Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
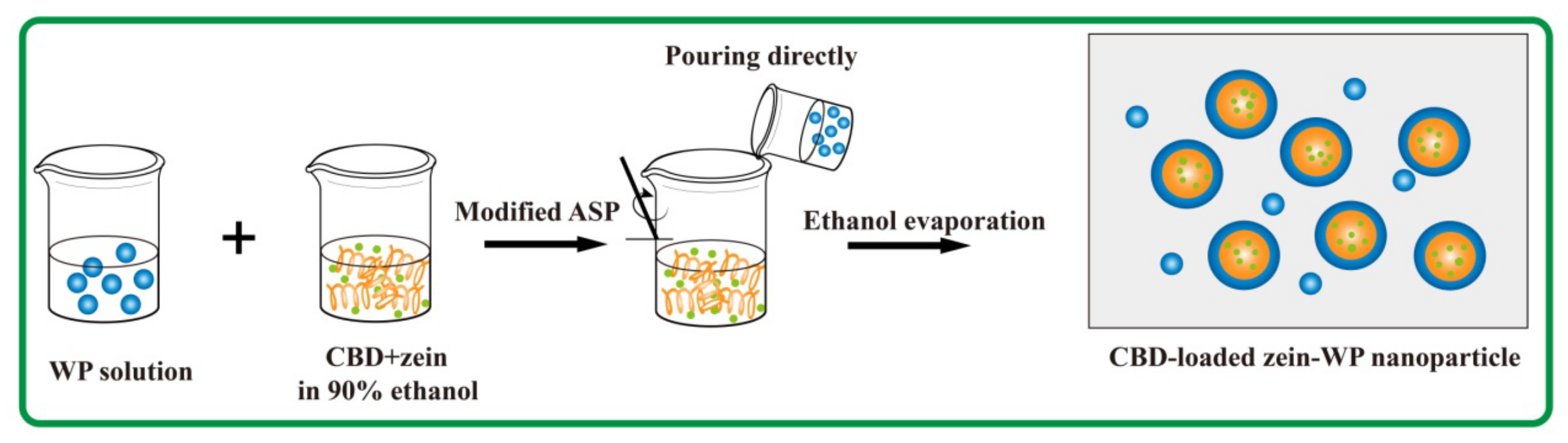
2.2. Fabrication of CBD-Loaded Composite Nanoparticles
2.3. Particle Size and Zeta-Potential
2.4. High Performance Liquid Chromatography (HPLC)
2.5. Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.6. Water Solubility
2.7. Re-Dispersibility of Freeze-Dried Nanoparticles
2.8. X-ray Diffraction (XRD)
2.9. Fourier Transform Infrared Spectrometry (FT-IR)
2.10. Transmission Electron Microscopy (TEM)
2.11. Stability
2.12. In Vivo Bioavailability Study
2.13. Statistical Analysis
3. Results and Discussion
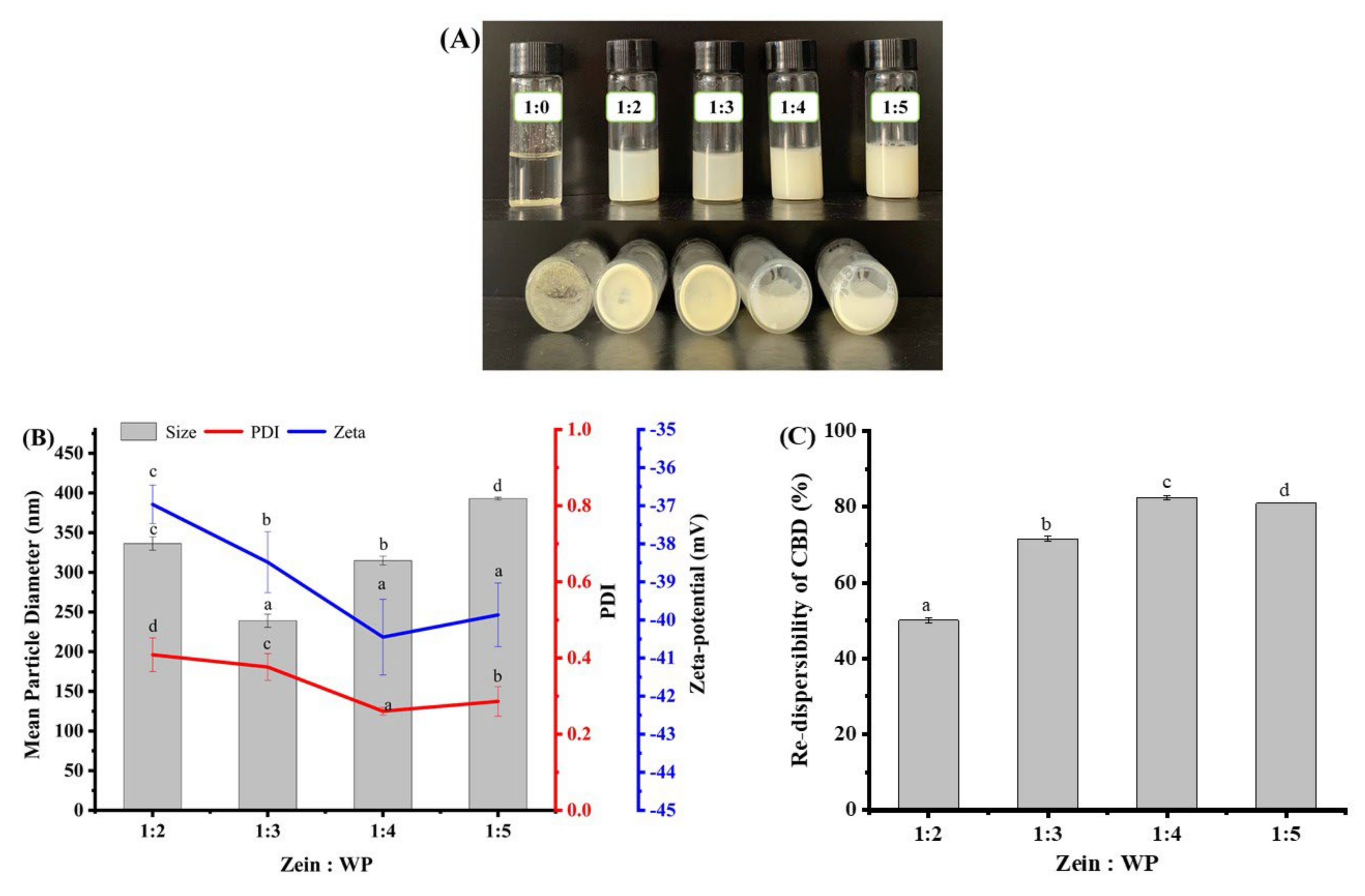
3.1. Particle Size and Zeta-Potential
3.2. Encapsulation Efficiency (EE), Loading Capacity (LC) and Water Solubility
3.3. Re-Dispersibility of Freeze-Dried Composite Nanoparticles
3.4. XRD Diffractogram
3.5. FT-IR Spectra
3.6. Microstructure Observed by TEM
3.7. Physicochemical Stability
3.8. Storage Stability
3.9. Bioavailability Analysis of CBD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mechoulam, R.; Hanus, L. Cannabidiol: An overview of some chemical and pharmacological aspects. Part I: Chemical aspects. Chem. Phys. Lipids 2002, 121, 35–43. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards better delivery of cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Hobbs, J.M.; Vazquez, A.R.; Remijan, N.D.; Trotter, R.E.; Weir, T.L. Evaluation of pharmacokinetics and acute antinflammatory potential of two oral cannabidiol preparations in healthy adults. Phytother. Res. 2020, 34, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Tajima, M.; Sugiyama, E.; Sato, V.H.; Sato, H. Development of a novel nanoemulsion formulation to improve intestinal absorption of cannabidiol. Med. Cannabis Cannabinoids 2019, 2, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Izgelov, D.; Davidson, E.; Barasch, D.; Regev, A.; Domb, A.J.; Hoffman, A. Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. Eur. J. Pharm. Biopharm. 2020, 154, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Fei, T.; Wan, Z.; Wang, T. Dispersing insoluble yolk low-density lipoprotein (LDL) recovered by complexing with carboxymethylcellulose (CMC) for the nanoencapsulation of hemp cannabidiol (CBD) through emulsification at neutral pH. Food Hydrocoll. 2021, 116, 106656. [Google Scholar] [CrossRef]
- Sharkawy, A.; Silva, A.M.; Rodrigues, F.; Barreiro, F.; Rodrigues, A. Pickering emulsions stabilized with chitosan/collagen peptides nanoparticles as green topical delivery vehicles for cannabidiol (CBD). Colloid Surf. A 2021, 631, 127677. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, D.J.; Guo, M.B.; Liu, J.; Chen, X.; Guo, R.; Xu, Y.P.; Zhang, Q.Y.; Liu, Y.; Guo, H.Y.; et al. Structural analysis and cytotoxicity of host-guest inclusion complexes of cannabidiol with three native cyclodextrins. J. Drug Deliv. Sci. Technol. 2019, 51, 337–344. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Q.F. Bioavailability enhancement of astilbin in rats through zein-caseinate nanoparticles. J. Agric. Food Chem. 2019, 67, 5746–5753. [Google Scholar] [CrossRef]
