Incorporation of Limosilactobacillus fermentum UCO-979C with Anti-Helicobacter pylori and Immunomodulatory Activities in Various Ice Cream Bases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Ice Cream Production
2.3. Viability of L. fermentum UCO-979C in Ice Cream
2.4. Anti-H. pylori Activity of the Probiotic Strain Recovered from Ice Cream
2.5. Immunomodulatory Activity of the Probiotic Strain Recovered from Ice Cream
2.6. Chemical and Physical Analysis of Ice Cream
2.6.1. Chemical Analysis
2.6.2. Physical Analysis
2.7. Statistical Analysis
3. Results
3.1. Viability of L. fermentum UCO-979C in Ice Cream
3.2. Anti-H. pylori Activity of L. fermentum UCO-979C
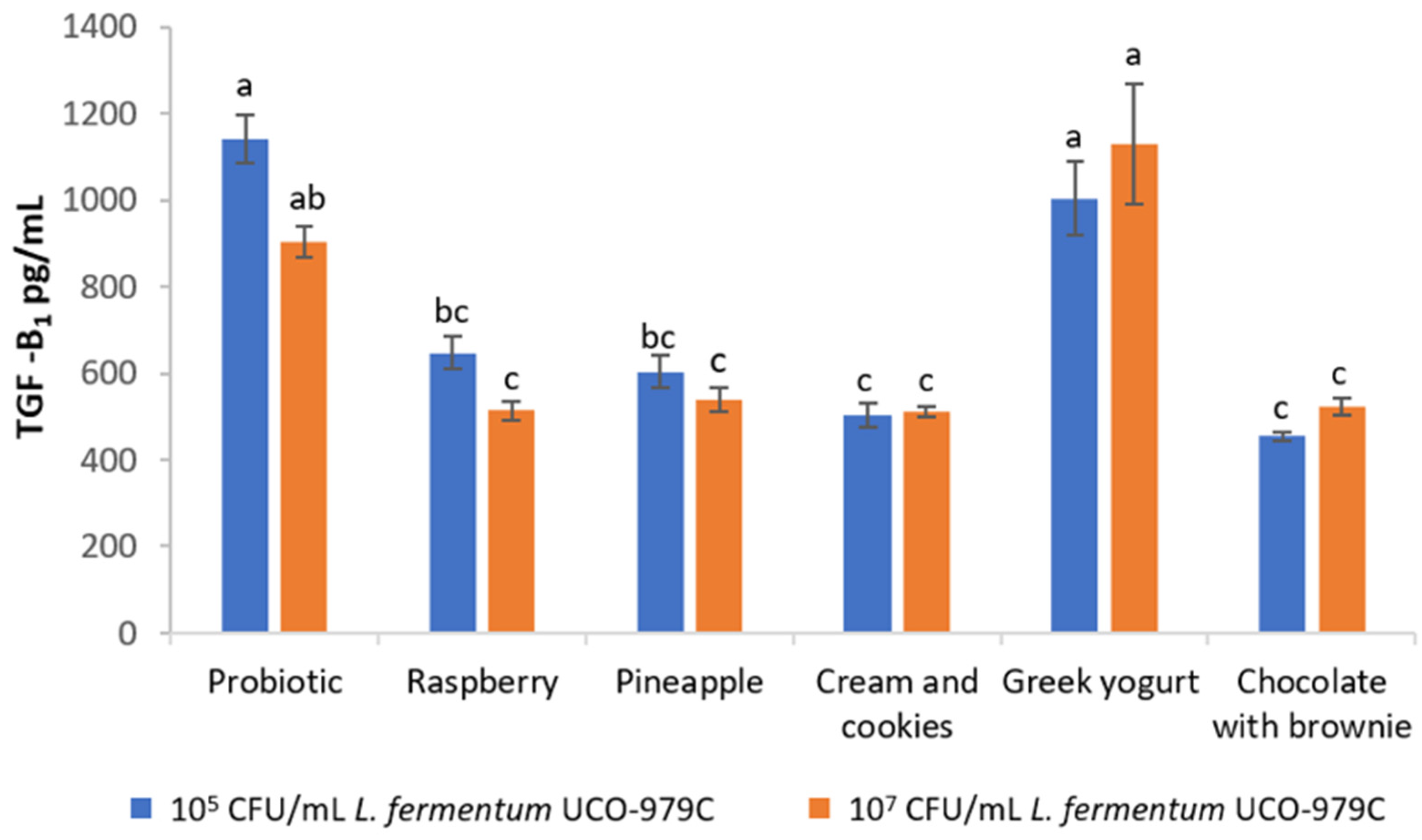
3.3. Immunomodulatory Activity of L. fermentum UCO-979C
3.4. Physicochemical Analysis
3.4.1. Proximal
3.4.2. Color
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Otero, R.W.; Gómez, Z.M.; Otero, P.L.; Trespalacios, R.A. Helicobacter pylori: ¿cómo se trata en el 2018? Rev. Gastroenterol. Perú 2018, 38, 54–63. [Google Scholar]
- Molina, J.; Corti, R.; Doweck, J.; McNicholl, A.; Gisbert, J. Avances recientes en el tratamiento de la infección por Helicobacter pylori. Acta Gastroenterol. Latinoam. 2017, 47, 75–85. [Google Scholar]
- Goderska, K.; Agudo, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2017, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cosme, A.; Montes, M.; Ibarra, B.; Tamayo, E.; Alonso, H.; Mendarte, U.; Lizasoan, J.; Herreros-Villanueva, M.; Bujanda, L. Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World J. Gastroenterol. 2017, 23, 3367–3373. [Google Scholar] [CrossRef]
- Aljeboury, G. In Vivo Study of Probiotic Role in Protection and Prevention of Helicobacter pylori that Cause Stomach Ulcer in Rats. Ann. Rom. Soc. Cell Biol. 2021, 25, 11717–11722. [Google Scholar]
- Ayala, G.; Escobedo-Hinojosa, W.I.; de la Cruz-Herrera, C.F.; Romero, I. Exploring alternative treatments for Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Sui, L.; Mu, G.; Qian, F.; Zhu, X. Screening of potential probiotics with anti-Helicobacter pylori activity from infant feces through principal component analysis. Food Biosci. 2021, 42, 101045. [Google Scholar] [CrossRef]
- Ji, J.; Yang, H. Using Probiotics as Supplementation for Helicobacter pylori Antibiotic Therapy. Int. J. Mol. Sci. 2020, 21, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food; FAO: Rome, Italy, 2002; pp. 1–11.
