihara, S.; Sasaki, H.; Aoyagi, Y.; Sugahara, T. Nitrogen-to-Protein Conversion Factors for Some Cereal Products in Japan. J. Food Sci. 2008, 73, C204–C209. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis: Official Method for Protein; Method No. 920.87; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative Potential, Nutritional Value and Sensory Profiles of Confectionery Fortified with Green and Yellow Tea Leaves (Camellia Sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef]
- EN14084:2003. Foodstuffs—Determination of Trace Elements—Determination of Lead, Cadmium, Zinc, Copper and Iron by Atomic Absorption Spectrometry (AAS) after Microwave Digestion; Polish Committee for Standardization: Warsaw, Poland, 2003. [Google Scholar]
- European Union. Regulation (EU) No. 1169/2011 of the European Parliament and of the Council of 25 October 2011. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Shahidi, F.; Naczk, M. Methods of analysis and quantification of phenolic compounds. In Food Phenolic: Sources, Chemistry, Effects and Applications; Technomic Publishing Company: Lancaster, PA, USA, 1995; pp. 287–293. [Google Scholar]
- Jan, U.; Gani, A.; Ahmad, M.; Shah, U.; Baba, W.N.; Masoodi, F.A.; Maqsood, S.; Gani, A.; Wani, I.A.; Wani, S.M. Characterization of Cookies Made from Wheat Flour Blended with Buckwheat Flour and Effect on Antioxidant Properties. J. Food Sci. Technol. 2015, 52, 6334–6344. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Korczak, J. Oxygen Radical Absorbance Capacity of Selected Food Products. Acta Sci. Pol. Technol. Aliment. 2013, 12, 175–180. [Google Scholar]
- Gramza-Michalowska, A.; Sidor, A.; Regula, J.; Kulczynski, B. PCL Assay Application in Superoxide Anion-Radical Scavenging Capacity of Tea Camellia Sinensis Extracts. Acta Sci. Pol. Technol. Aliment. 2015, 14, 331–341. [Google Scholar] [CrossRef]
- Bugaud, C.; Maraval, I.; Daribo, M.O.; Leclerc, N.; Salmon, F. Optimal and Acceptable Levels of Sweetness, Sourness, Firmness, Mealiness and Banana Aroma in Dessert Banana (Musa sp.). Sci. Hortic. 2016, 211, 399–409. [Google Scholar] [CrossRef]
- Patil, S.S.; Brennan, M.A.; Mason, S.L.; Brennan, C.S. The Effects of Fortification of Legumes and Extrusion on the Protein Digestibility of Wheat Based Snack. Foods 2016, 5, 26. [Google Scholar] [CrossRef]
- Chillo, S.; Monro, J.A.; Mishra, S.; Henry, C.J. Effect of Incorporating Legume Flour into Semolina Spaghetti on Its Cooking Quality and Glycaemic Impact Measured in vitro. Int. J. Food Sci. Nutr. 2010, 61, 149–160. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The Impact of Germination and Dehulling on Nutrients, Antinutrients, in vitro Iron and Calcium Bioavailability and in vitro Starch and Protein Digestibility of Some Legume Seeds. LWT Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Curtain, F.; Grafenauer, S. Comprehensive Nutrition Review of Grain-Based Muesli Bars in Australia: An Audit of Supermarket Products. Foods 2019, 8, 370. [Google Scholar] [CrossRef]
- Soni, D.; Saxena, G. Standardization and Development of Nutritious Snack Bar for Varied Age Groups. Res. Rev. J. Food Sci. Technol. 2018, 7, 22–28. [Google Scholar]