- Wei, Y.; Zhan, X.Y.; Dai, L.; Zhang, L.; Mao, L.K.; Yuan, F.; Liu, J.F.; Gao, Y.X. Formation mechanism and environmental stability of whey protein isolate-zein core-shell complex nanoparticles using the pH-shifting method. Lwt-Food Sci. Technol. 2021, 139, 110605. [Google Scholar] [CrossRef]
- Liu, Q.G.; Cheng, J.J.; Sun, X.M.; Guo, M.R. Preparation, characterization, and antioxidant activity of zein nanoparticles stabilized by whey protein nanofibrils. Int. J. Biol. Macromol. 2021, 167, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Reineke, J.; Kaushik, R.; Woyengo, T.; Baride, A.; Alqahtani, M.S.; Perumal, O. Bioadhesive food protein nanoparticles as pediatric oral drug delivery system. ACS Appl. Mater. Interfaces 2019, 11, 18062–18073. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Yin, S.W.; Yin, Y.C.; Tang, C.H.; Yang, X.Q.; Wen, S.H. Preparation of water-soluble antimicrobial zein nanoparticles by a modified antisolvent approach and their characterization. J. Food Eng. 2013, 119, 343–352. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Y.Y.; Chen, G.W.; Shi, Y.G.; Li, X.M.; Zhang, H.; Shen, Y.L. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate beta-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA). J. Pharm. Biomed. 2018, 149, 532–540. [Google Scholar] [CrossRef]
- Zhu, Q.M.; Lu, H.Q.; Zhu, J.Y.; Zhang, M.; Yin, L.J. Development and characterization of pickering emulsion stabilized by zein/corn fiber gum (CFG) complex colloidal particles. Food Hydrocoll. 2019, 91, 204–213. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhao, Z.L.; Xia, G.B.; Xue, F.; Chen, C.; Zhang, Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7,8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2020, 146, 179–192. [Google Scholar] [CrossRef]
- Zhong, Q.X.; Jin, M.F. Nanoscalar structures of spray-dried zein microcapsules and in vitro release kinetics of the encapsulated lysozyme as affected by formulations. J. Agric. Food Chem. 2009, 57, 3886–3894. [Google Scholar] [CrossRef]