- Cruz, A.; Antunes, A.; Sousa, A.; Faria, J.; Saad, S. Ice cream as a probiotic food carrier. Food Resarch Int. 2009, 42, 1233–1239. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Yang, Z. Lactic Acid Bacteria in Health and Disease. Lact. Acid Bact. 2014, 5, 303–374. [Google Scholar] [CrossRef]
- Panwar, D.; Shubhashini, A.; Kapoor, M. Enhanced survival of Lactobacillus spp. in beta-manno-oligosaccharides-enriched low-fat ice cream under simulated gastrointestinal stress. J. Food Processing Preserv. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Dos Santos, E.; de Araújo, E.; da Conceicao, L.; de Moraes, C.; de Carvalho, A. Survival of Lactobacillus delbrueckii UFV H2b20 in ice cream produced with different fat levels and after submission to stress acid and bile salts. J. Func. Food 2012, 5, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.; Henriquez, P.; Retamal, C.; Pineda, S.; Delgado, C.; Gonzales, C. Propiedades probióticas de Lactobacillus spp. aislados de biopsias gástricas de pacientes con y sin infección por Helicobacter pylori. Rev. Med. Chil. 2009, 137, 369–376. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Navarro, K.; Sanhueza, E.; Pineda, S.; Pastene, E.; Quezada, M.; Henríquez, K.; Karlyshev, A.; Villena, J.; González, C. Characterization of Lactobacillus fermentum UCO-979C, a probiotic strain with a potent anti-Helicobacter pylori activity. Electron. J. Biotechnol. 2017, 25, 75–83. [Google Scholar] [CrossRef]
- Garcia, V.; Zelaya, H.; Ilabaca, A.; Espinoza, M.; Komatsu, R.; Albarracín, L.; Kitazawa, H.; Garcia, A.; Villena, J. Lactobacillus fermentum UCO-979C beneficially modulates the innate immune response triggered by Helicobacter pylori infection in vitro. Benef. Microbies 2018, 9, 829–841. [Google Scholar] [CrossRef]
- Merino, J.; Garcia, A.; Pastene, E.; Salas, A.; Saez, K.; Gonzalez, C. Lactobacillus fermentum UCO-979C strongly inhibited Helicobacter pylori SS1 in Meriones unguiculatus. Benef. Microbes 2018, 9, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Marcial, G.; Albarracin, L.; Tomokiyo, M.; Clua, P.; Takahashi, H.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms 2020, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Sanhueza, E.; Paredes-Osses, E.; González, C.L.; García, A. Effect of pH in the survival of Lactobacillus salivarius strain UCO_979C wild type and the pH acid acclimated variant. Electron. J. Biotechnol. 2015, 18, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Vega, M.; Rocha, J.; Sáez, K.; Smith, C.; Gutierrez, C.; García, A. Encapsulation, with and without oil, of biofilm forming Lactobacillus fermentum UCO-979C strain in alginate-xanthan gum and its anti- Helicobacter pylori effect. J. Funct. Foods 2018, 46, 504–513. [Google Scholar] [CrossRef]
