Sustainable Extractions for Maximizing Content of Antioxidant Phytochemicals from Black and Red Currants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Reagents
2.3. Extraction Techniques
2.3.1. Solid–Liquid Extraction
2.3.2. Pressurized-Liquid Extraction (PLE)
2.3.3. Microwave-Assisted Extraction
2.3.4. Ultrasound-Assisted Extraction
2.4. Experimental Design
2.5. Extraction Yield
2.6. Analyses of Bioactive Compounds
2.7. Analyses of Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
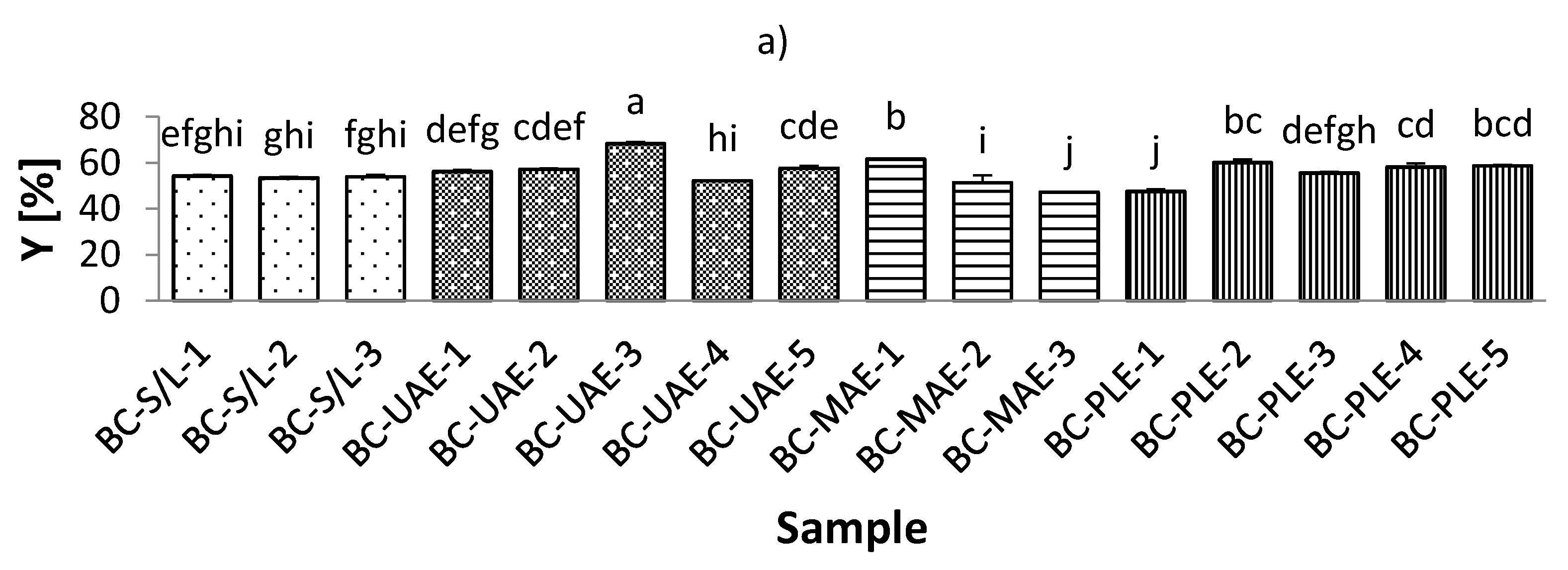
3.1. Extraction Yield
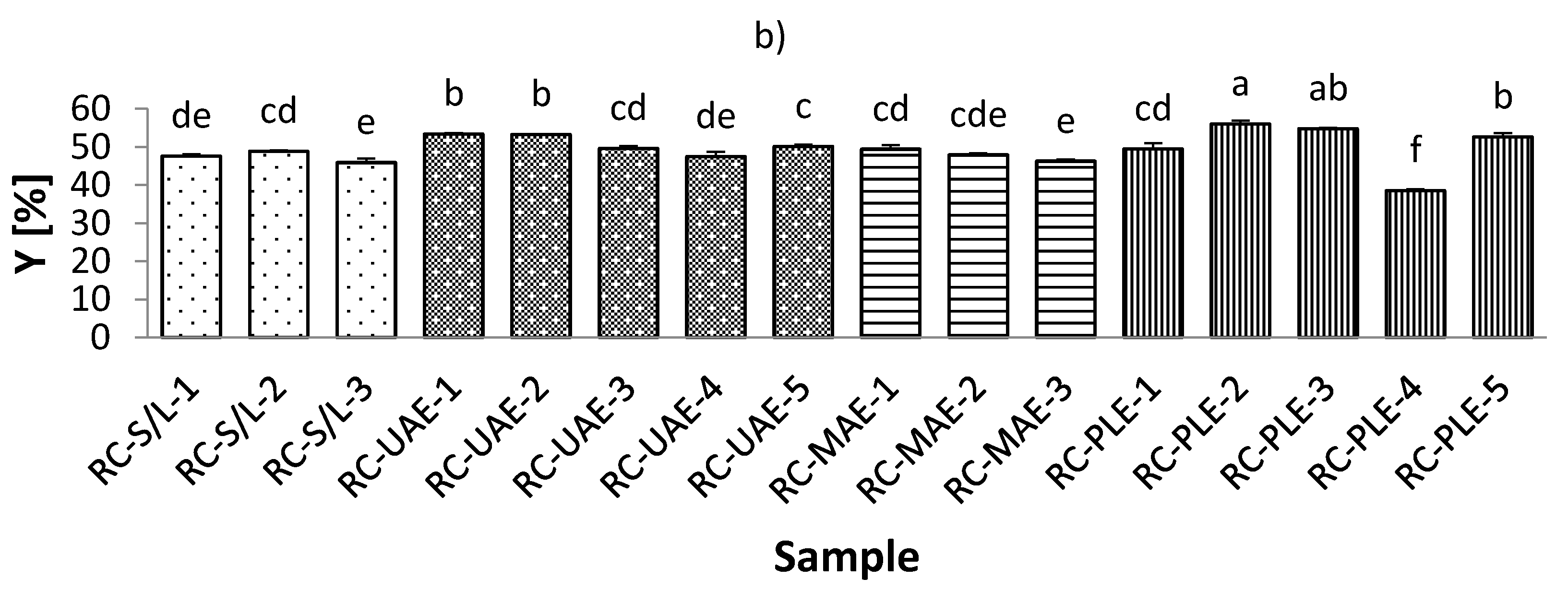
3.2. Total Phenolic Content
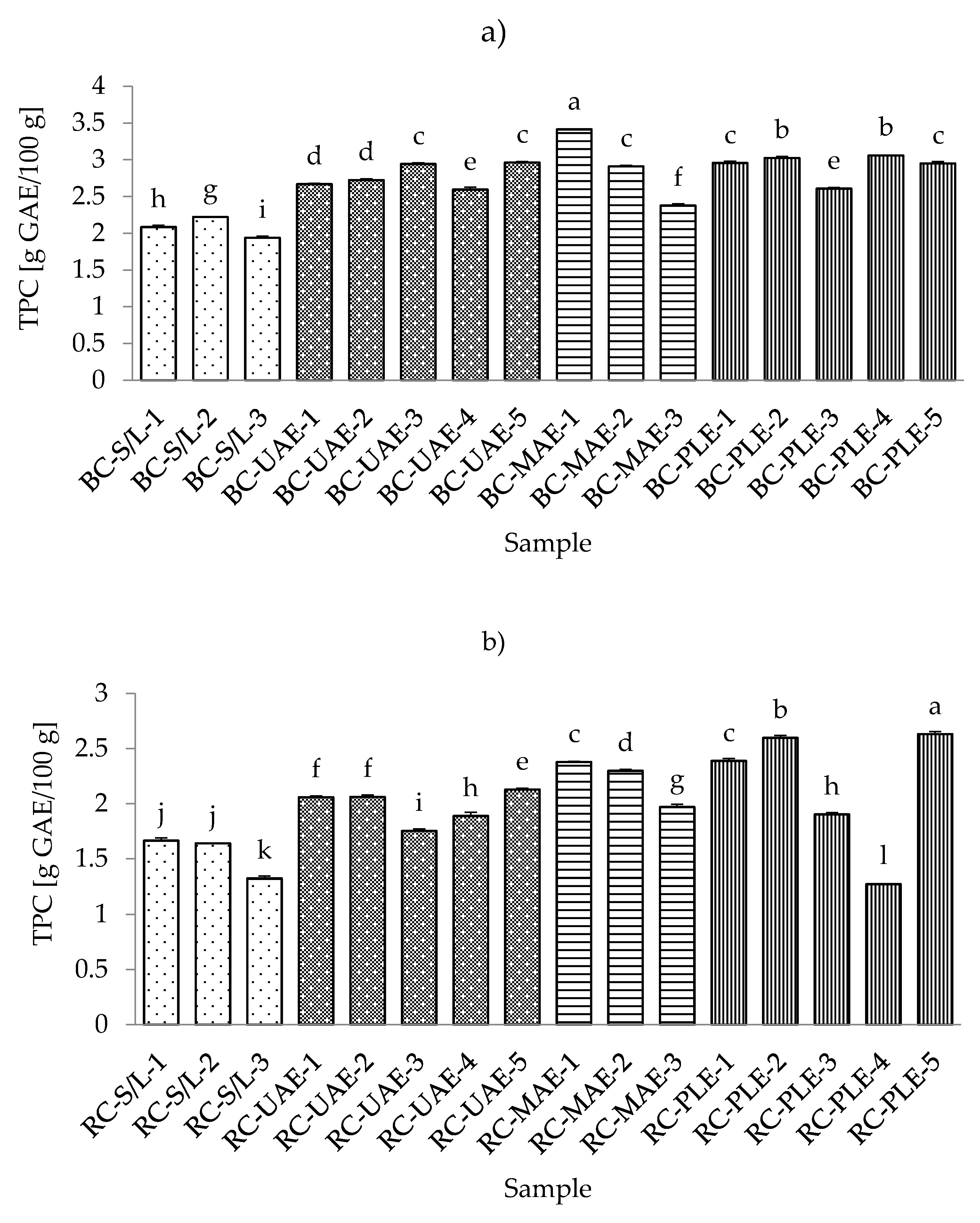
3.3. Total Flavonoid Content
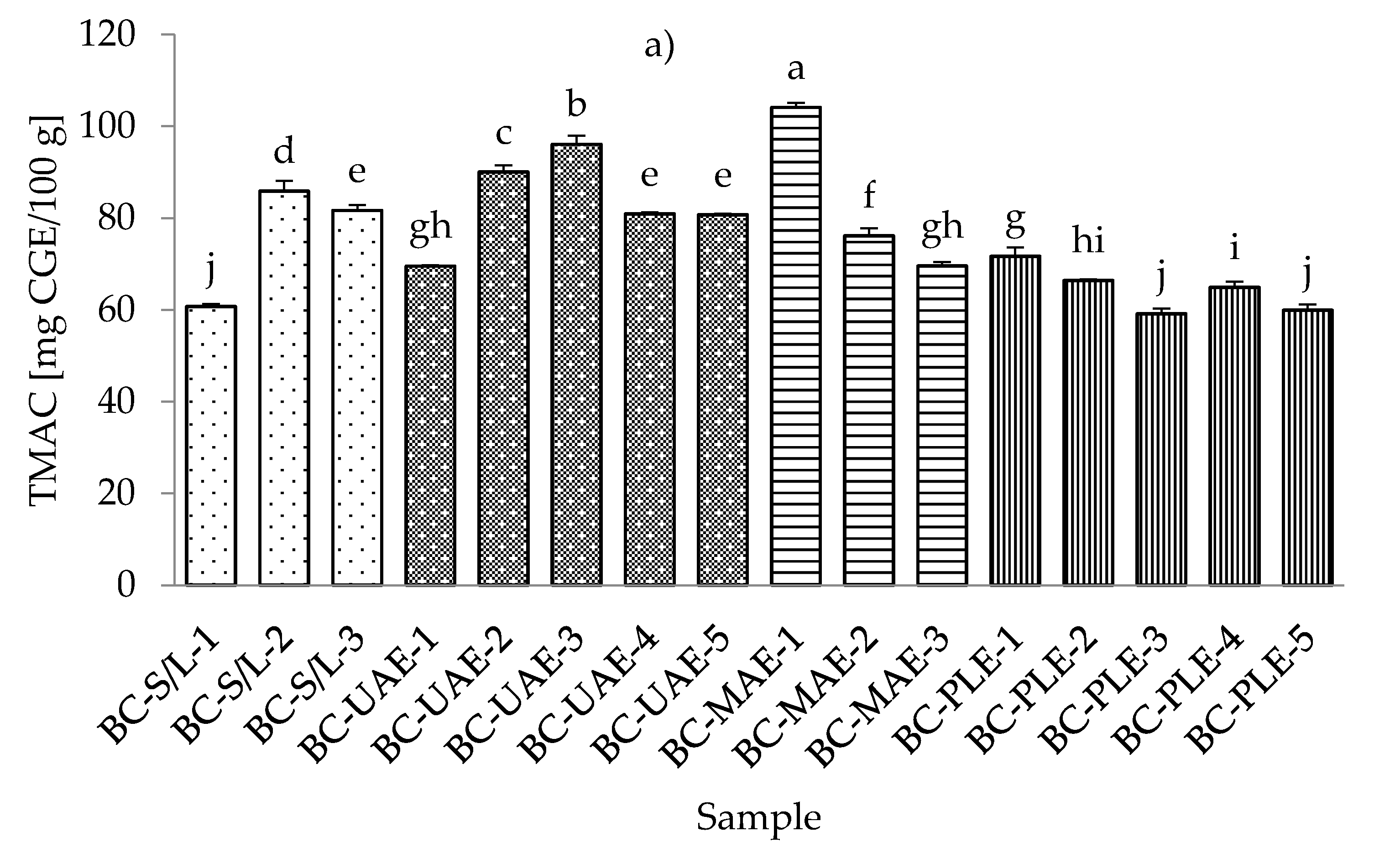
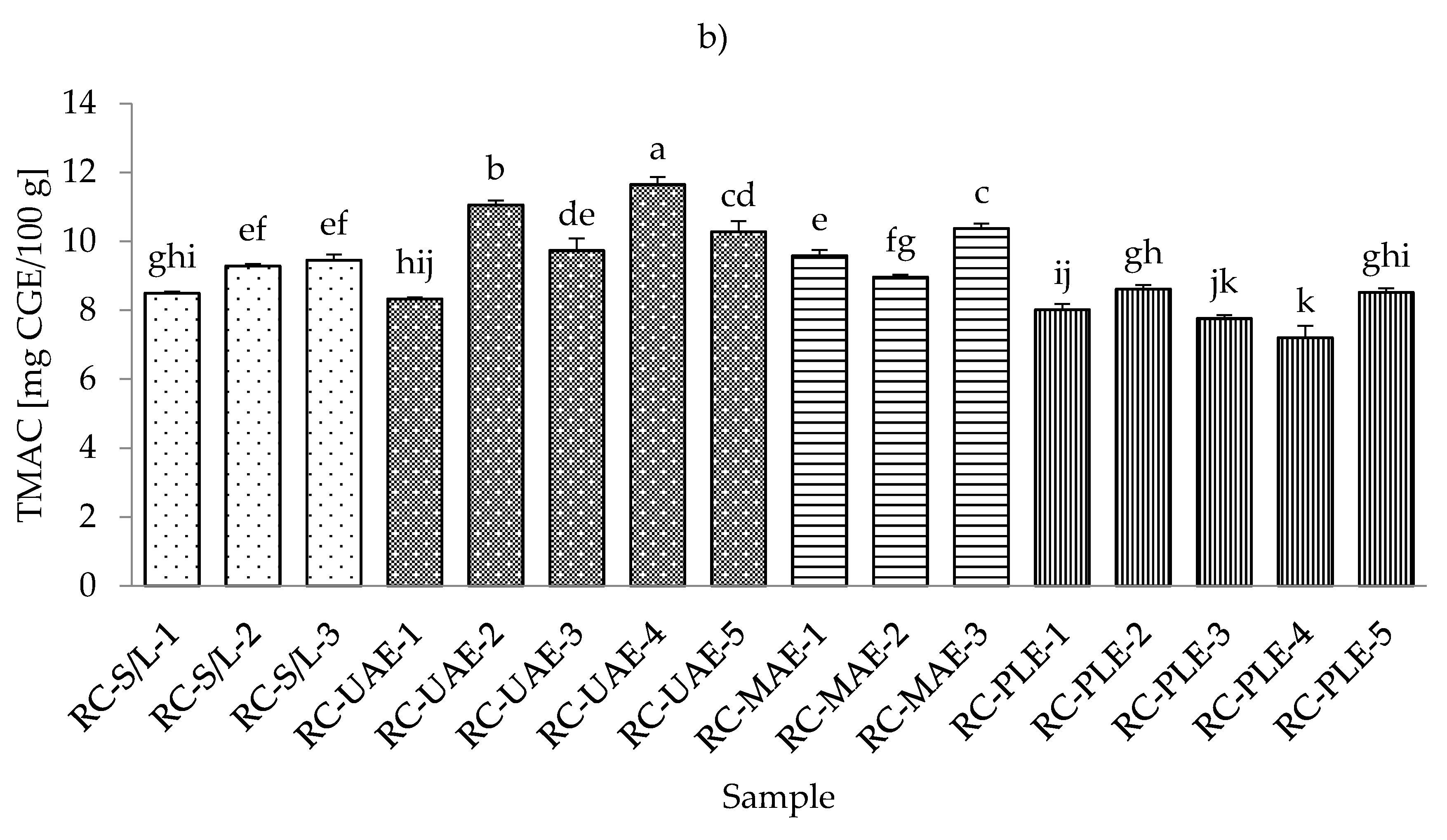
3.4. Total Monomeric Anthocyanin Content
3.5. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djordjević, B.; Rakonjac, V.; Fotirić Akšić, M.; Šavikin, K.; Vulić, T. Pomological and biochemical characterization of European currant berry (Ribes sp.) cultivars. Sci. Hortic. 2014, 165, 156–162. [Google Scholar] [CrossRef]
- Knyazev, S.; Panfilova, O.; Tsoy, M.; Golyaeva, O.; Loretts, O.; Kukhar, V.; Panfilova, O.; Tsoy, M. Currant growing technology and mechanized harvesting-review. E3S Web Conf. 2021, 254, 07002. [Google Scholar] [CrossRef]
- Vakula, A.; Drašković-Berger, M.; Daničić, T.; Tepić-Horecki, A.; Pavlić, B.; Jokanović, M.; Šumić, Z. Vacuum drying of red currant (Ribes rubrum L.): Physical and chemical properties and kinetic modeling. Food Feed. Res. 2019, 46, 91–98. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Rugină, D.; Stanilă, A.; Dulf, F.; Bunea, A.; Socaci, S.A.; Socaciu, C.; Pintea, A. Phytochemical characterization of commercial processed blueberry, blackberry, blackcurrant, cranberry, and raspberry and their antioxidant activity. Antioxidants 2019, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.-M.; Veteläinen, M. High variability in flavonoid contents and composition between different North-European currant (Ribes spp.) varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef]
- Yucel, E.E.; Kaya, C. Effect of jam and marmalade processing and storage on the phytochemical properties of currant cultivars (Ribes spp.). J. Food Processing Preserv. 2021. [Google Scholar] [CrossRef]
- Ersoy, N.; Kupe, M.; Gundogdu, M.; Ilhan, G.; Ercisli, S. Phytochemical and antioxidant diversity in fruits of currant (Ribes spp.). Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Laaksonen, O.; Haikonen, H.; Vanag, A.; Ejaz, H.; Linderborg, K.; Karhu, S.; Yang, B. Compositional diversity among blackcurrant (Ribes nigrum) cultivars originating from European countries. J. Agric. Food Chem. 2019, 67, 5621–5633. [Google Scholar] [CrossRef] [Green Version]
- Zdunić, G.; Šavikin, K.; Pljevljakušić, D.; Djordjević, B. Black (Ribes nigrum L.) and red currant (Ribes rubrum L.) cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2016; pp. 101–126. [Google Scholar]
- Laczkó-Zöld, E.; Komlósi, A.; Ülkei, T.; Fogarasi, E.; Croitoru, M.; Fülöp, I.; Domokos, E.; Ştefănescu, R.; Varga, E. Extractability of polyphenols from black currant, red currant and gooseberry and their antioxidant activity. Acta Biol. Hung. 2018, 69, 156–169. [Google Scholar] [CrossRef]