- Yuan, Y.K.; Ma, M.J.; Zhang, S.Z.; Liu, C.Z.; Chen, P.; Li, H.; Wang, D.F.; Xu, Y. Effect of sophorolipid on the curcumin-loaded ternary composite nanoparticles self-assembled from zein and chondroitin sulfate. Food Hydrocoll. 2021, 113, 106493. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.L.; Li, F.; Shi, N.Q.; Li, C.L.; Yu, X.H.; Chen, Y.; Kong, W. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, C.X.; Dai, L.; Zhan, X.Y.; Gao, Y.X. Structure, physicochemical stability and in vitro simulated gastrointestinal digestion properties of beta-carotene loaded zein-propylene glycol alginate composite nanoparticles fabricated by emulsification-evaporation method. Food Hydrocoll. 2018, 81, 149–158. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.K.; Zhu, J.X.; Wang, T.; Wang, D.F.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.R.; Wei, Y.; Sun, C.X.; Mao, L.K.; Gao, Y.X. Fabrication of zein and rhamnolipid complex nanoparticles to enhance the stability and in vitro release of curcumin. Food Hydrocoll. 2018, 77, 617–628. [Google Scholar] [CrossRef]
- Wang, L.; Xue, J.; Zhang, Y. Preparation and characterization of curcumin loaded caseinate/zein nanocomposite film using pH-driven method. Ind. Crops Prod. 2019, 130, 71–80. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.L.; Chang, T.R.; You, Y.; Wang, X.D.; Wang, L.W.; Yuan, X.F.; Tan, M.H.; Wang, P.D.; Xu, P.W.; et al. Inclusion complexes of cannabidiol with beta-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Zhou, J.F.; Zheng, G.D.; Wang, W.J.; Yin, Z.P.; Chen, J.G.; Li, J.E.; Zhang, Q.F. Physicochemical properties and bioavailability comparison of two quercetin loading zein nanoparticles with outer shell of caseinate and chitosan. Food Hydrocoll. 2021, 120, 106959. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Dai, L.; Zhang, L.; Gao, Y.X. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020, 106, 105839. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 2: Stability and functionality. Food Hydrocoll. 2015, 49, 127–134. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, L.Y.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Weng, Q.X.; Cai, X.X.; Zhang, F.; Wang, S.Y. Fabrication of self-assembled Radix Pseudostellariae protein nanoparticles and the entrapment of curcumin. Food Chem. 2019, 274, 796–802. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condit.tion through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Chen, S.; Sun, C.X.; Wang, Y.Q.; Han, Y.H.; Dai, L.; Abliz, A.; Gao, Y.X. Quercetagetin-loaded composite nanoparticles based on zein and hyaluronic acid: Formation, characterization, and physicochemical stability. J. Agric. Food Chem. 2018, 66, 7441–7450. [Google Scholar] [CrossRef]
- Liu, Q.G.; Jing, Y.Q.; Han, C.P.; Zhang, H.; Tian, Y.M. Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Yuan, Y.K.; Li, H.; Zhu, J.X.; Liu, C.Z.; Sun, X.; Wang, D.F.; Xu, Y. Fabrication and characterization of zein nanoparticles by dextran sulfate coating as vehicles for delivery of curcumin. Int. J. Biol. Macromol. 2020, 151, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Sun, X.M.; Gao, F.; Wang, J.Q.; Wang, C.N. Systematical characterization of physiochemical and rheological properties of thermal-induced polymerized whey protein. J. Sci. Food Agric. 2019, 99, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Kosovic, E.; Sykora, D.; Kuchar, M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics 2021, 13, 412. [Google Scholar] [CrossRef]