- Gaudana, S.B.; Dhanani, A.S.; Bagchi, T. Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br. J. Nutr. 2010, 103, 1620–1628. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC: Gaithersburg, MD, USA, 2012; Volume 2. [Google Scholar]
- Abd El-Rahman, A.; Madkor, S.; Ibrahim, F.; Kilara, A. Physical Characteristics of Frozen Desserts Made with Cream, Anhydrous Milk Fat, or Milk Fat Fractions. J. Dairy Sci. 1997, 80, 1926–1935. [Google Scholar] [CrossRef]
- Lee, H.; Coates, G. Effect of thermal pasteurization on Valencia orange juice color and pigments. Lebensm.-Wiss. Und-Technol.-Food Sci. Technol. 2003, 36, 153–156. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, H.; Kumar, N.; Kaur, M. The effect of inulin as a fat replacer on the quality of low-fat ice cream. Int. J. Dairy Technol. 2015, 68, 374–380. [Google Scholar] [CrossRef]
- Mathias, K.; Ah, K. El color en los alimentos un criterio de calidad medible. AgroSur 2014, 42, 39–48. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Cankurt, H.; Tornuk, F. Interaction Between Some Phenolic Compounds and Probiotic Bacterium in Functional Ice Cream Production. Food Bioprocess Technol. 2012, 5, 2964–2971. [Google Scholar] [CrossRef]
- MINSAL. Norma Técnica Sobre Directrices Nutricionales para Declarar Propiedades Saludables de los Alimentos. Minist. De Salud Pública De Chile. 2017. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1105664&buscar=860%2Bministerio%2Bde%2Bsalud (accessed on 2 November 2021).
- Chiquetti, R.L.; Castro, E.M.; Valério, G.; Bernini, L.J.; Suguimoto, H.; Santana, E.; Aragon-Alegro, L.; Souza, C. Viability of the probiotic Lactobacillus acidophilus La-5 in ice cream: Effect of lactose hydrolysis and overrun. Int. Food Res. J. 2016, 23, 2631–2637. [Google Scholar]
- Homayouni, A.; Azizi, A.; Javadi, M.; Mahdipour, S.; Ejtahed, H. Factors Influencing Probiotic Survival in Ice Cream: A Review. Int. J. Dairy Sci. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mortazavian, A.M.; Khosrokhavar, R.; da Cruz, A.G. Probiotic ice cream: Viability of probiotic bacteria and sensory properties. Ann. Microbiol. 2011, 61, 411–424. [Google Scholar] [CrossRef]
- TurguT, T.; Cakmakci, S. Investigation of the possible use of probiotics in ice cream manufacture. Int. J. Dairy Technol. 2009, 62, 444–451. [Google Scholar] [CrossRef]