- Hall, C.; Hillen, C.; Robinson, J. Composition, Nutritional Value and Health Benefits of Pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Perera, D.R.G.; Gunawardana, D.; Jayatissa, R.; Silva, A.B.G. Iron Content of Some Popular Cooked Foods Consumed by the Rural School Children in Sri Lanka. J. Food Qual. 2019, 2019, 6972745. [Google Scholar] [CrossRef]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Grewal, S.K.; Sharma, K.P.; Bharadwaj, R.D.; Hegde, V.; Tripathi, S.; Singh, S.; Kumar Jain, P.; Kumar Agrawal, P.; Mondal, B. Understanding Genotypic Variation and Identification of Promising Genotypes for Iron and Zinc Content in Chickpea (Cicer arietinum L.). J. Food Compos. Anal. 2020, 88, 103458. [Google Scholar] [CrossRef]
- Sozer, N.; Holopaien-Mantila, U.; Poutanen, K. Traditional and New Food Uses of Pulses. Cereal Chem. 2017, 94, 66–73. [Google Scholar] [CrossRef]
- Kassouf, A.; Chebib, H.; Lebbos, N.; Ouaini, R. Migration of Iron, Lead, Cadmium and Tin from Tinplate-Coated Cans into Chickpeas. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 1987–1992. [Google Scholar] [CrossRef]
- Jha, A.B.; Warkentin, T.D. Biofortification of Pulse Crops: Status and Future Perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for Iron. EFSA J. 2015, 13, 4254. [Google Scholar] [CrossRef]
- Adelpilerood, S.; Prakash, J. Ionizability and Bioaccessibility of Externally Added Iron in Legumes and Their Water Soluble Protein Fractions. Int. J. Food Prop. 2013, 16, 1543–1551. [Google Scholar] [CrossRef][Green Version]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic Strain Lactobacillus plantarum 299v Increases Iron Absorption from an Iron-Supplemented Fruit Drink: A Double-Isotope Cross-over Single-Blind Study in Women of Reproductive Age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas and Chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Dai, F.J.; Chau, C.F. Classification and Regulatory Perspectives of Dietary Fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef]
- Ramulu, P.; Udayasekhara Rao, P. Effect of Proccesing on Dietary Fiber Content of Cereals and Pulses. Plant Foods Hum. Nutr. 1997, 50, 249–257. [Google Scholar] [CrossRef]
- Zhang, F. Iron Absorption and Regulatory Mechanisms: Effects of Fructooligosaccharide and Other Prebiotics. Ph.D. Thesis, Hong Kong Baptist University, Hong Kong, China, 2017. [Google Scholar]
- Kaur, R.; Prasad, K. Technological, Processing and Nutritional Aspects of Chickpea (Cicer arietinum)—A Review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Arnold, M.; Rajagukguk, Y.V.; Gramza-Michałowska, A. Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review. Foods 2021, 10, 656. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Rattan, B.; Singh, J.P.; Kaur, A.; Singh, B. Effect of Chickpea and Spinach on Extrusion Behavior of Corn Grit. J. Food Sci. Technol. 2019, 56, 2257–2266. [Google Scholar] [CrossRef]
- Ladjal Ettoumi, Y.; Chibane, M. Some Physicochemical and Functional Properties of Pea, Chickpea and Lentil Whole Flours. Int. Food Res. J. 2015, 22, 987–996. [Google Scholar]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Marathe, S.A.; Rajalakshmi, V.; Jamdar, S.N.; Sharma, A. Comparative Study on Antioxidant Activity of Different Varieties of Commonly Consumed Legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. Functional Extruded Products from Rice Flour, Pulse Flour and Tomato Powder Blends. Master’s Thesis, McGill University, Montreal, QC, Canada, 2017. [Google Scholar]