- Eksi Karaagac, H.; Cavus, F.; Kadioglu, B.; Ugur, N.; Tokat, E.; Sahan, Y. Evaluation of nutritional, color and volatiles properties of currant (Ribes spp.) cultivars in Turkey. Food Sci. Technol. 2021, 41, 304–313. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A review on extraction techniques and its future applications in industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Pell, R.; Tijsseling, L.; Goodenough, K.; Wall, F.; Dehaine, Q.; Grant, A.; Deak, D.; Yan, X.; Whattoff, P. Towards sustainable extraction of technology materials through integrated approaches. Nat. Rev. Earth Environ. 2021, 2, 665–679. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2019, 60, 1826–1841. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative hurdle technologies for the preservation of functional fruit juices. Foods 2020, 9, 699. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.; Barba, F.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Int. 2020, 1–31. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Jarillo, J.A.; Carrera, C.; Ferreiro-González, M.; Álvarez, J.Á.; Palma, M.; Ayuso, J.; Barbero, G.F.; Espada-Bellido, E. Optimization of a novel method based on ultrasound-assisted extraction for the quantification of anthocyanins and total phenolic compounds in blueberry samples (Vaccinium corymbosum L.). Foods 2020, 9, 1763. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, N.; Viera, V.B.; Mello, R.; dos Santos, R.C.V.; Vaucher, R.A.; Dressler, V.L.; Bizzi, C.A.; Fries, L.L.M. Microwave-assisted extraction of bioactive compounds from blueberry (Vaccinium ashei Reade) and their antioxidant and antimicrobial capacity. Int. Food Res. J. 2017, 24, 2526–2533. [Google Scholar]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids: Potential therapeutic agents by their antioxidant capacity. Bioactive compounds: Health benefits and potential applications. In Bioactive Compounds—Health Benefits and Potential Applications; Segura Campos, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 265–288. [Google Scholar]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Otero-Pareja, M.; Casas, L.; Fernández-Ponce, M.; Mantell, C.; Ossa, E. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of bioactive compounds from strawberry (Fragaria × ananassa) pomace by conventional and pressurized liquid extraction and assessment their bioactivity in human cell cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef]
- Šumić, Z.; Tepić, A.; Vidović, S.; Jokić, S.; Malbaša, R. Optimization of frozen sour cherries vacuum drying process. Food Chem. 2013, 136, 55–63. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-Liquid Extraction as an efficient method for valorization of Thymus serpyllum herbal dust towards sustainable production of antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef]
- Pavlić, B.; Kaplan, M.; Bera, O.; Oktem Olgun, E.; Canli, O.; Milosavljević, N.; Antić, B.; Zeković, Z. Microwave-assisted extraction of peppermint polyphenols—Artificial neural networks approach. Food Bioprod. Processing 2019, 118, 258–269. [Google Scholar] [CrossRef]