- Champagne, C. Some Technological Challenges in the Addition of Probiotic Bacteria to Foods. Prebiotics Probiotics Sci. Technol. 2009, 761–804. [Google Scholar] [CrossRef]
- Ayar, A.; Sıçramaz, H.; Öztürk, S.; Yilmaz, S. Probiotic properties of ice creams produced with dietary fibres from by-products of the food industry. Int. J. Dairy Technol. 2017, 71, 174–182. [Google Scholar] [CrossRef]
- Di Criscio, T.; Fratianni, A.; Mignogna, R.; Cinquanta, L.; Coppola, R.; Sorrentino, E.; Panfili, G. Production of functional probiotic, prebiotic, and synbiotic ice creams. J. Dairy Sci. 2010, 93, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Bujna, E.; Fekete, N.; Tran, A.; Rezessy, J.; Prasad, R.; Nguyen, Q. Probiotic Beverage From Pineapple Juice Fermented With Lactobacillus and Bifidobacterium Strains. Front. Nutr. 2019, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, G.; Hernandez, L.; Fernandez, S. Froto, M.; Vasquez, L. Estrategias para mejorar la sobrevivencia de probioticos en helados. Rev. Cienc. Biològicas Salud. 2013, 15, 31–38. [Google Scholar] [CrossRef]
- Magarinos, H.; Selaive, S.; Costa, M.; Flores, M.; Pizarro, O. Viability of probiotic micro-organisms (Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp lactis Bb-12) in ice cream. Int. J. Dairy Technol. 2007, 60, 128–134. [Google Scholar] [CrossRef]
- Siow, L.; Lee, K. Canned, frozen and dried pineapple. In Handbook of Pineapple Technology: Production, Postharvest Science, Processing and Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 13, pp. 126–139. [Google Scholar]
- Meybodi, N.; Mortazavian, A.; Sohrabvandi, S.; da Cruz, A.G.; Mohammadi, R. Probiotic Supplements and Food Products: Comparison for Different Targets. Appl. Food Biotechnol. 2017, 4, 123–131. [Google Scholar] [CrossRef]
- Wada, T.; Aiba, Y.; Shimizu, K.; Takagi, A.; Miwa, T.; Koga, Y. The therapeutic effect of bovine lactoferrin in the host infected with Helicobacter pylori. Scand. J. Gastroenterol. 1999, 34, 238–243. [Google Scholar]
- Takeuchi, H.; Trang, V.T.; Morimoto, N.; Nishida, Y.; Matsumura, Y.; Sugiura, T. Natural products and food components with anti-Helicobacter pylori activities. World J. Gastroenterol. 2014, 20, 8971–8978. [Google Scholar] [CrossRef]
- Lawal, T.; Olorunnipa, T.; Adeniyi, B. Susceptibility testing and bactericidal activities of Theobrorna cacao Linn. (cocoa) on Helicobacter pylori in an in vitro study. J. Herb. Med. 2014, 4, 201–207. [Google Scholar] [CrossRef]