- Zhong, Y.; Shahidi, F. Methods for the Assessment of Antioxidant Activity in Foods; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Zou, Y.; Chang, S.K.C.; Gu, Y.; Qian, S.Y. Antioxidant Activity and Phenolic Compositions of Lentil (Lens culinaris var. Morton) Extract and Its Fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Bragança, G.C.M.; Ziegler, V.; Ávila, B.P.; Monks, J.L.F.; Peres, W.; Elias, M.C. Multivariate Analysis of the Conditions of Temperature, Moisture and Storage Time in the Technological, Chemical, Nutritional Parameters and Phytochemical of Green Lentils. J. Stored Prod. Res. 2020, 87, 101617. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Changes in the Content and Composition of Anthocyanins in Red Cabbage and Its Antioxidant Capacity during Fermentation, Storage and Stewing. Food Chem. 2015, 167, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, L.M.; Huerta-Ocampo, J.Á.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-García, M.A.; González-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G.; et al. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum l. Cv Blanoro) Stored under N2 and Co2 Atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Hegedus, A.; Stefanovits-Bányai, É. Application of and Correlation among Antioxidant and Antiradical Assays for Characterizing Antioxidant Capacity of Berries. Sci. Hortic. 2010, 125, 332–336. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the Antioxidant Capacity of Chickpeas by Solid State Fermentation with Cordyceps Militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Sánchez-Magaña, L.M.; Cuevas-Rodríguez, E.O.; Gutiérrez-Dorado, R.; Ayala-Rodríguez, A.E.; Valdez-Ortiz, A.; Milán-Carrillo, J.; Reyes-Moreno, C. Solid-State Bioconversion of Chickpea (Cicer arietinum L.) by Rhizopus oligosporus to Improve Total Phenolic Content, Antioxidant Activity and Hypoglycemic Functionality. Int. J. Food Sci. Nutr. 2014, 65, 558–564. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Phenolic Substance Characterization and Chemical and Cell-Based Antioxidant Activities of 11 Lentils Grown in the Northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef]
- Menga, V.; Codianni, P.; Fares, C. Agronomic Management under Organic Farming May Affect the Bioactive Compounds of Lentil (Lens culinaris L.) and Grass Pea (Lathyrus Communis L.)? Sustainability 2014, 6, 1059–1075. [Google Scholar] [CrossRef]
- Singh, N. Pulses: An Overview. J. Food Sci. Technol. 2017, 54, 853–857. [Google Scholar] [CrossRef]
- Heiras-Palazuelos, M.J.; Ochoa-Lugo, M.I.; Gutiérrez-Dorado, R.; López-Valenzuela, J.A.; Mora-Rochín, S.; Milán-Carrillo, J.; Garzón-Tiznado, J.A.; Reyes-Moreno, C. Technological Properties, Antioxidant Activity and Total Phenolic and Flavonoid Content of Pigmented Chickpea (Cicer arietinum L.) Cultivars. Int. J. Food Sci. Nutr. 2013, 64, 69–76. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty Acid, Carotenoid and Tocopherol Compositions of 20 Canadian Lentil Cultivars and Synergistic Contribution to Antioxidant Activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef]
- Agil, R.; Gaget, A.; Gliwa, J.; Avis, T.J.; Willmore, W.G.; Hosseinian, F. Lentils Enhance Probiotic Growth in Yogurt and Provide Added Benefit of Antioxidant Protection. LWT Food Sci. Technol. 2013, 50, 45–49. [Google Scholar] [CrossRef]
- García-Casal, M.N.; Leets, I. Carotenoids, but Not Vitamin A, Improve Iron Uptake and Ferritin Synthesis by Caco-2 Cells from Ferrous Fumarate and NaFe-EDTA. J. Food Sci. 2014, 79, H706–H712. [Google Scholar] [CrossRef]
- Ahmad, S. Functional Food Products and Sustainable Health; Springer: Berlin, Germany, 2020. [Google Scholar]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive Compounds, Antioxidant Activity, and Biological Effects of European Cranberry (Vaccinium Oxycoccos). Molecules 2019, 24, 24. [Google Scholar] [CrossRef]