- Milić, A.; Daničić, T.; Tepić Horecki, A.; Šumić, Z.; Bursać Kovačević, D.; Putnik, P.; Pavlić, B. Maximizing contents of phytochemicals obtained from dried sour cherries by ultrasound-assisted extraction. Separations 2021, 8, 155. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Harborne, J.B. Methods of plant analysis. In Phytochemical Methods—A Guide to Modern Techniques of Plant Analysis; Harborne, J.B., Ed.; Springer: Berlin, Germany, 1984; pp. 1–36. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef] [Green Version]
- Cvejić, J.; Atanacković Krstonošić, M.; Mikulić, M.; Miljić, U. Polyphenols. In Nutraceutical and Functional Food Components; Galanakis, C.M., Ed.; Academic Press: New York, NY, USA, 2022; pp. 243–312. [Google Scholar]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2009, 58, 3901–3909. [Google Scholar] [CrossRef]
- Gavrilova, V.; Kajdžanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC−DAD−ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef]
- Määttä, K.; Kamal-Eldin, A.; Törrönen, R. Phenolic compounds in berries of black, red, green, and white currants (Ribes sp.). Antioxid. Redox Signal. 2001, 3, 981–993. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-ethanolic mixtures for the recovery of phenols from mediterranean plant materials. Food Bioprocess Technol. 2010, 5, 1384–1393. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
| Sample | Extraction Technique | Extraction Parameters | Factor | |