- Akyon, Y. Effect of antioxidants on the immune response of Helicobacter pylori. Clin. Microbiol. Infection. 2002, 8, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Mol. Cell. Biochem. 2004, 265, 19–26. [Google Scholar] [CrossRef]
- Anusha, M.; Shivanna, N.; Kumar, G.; Anilakumar, K. Efficiency of selected food ingredients on protein efficiency ratio, glycemic index and in vitro digestive properties. J. Food Sci. Technol.-Mysore 2018, 55, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Berner, D.; Viernstein, H. Effect of protective agents on the viability of Lactococcus lactic subjected to freeze-thawing and freeze-drying. Sci. Pharm. 2006, 74, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, P.; Lai, F.; Tsai, P.; Sheu, B. Probiotics-Containing Yogurt Ingestion and H. pylori Eradication Can Restore Fecal Faecalibacterium prausnitzii Dysbiosis in H. pylori -Infected Children. Biomedicines 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Alamprese, C.; Foschino, R.; Rossi, M.; Pompei, C.; Savani, L. Survival of Lactobacillus johnsonii La1 and influence of its addition in retail-manufactured ice cream produced with different sugar and fat concentrations. Int. Dairy J. 2002, 12, 201–208. [Google Scholar] [CrossRef]
- Salas, M.; Sanhueza, E.; Retamal, A.; Gonzales, C.; Urrutia, H.; García, A. Probiotic Lactobacillus fermentum UCO-979C biofilm formation on AGS and Caco-2 cells and Helicobacter pylori inhibition. J. Bioadhesion Biofilm Res. 2016, 32, 1245–1257. [Google Scholar] [CrossRef]
- Guergoletto, K.; Sivieri, K.; Tsuruda, A.; Martins, E.; de Souza, J.; Roig, S.; Hirooka, E.; Garcia, S. Dried Probiotics for Use in Functional Food Applications. In Food Industrial Processes—Methods and Equipment; InTechOpen: London, UK, 2012; Volume 21, pp. 227–250. [Google Scholar]
- Goff, H.; Hartel, R. Ice Cream Structure. Ice Cream 2013, 11, 313–352. [Google Scholar] [CrossRef]
- Alvarez, V. Ice Cream and Related Products. In The Sensory Evaluation of Dairy Products, 2nd ed.; Clark, S., Costello, M., Drake, M., Bodyfelt, F., Eds.; Springer: New York, NY, USA, 2008; Volume 10, pp. 271–331. [Google Scholar]
- Erkaya, T.; Dagdemir, E.; Sengul, M. Influence of Cape gooseberry (Physalis peruviana L.) addition on the chemical and sensory characteristics and mineral concentrations of ice cream. Food Res. Int. 2012, 45, 331–335. [Google Scholar] [CrossRef]