- Escuredo, O.; Seijo, M.C.; Salvador, J.; González-Martín, M.I. Near Infrared Spectroscopy for Prediction of Antioxidant Compounds in the Honey. Food Chem. 2013, 141, 3409–3414. [Google Scholar] [CrossRef]
- Salvador, I.; Massarioli, A.P.; Silva, A.P.S.; Malaguetta, H.; Melo, P.S.; Alencar, S.M. Can We Conserve Trans-Resveratrol Content and Antioxidant Activity during Industrial Production of Chocolate? J. Sci. Food Agric. 2019, 99, 83–89. [Google Scholar] [CrossRef]
- Hitayezu, R.; Baakdah, M.M.; Kinnin, J.; Henderson, K.; Tsopmo, A. Antioxidant Activity, Avenanthramide and Phenolic Acid Contents of Oat Milling Fractions. J. Cereal Sci. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Klu, Y.A.K.; Williams, J.H.; Phillips, R.D.; Chen, J. Survival of Lactobacillus Rhamnosus GG as Influenced by Storage Conditions and Product Matrixes. J. Food Sci. 2012, 77, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C. A Review of Dose-Responses of Probiotics in Human Studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mirali, M.; Purves, R.W.; Vandenberg, A. Phenolic Profiling of Green Lentil (Lens culinaris Medic.) Seeds Subjected to Long-Term Storage. Eur. Food Res. Technol. 2016, 242, 2161–2170. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Manthey, F.A.; Chang, S.K.C.; Hou, H.J.; Yuan, S.H. Quality Characteristics of Spaghetti as Affected by Green and Yellow Pea, Lentil, and Chickpea Flours. J. Food Sci. 2005, 70, s371–s376. [Google Scholar] [CrossRef]
- Hellwig, M.; Kühn, L.; Henle, T. Individual Maillard Reaction Products as Indicators of Heat Treatment of Pasta—A Survey of Commercial Products. J. Food Compos. Anal. 2018, 72, 83–92. [Google Scholar] [CrossRef]
- Gan, Y.T.; Miller, P.R.; McDonald, C.L. Response of Kabuli Chickpea to Seed Size and Planting Depth. Can. J. Plant Sci. 2003, 83, 39–46. [Google Scholar] [CrossRef]
- Firatligil-Durmuş, E.; Šárka, E.; Bubník, Z. Image Vision Technology for the Characterisation of Shape and Geometrical Properties of Two Varieties of Lentil Grown in Turkey. Czech J. Food Sci. 2008, 26, 109–116. [Google Scholar] [CrossRef]
- Carrizo, D.; Taborda, G.; Nerín, C.; Bosetti, O. Extension of Shelf Life of Two Fatty Foods Using a New Antioxidant Multilayer Packaging Containing Green Tea Extract. Innov. Food Sci. Emerg. Technol. 2016, 33, 534–541. [Google Scholar] [CrossRef]
- Guraya, H.S.; Patindol, J.A. Storage Stability of Flour-Blasted Brown Rice. Cereal Chem. 2011, 88, 56–63. [Google Scholar] [CrossRef]


| Ingredients | Weight (g) |
|---|---|
| Chickpeas or green lentils | 85.0 |
| Rolled oat meal | 74.0 |
| High-Fructose Corn Syrup (HFCS) | 53.0 |
| Dried cranberry | 38.0 |
| Almond slices | 34.0 |
| Honey | 30.0 |
| Puffed rice | 27.0 |
| Vanilla essence | 3.0 |
| Cinnamon powder | 1.0 |
| Mixture Added After Baking | |
| Dark chocolate (55% cocoa solid) | 60.0 |
| Probiotic (Lactiplantibacillus plantarum) | 0.15 |
| Notes: 12 portions (±33 g per serving) | |
| Nutrient | Chickpea-Based Snack Bar | Green Lentil-Based Snack Bar | |
|---|---|---|---|
| Protein | [g/100 g dry basis] | 4.49 ± 0.14 a | 5.19 ± 0.32 b |
| Lipid | 18.37 ± 1.32 b | 15.45 ± 1.16 a | |
| Carbohydrates | 44.35 ± 1.53 a | 49.19 ± 1.74 a | |
| Ash | 1.91 ± 0.07 b | 1.54 ± 0.04 a | |
| Moisture | 4.33 ± 0.10 a | 6.00 ± 0.33 b | |
| Energy value | [kcal/100 g dry basis] | 414.56 | 401.12 |
| Minerals | |||
| Fe | [mg/100 g dry basis] | 3.08 ± 0.07 b | 2.31 ± 0.04 a |
| Dietary Fibre Content | Chickpea-Based Snack Bar | Green Lentil-Based Snack Bar | |