|---|---|---|---|---|
| Black Currant | Red Currant | |||
| BC-S/L-1 | RC-S/L-1 | S/L | 1:10 (m:v) solid-to-liquid ratio, 24 h, room temperature, 150 rpm (shaker) | 30% ethanol |
| BC-S/L-2 | RC-S/L-2 | 50% ethanol | ||
| BC-S/L-3 | RC-S/L-3 | 70% ethanol | ||
| BC-UAE-1 | RC-UAE-1 | UAE | 1:10 (m:v) solid-to-liquid ratio, 30 min, 50 °C, 60 W/L (ultrasonic power), 40 kHz frequency | 30% ethanol |
| BC-UAE-2 | RC-UAE-2 | 50% ethanol | ||
| BC-UAE-3 | RC-UAE-3 | 70% ethanol | ||
| BC-UAE-4 | RC-UAE-4 | 1:10 (m:v) solid-to-liquid ratio, 30 min, 50% ethanol, 60 W/L (ultrasonic power) | 30 °C | |
| BC-UAE-5 | RC-UAE-5 | 70 °C | ||
| BC-MAE-1 | RC-MAE-1 | MAE | 1:10 (m:v) solid-to-liquid ratio, 10 min, 600 W (microwave power) | 30% ethanol |
| BC-MAE-2 | RC-MAE-2 | 50% ethanol | ||
| BC-MAE-3 | RC-MAE-3 | 70% ethanol | ||
| BC-PLE-1 | RC-PLE-1 | PLE | 5 g sample, 1:20 (m:v) solid-to-liquid ratio, 2 cycles, 100% rinse, 100°C, 10 min dynamic extraction time | 30% ethanol |
| BC-PLE-2 BC-PLE-3 | RC-PLE-2 RC-PLE-3 | 50% ethanol 70% ethanol | ||
| BC-PLE-4 | RC-PLE-4 | 5 g sample, 1:20 (m:v) solid-to-liquid ratio, 2 cycles, 100% rinse, 50% ethanol, 10 min dynamic extraction time | 80 °C | |
| BC-PLE-5 | RC-PLE-5 | 120 °C | ||
| Sample | DPPH [µM TE/g] | FRAP [µM Fe2+/g] | ABTS [µM TE/g] |
|---|---|---|---|
| BC-S/L-1 | 73.51 ± 0.92 g | 59.66 ± 0.06 j | 146.34 ± 1.83 j |
| BC-S/L-2 | 75.81 ± 0.92 f | 61.56 ± 0.33 i | 154.19 ± 1.44 h, i |
| BC-S/L-3 | 59.82 ± 1.06 h | 57.17 ± 0.32 k | 101.99 ± 1.83 k |
| BC-UAE-1 | 86.10 ± 0.90 d | 75.46 ± 0.21 c | 151.65 ± 2.23 i, j |