- Hernandez, J.; Frutos, M. Degradation kinetics of pigment, colour and stability of the antioxidant capacity in juice model systems from six anthocyanin sources. Int. J. Food Sci. Technol. 2011, 46, 2550–2557. [Google Scholar] [CrossRef]
- Ferreira, E.; Siqueira, H.; Boas, E.V.; Hermes, V.; Rios, A. Bioactive Compounds and Antioxidant Activity of Pineapple fruit of Different Cultivars. Rev. Bras. Frutic. 2016, 38, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bartolome, A.; Ruperez, P.; Fuster, C. Freezing rate and frozen storage effects on color and sensory characteristics of pineapple fruit slices. J. Food Sci. 1996, 61, 154–156. [Google Scholar] [CrossRef]
- Krahl, T.; Fuhrmann, H.; Dimassi, S. Ice Cream. In Handbook on Natural Pigments in Food and Beverages; German, E., Ed.; Woodhead Publish Ltd.: Sawston, UK, 2016; Volume 9, pp. 197–207. [Google Scholar]
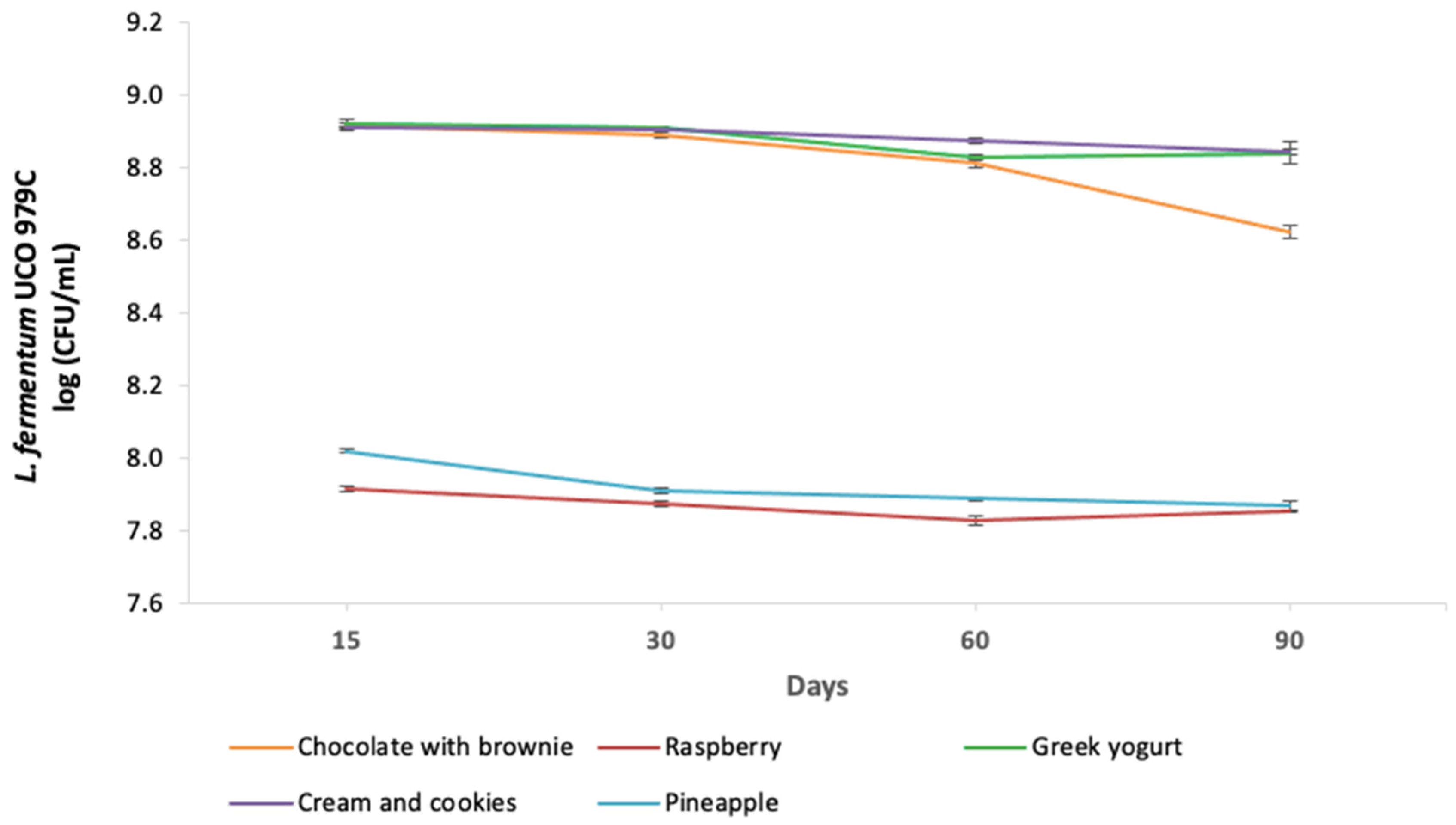
| Time (Days) | 15 | 30 | 60 | 90 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Chocolate with brownie | 8.917 a | 0.007 | 8.890 a | 0.009 | 8.812 b | 0.018 | 8.623 c | 0.031 |
| Raspberry | 7.915 a | 0.011 | 7.874 ab | 0.016 | 7.828 b | 0.024 | 7.855 b | 0.006 |
| Greek yogurt | 8.920 a | 0.024 | 8.909 a | 0.005 | 8.828 b | 0.009 | 8.840 b | 0.055 |
| Cookies and cream | 8.908 a | 0.005 | 8.904 a | 0.002 | 8.876 ab | 0.014 | 8.844 b | 0.010 |
| Pineapple | 8.021 a | 0.008 | 7.912 b | 0.013 | 7.890 b | 0.009 | 7.869 b | 0.020 |
| Factor | MRS + Probiotic Bacteria (mm) | MRS without Probiotic Bacteria (mm) | Supernatant (mm) | Pellet (mm) | Saline Solution (mm) | Probiotic Inhibition Halo (mm) (a) | Supernatant Inhibition Halo (mm) (b) |
|---|---|---|---|---|---|---|---|
| Chocolate with brownie | 4.5 1 | 3 | 5.4 | s/n | s/n | 1.5 (+) | 2.4 (++) |
| Greek yogurt | 4.5 | 3 | 5.8 | s/n | s/n | 1.8 (+) | 2.8 (++) |
| Cookies and cream | 4.5 | 3 | 5.6 | s/n | s/n | 1.5 (+) | 2.6 (++) |
| Raspberry | 3.5 | 3 | 4.3 | s/n | s/n | 0.5 (+/−) | 1.3 (+) |
| Pineapple | 3.3 | 3 | 4 | s/n | s/n | 0.3 (+/−) | 1 (+) |
| L. fermentum2 UCO-979C | 5 | 3 | 5.1 | s/n | s/n | 2 (+) | 2.1 (++) |
| Fruit-Based Ice Creams | ||||||
|---|---|---|---|---|---|---|
| Raspberry | Pineapple | |||||
| Analysis | Probiotic | Control | p (Value) | Probiotic | Control | p (Value) |
| Ash % | 0.165 ± 0.007 | 0.120 ± 0.014 | 0.0565 | 0.140 ± 0.014 | 0.095 ± 0.007 | 0.0565 |
| Fat % | 0.100 ± 0.000 | 0.100 ± 0.000 | - | 0.150 ± 0.071 | 0.065 ± 0.021 | 0.2450 |
| Fiber % | 0.980 ± 0.127 | 1.015 ± 0.007 | 0.7352 | 1.700 ± 0.141 | 1.475 ± 0.050 | 0.1677 |