|---|---|---|---|
| NDF | [g/100 g dry basis] | 9.58 ± 0.09 a | 16.06 ± 0.76 b |
| ADF | 6.06 ± 0.17 a | 7.09 ± 0.39 b | |
| ADL | 2.75 ± 0.49 a | 3.36 ± 0.49 a | |
| ADC | 3.31 ± 0.34 a | 3.73 ± 0.45 a | |
| Hemicellulose | 3.53 ± 0.13 a | 8.97 ± 0.99 b | |
| SDF | 12.48 ± 0.55 a | 12.83 ± 0.56 a | |
| IDF | 11.78 ± 0.35 b | 7.24 ± 0.64 a | |
| TDF | 26.92 ± 0.40 b | 22.27 ± 1.20 a |
| Assay | Storage Time (Month) | Chickpea-Based Snack Bar | Green Lentil-Based Snack Bar | ||
|---|---|---|---|---|---|
| [mg GAE/100 g] | 0 | 293.16 ± 4.05 cA | 305.90 ± 3.02 bB | ||
| TPC | 1 | 210.01 ± 1.63 bA | 277.20 ± 5.59 aB | ||
| 2 | 179.89 ± 2.32 aA | 280.52 ± 0.88 aB | |||
| [mg TE/100 g] | 0 | 577.98 ± 8.28 cB | 512.75 ± 35.51 aA | ||
| ABTS | 1 | 413.21 ± 8.19 bA | 483.57 ± 21.74 aB | ||
| 2 | 306.61 ± 24.14 aA | 446.54 ± 37.26 aB | |||
| 0 | 393.74 ± 4.45 cA | 434.65 ± 3.11 cB | |||
| DPPH | 1 | 277.40 ± 4.75 bA | 401.94 ± 1.55 bB | ||
| 2 | 236.69 ± 2.37 aA | 387.78 ± 5.33 aB | |||
| 0 | 2493.29 ± 64.46 cB | 2287.67 ± 61.19 bA | |||
| ORAC | 1 | 1836.66 ± 40.12 bA | 2080.14 ± 80.11 aB | ||
| 2 | 1425.79 ± 24.99 aA | 2109.63 ± 16.33 abB | |||
| PCL | 0 | 241.58 ± 12.01 cA | 231.39 ± 17.18 bA | ||
| ACW | 1 | 161.43 ± 5.52 bB | 146.46 ± 6.96 aA | ||
| 2 | 107.22 ± 5.40 aA | 151.83 ± 1.83 aB | |||
| 0 | 318.12 ± 5.73 cB | 295.84 ± 1.96 bA | |||
| ACL | 1 | 223.33 ± 10.13 bA | 251.57 ± 4.26 aA | ||
| 2 | 198.00 ± 3.65 aA | 248.31 ± 16.86 aB | |||
| 0 | 559.70 ± 12.70 cA | 527.23 ± 77.06 bA | |||
| IAC | 1 | 384.76 ± 5.43 bA | 398.03 ± 8.10 aA | ||
| 2 | 305.22 ± 8.07 aA | 399.70 ± 16.50 aB | |||
| Sample | TPC with - | Pearson’s Correlation | Sig. 2-Tailed |
|---|---|---|---|
| Chickpea-based snack bar | ABTS | 0.986 | p < 0.01 |
| DPPH | 0.996 | p < 0.01 | |
| ORAC | 0.988 | p < 0.01 | |
| PCL-ACW | 0.982 | p < 0.01 | |
| PCL-ACL | 0.992 | p < 0.01 | |
| PCL-IAC | 0.997 | p < 0.01 | |
| Green lentil-based snack bar | ABTS | 0.615 | p < 0.05 |
| DPPH | 0.884 | p < 0.01 | |
| ORAC | 0.881 | p < 0.01 | |
| PCL-ACW | 0.953 | p < 0.01 | |
| PCL-ACL | 0.859 | p < 0.01 | |
| PCL-IAC | 0.941 | p < 0.01 |
| Storage Time (Month) | Chickpeas | Green Lentils | Lactiplantibacillus plantarum Capsule | |||
|---|---|---|---|---|---|---|
| cfu/g | log | cfu/g | log | cfu/Capsule | log | |
| 0 | 3.04 × 106 | 6.48 | 1.61 × 106 | 6.21 | 2.28 × 106 | 6.36 |
| 1 | 7.12 × 105 | 5.85 | 6.27 × 105 | 5.80 | ||
| 2 | 5.62 × 105 | 5.75 | 1.17 × 105 | 5.07 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajagukguk, Y.V.; Arnold, M.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Schmidt, M.; Gramza-Michałowska, A. Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus plantarum. Foods 2022, 11, 309. https://doi.org/10.3390/foods11030309
Rajagukguk YV, Arnold M, Sidor A, Kulczyński B, Brzozowska A, Schmidt M, Gramza-Michałowska A. Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus plantarum. Foods. 2022; 11(3):309. https://doi.org/10.3390/foods11030309
Chicago/Turabian StyleRajagukguk, Yolanda Victoria, Marcellus Arnold, Andrzej Sidor, Bartosz Kulczyński, Anna Brzozowska, Marcin Schmidt, and Anna Gramza-Michałowska. 2022. "Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus plantarum" Foods 11, no. 3: 309. https://doi.org/10.3390/foods11030309
APA StyleRajagukguk, Y. V., Arnold, M., Sidor, A., Kulczyński, B., Brzozowska, A., Schmidt, M., & Gramza-Michałowska, A. (2022). Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus plantarum. Foods, 11(3), 309. https://doi.org/10.3390/foods11030309