| BC-UAE-2 | 80.74 ± 0.64 e | 69.39 ± 0.32 g, h | 157.43 ± 1.39 h |
| BC-UAE-3 | 75.55 ± 0.88 f, g | 71.35 ± 0.36 f | 171.52 ± 2.23 g |
| BC-UAE-4 | 76.74 ± 0.39 f | 70.04 ± 0.32 g | 174.75 ± 1.39 g |
| BC-UAE-5 | 94.52 ± 0.59 b, c | 73.08 ± 0.31 e | 207.78 ± 1.74 c |
| BC-MAE-1 | 96.39 ± 0.82 b | 87.12 ± 0.39 a | 222.79 ± 2.23 a |
| BC-MAE-2 | 86.02 ± 0.51 d | 78.32 ± 0.26 b | 209.39 ± 1.39 b, c |
| BC-MAE-3 | 75.30 ± 0.44 f, g | 57.24 ± 0.31 k | 155.81 ± 1.74 h, i |
| BC-PLE-1 | 94.10 ± 1.03 c | 87.50 ± 0.12 a | 196.00 ± 2.62 e |
| BC-PLE-2 | 92.82 ± 0.64 c | 72.63 ± 0.22 e | 201.54 ± 0.80 d |
| BC-PLE-3 | 81.85 ± 0.53 e | 68.97 ± 0.26 h | 159.51 ± 1.39 h |
| BC-PLE-4 | 92.57 ± 0.39 c | 74.56 ± 0.26 d | 185.84 ± 2.08 f |
| BC-PLE-5 | 107.7 ± 0.92 a | 76.08 ± 0.27 c | 213.32 ± 0.80 b |
| Sample | DPPH [µM TE/g] | FRAP [µM Fe2+/g] | ABTS [µM TE/g] |
|---|---|---|---|
| RC-S/L-1 | 58.46 ± 0.68 i | 48.52 ± 0.39 f | 106.38 ± 2.62 f |
| RC-S/L-2 | 56.76 ± 0.29 i | 43.03 ± 0.36 i | 90.22 ± 2.40 h |
| RC-S/L-3 | 49.53 ± 0.26 k | 40.10 ± 0.43 j | 73.12 ± 2.43 j |
| RC-UAE-1 | 70.88 ± 1.03 f | 52.07 ± 0.37 d | 103.38 ± 1.20 f, g |
| RC-UAE-2 | 74.96 ± 0.78 e | 46.41 ± 0.49 g | 138.72 ± 3.02 c |
| RC-UAE-3 | 63.39 ± 0.64 h | 49.90 ± 0.27 e | 80.98 ± 1.44 i |
| RC-UAE-4 | 67.81 ± 0.39 g | 51.55 ± 0.36 d | 97.14 ± 2.08 g |
| RC-UAE-5 | 85.42 ± 0.78 c | 56.59 ± 0.32 c | 187.91 ± 2.40 a |
| RC-MAE-1 | 93.76 ± 1.06 b | 63.70 ± 0.30 a | 155.58 ± 1.44 b |
| RC-MAE-2 | 96.56 ± 0.53 a | 57.24 ± 0.18 c | 126.94 ± 2.77 d |
| RC-MAE-3 | 73.26 ± 0.92 e | 46.89 ± 0.18 g | 115.85 ± 2.08 e |