| Moisture % | 76.820 ± 0.382 | 77.250 ± 0.396 | 0.3841 | 72.460 ± 0.580 | 74.335 ± 0.587 | 0.0847 |
| Protein % | 0.455 ± 0.021 | 0.305 ± 0.021 | 0.0194 * | 0.270 ± 0.085 | 0.260 ± 0.071 | 0.9098 |
| Sugar (g) | 19.246 ± 0.024 | 19.238 ± 1.197 | 0.9946 | 23.920 ± 0.981 | 21.592 ± 0.233 | 0.0823 |
| pH % | 3.135 ± 0.007 | 3.420 ± 0.028 | 0.0052 * | 3.670 ± 0.014 | 3.990 ± 0.014 | 0.0019 * |
| Lactic Acid % | 0.868 ± 0.032 | 0.733 ± 0.015 | 0.0428 * | 0.307 ± 0.004 | 0.259 ± 0.010 | 0.0253 * |
| Dairy-Based Ice Creams | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chocolate with Brownie | Cookies and Cream | Greek Yogurt | |||||||
| Analysis | Probiotic | Control | p (Value) | Probiotic | Control | p (Value) | Probiotic | Control | p (Value) |
| Ash % | 1.550 ± 0.028 | 1.490 ± 0.014 | 0.1153 | 1.100 ± 0.000 | 1.060 ± 0.014 | - | 0.960 ± 0.014 | 0.920 ± 0.014 | 0.1056 |
| Fat % | 6.100 ± 0.283 | 4.400 ± 0.283 | 0.0266 * | 3.200 ± 0.283 | 2.550 ± 0.212 | 0.1215 | 1.100 ± 0.000 | 0.650 ± 0.071 | 0.0704 |
| Fiber % | 0.860 ± 0.000 | 0.850 ± 0.014 | - | 1.285 ± 0.035 | 1.355 ± 0.035 | 0.1863 | 0.550 ± 0.042 | 0.625 ± 0.007 | 0.1325 |
| Moisture % | 60.655 ± 3.331 | 57.250 ± 1.004 | 0.3005 | 59.315 ± 0.035 | 57.895 ± 2.044 | 0.5056 | 63.930 ± 0.127 | 65.265 ± 0.007 | 0.0045 * |
| Protein % | 6.075 ± 0.050 | 6.445 ± 0.304 | 0.2315 | 4.855 ± 0.149 | 4.850 ± 0.057 | 0.9685 | 4.195 ± 0.219 | 4.105 ± 0.163 | 0.6868 |
| Sugar (g) | 19.159 ± 0.172 | 20.829 ± 0.284 | 0.0192 * | 20.577 ± 0.399 | 19.971 ± 1.594 | 0.6537 | 19.180 ± 1.318 | 21.441 ± 0.024 | 0.2489 |
| pH % | 6.545 ± 0.007 | 6.605 ± 0.007 | 0.0136 * | 6.780 ± 0.028 | 6.980 ± 0.014 | 0.0123 * | 5.565 ± 0.021 | 5.655 ± 0.021 | 0.0513 |
| Lactic Acid % | 0.291 ± 0.032 | 0.244 ± 0.031 | 0.2724 | 0.175 ± 0.000 | 0.090 ± 0.000 | - | 0.551 ± 0.031 | 0.514 ± 0.032 | 0.3537 |
| Chocolate with Brownie | Greek Yogurt | Cookies and Cream | Pineapple | Raspberry | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (Days) | Ice Cream Control | Ice Cream Probiotic | Ice Cream Control | Ice Cream Probiotic | Ice Cream Control | Ice Cream Probiotic | Ice Cream Control | Ice Cream Probiotic | Ice Cream Control | Ice Cream Probiotic | |
| L | 15 | 29.29 b | 33.36 a | 68.64 a | 70.47 a | 73.52 a | 73.51 ab | 63.49 a | 64.40 a | 33.55 a | 33.39 c |
| 30 | 29.44 b | 33.36 a | 66.51 b | 70.53 a | 73.45 a | 73.53 a | 62.40 b | 63.45 b | 33.57 a | 34.56 b | |
| 60 | 30.41 a | 33.53 a | 66.53 b | 70.42 a | 73.42 a | 73.32 b | 62.43 b | 63.47 b | 33.47 a | 35.44 a | |