- Fraguas-Sanchez, A.I.; Fernandez-Carballido, A.; Martin-Sabroso, C.; Torres-Suarez, A.I. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B 2020, 1150, 122188. [Google Scholar] [CrossRef]
- Xiao, J.; Nian, S.; Huang, Q.R. Assembly of kafirin/carboxymethyl chitosan nanoparticles to enhance the cellular uptake of curcumin. Food Hydrocoll. 2015, 51, 166–175. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.M.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar]
- Hlozek, T.; Uttl, L.; Kaderabek, L.; Balikova, M.; Lhotkova, E.; Horsley, R.R.; Novakova, P.; Sichova, K.; Stefkova, K.; Tyls, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC plus CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef]
- FDA. FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare (accessed on 31 July 2020).
- Sudhakar, S.; Chandran, S.V.; Selvamurugan, N.; Nazeer, R.A. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Biol. Macromol. 2020, 150, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, H.; Xu, C.M.; Gu, L.W. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Hebrard, G.; Hoffart, V.; Cardot, J.M.; Subirade, M.; Alric, M.; Beyssac, E. Investigation of coated whey protein/alginate beads as sustained release dosage form in simulated gastrointestinal environment. Drug Dev. Ind. Pharm. 2009, 35, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.T.; Nie, G.J.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y.L. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, J.; Sun, Y.; Freeman, K.; Mchenry, M.A.; Wang, C.; Guo, M. Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach. Foods 2022, 11, 376. https://doi.org/10.3390/foods11030376
Wang C, Wang J, Sun Y, Freeman K, Mchenry MA, Wang C, Guo M. Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach. Foods. 2022; 11(3):376. https://doi.org/10.3390/foods11030376
Chicago/Turabian StyleWang, Ce, Jia Wang, Yonghai Sun, Kalev Freeman, Monique Alyssa Mchenry, Cuina Wang, and Mingruo Guo. 2022. "Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach" Foods 11, no. 3: 376. https://doi.org/10.3390/foods11030376
APA StyleWang, C., Wang, J., Sun, Y., Freeman, K., Mchenry, M. A., Wang, C., & Guo, M. (2022). Enhanced Stability and Oral Bioavailability of Cannabidiol in Zein and Whey Protein Composite Nanoparticles by a Modified Anti-Solvent Approach. Foods, 11(3), 376. https://doi.org/10.3390/foods11030376