| RC-PLE-1 | 78.62 ± 0.44 d | 64.56 ± 0.33 a | 142.18 ± 2.08 c |
| RC-PLE-2 | 94.18 ± 0.77 b | 56.45 ± 0.42 c | 158.35 ± 1.06 b |
| RC-PLE-3 | 70.62 ± 0.59 f | 44.48 ± 0.12 h | 114.47 ± 3.02 e |
| RC-PLE-4 | 53.35 ± 0.68 j | 31.89 ± 0.10 k | 81.90 ± 2.08 i |
| RC-PLE-5 | 84.48 ± 0.68 c | 59.45 ± 0.42 b | 159.51 ± 0.80 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milić, A.; Daničić, T.; Tepić Horecki, A.; Šumić, Z.; Teslić, N.; Bursać Kovačević, D.; Putnik, P.; Pavlić, B. Sustainable Extractions for Maximizing Content of Antioxidant Phytochemicals from Black and Red Currants. Foods 2022, 11, 325. https://doi.org/10.3390/foods11030325
Milić A, Daničić T, Tepić Horecki A, Šumić Z, Teslić N, Bursać Kovačević D, Putnik P, Pavlić B. Sustainable Extractions for Maximizing Content of Antioxidant Phytochemicals from Black and Red Currants. Foods. 2022; 11(3):325. https://doi.org/10.3390/foods11030325
Chicago/Turabian StyleMilić, Anita, Tatjana Daničić, Aleksandra Tepić Horecki, Zdravko Šumić, Nemanja Teslić, Danijela Bursać Kovačević, Predrag Putnik, and Branimir Pavlić. 2022. "Sustainable Extractions for Maximizing Content of Antioxidant Phytochemicals from Black and Red Currants" Foods 11, no. 3: 325. https://doi.org/10.3390/foods11030325
APA StyleMilić, A., Daničić, T., Tepić Horecki, A., Šumić, Z., Teslić, N., Bursać Kovačević, D., Putnik, P., & Pavlić, B. (2022). Sustainable Extractions for Maximizing Content of Antioxidant Phytochemicals from Black and Red Currants. Foods, 11(3), 325. https://doi.org/10.3390/foods11030325