| 90 | 30.48 a | 33.42 a | 66.59 b | 69.52 b | 73.41 a | 73.47 ab | 61.50 b | 62.50 c | 33.94 ab | 35.51 a | |
| C | 15 | 10.67 b | 17.69 b | 5.08 b | 4.31 b | 10.77 b | 11.90 b | 21.09 c | 18.37 b | 37.64 b | 36.20 b |
| 30 | 12.08 a | 18.37 a | 5.15 b | 5.59 a | 11.41 b | 12.79 b | 19.23 a | 18.33 a | 37.40 b | 37.53 c | |
| 60 | 11.95 a | 17.65 b | 5.14 b | 5.56 a | 12.22 a | 13.19 a | 19.19 b | 17.45 b | 38.39 a | 38.40 b | |
| 90 | 12.02 a | 17.68 b | 5.74 a | 5.70 a | 12.19 a | 13.06 a | 19.24 b | 17.41 b | 38.47 a | 39.49 a | |
| h | 15 | 36.82 b | 40.79 c | 59.94 a | 53.23 ab | 127.67 a | 118.18 b | 105.17 b | 107.56 c | 77.05 b | 77.83 a |
| 30 | 44.98 a | 42.80 b | 60.67 a | 53.95 a | 124.97 b | 115.76 c | 106.12 a | 107.76 bc | 76.74 b | 76.99 c | |
| 60 | 44.88 a | 45.05 a | 61.23 a | 51.33 b | 121.89 c | 119.41 a | 106.14 a | 108.32 ab | 76.99 b | 77.32 bc | |
| 90 | 45.00 a | 45.05 a | 52.33 b | 51.39 b | 121.92 c | 119.10 a | 106.61 a | a 108.85 a | 77.35 a | 77.60 ab | |
| ΔE | 15 | - | - | - | - | - | - | - | - | - | - |
| 30 | 2.150 | 0.934 | 2.133 | 1.287 | 0.833 | 1.041 | 2.182 | 0.958 | 0.314 | 1.848 | |
| 60 | 2.326 | 1.330 | 2.120 | 1.272 | 1.856 | 1.331 | 2.198 | 1.337 | 0.762 | 3.025 | |
| 90 | 2.422 | 1.320 | 2.273 | 1.694 | 1.830 | 1.182 | 2.762 | 2.174 | 1.329 | 3.926 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paucar-Carrión, C.; Espinoza-Monje, M.; Gutiérrez-Zamorano, C.; Sánchez-Alonzo, K.; Carvajal, R.I.; Rogel-Castillo, C.; Sáez-Carrillo, K.; García-Cancino, A. Incorporation of Limosilactobacillus fermentum UCO-979C with Anti-Helicobacter pylori and Immunomodulatory Activities in Various Ice Cream Bases. Foods 2022, 11, 333. https://doi.org/10.3390/foods11030333
Paucar-Carrión C, Espinoza-Monje M, Gutiérrez-Zamorano C, Sánchez-Alonzo K, Carvajal RI, Rogel-Castillo C, Sáez-Carrillo K, García-Cancino A. Incorporation of Limosilactobacillus fermentum UCO-979C with Anti-Helicobacter pylori and Immunomodulatory Activities in Various Ice Cream Bases. Foods. 2022; 11(3):333. https://doi.org/10.3390/foods11030333
Chicago/Turabian StylePaucar-Carrión, Cristina, Marcela Espinoza-Monje, Cristian Gutiérrez-Zamorano, Kimberly Sánchez-Alonzo, Romina I. Carvajal, Cristian Rogel-Castillo, Katia Sáez-Carrillo, and Apolinaria García-Cancino. 2022. "Incorporation of Limosilactobacillus fermentum UCO-979C with Anti-Helicobacter pylori and Immunomodulatory Activities in Various Ice Cream Bases" Foods 11, no. 3: 333. https://doi.org/10.3390/foods11030333







