Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications
Abstract
1. Introduction
2. HCs in Food Products
- Plant-based: tree cellulose; exudates such as gum Arabic, karaya, ghatti, and tragacanth; seeds such as guar, locust bean, tara, tamarind gum, or konjac mannan; tuber starches; and fruit pectin.
- Algal: brown seaweed alginate, red seaweed agar, and carrageenan.
- Microbial: curdlan, dextran, cellulose, xanthan, or gellan gum.
- Animal sourced: gelatin, caseinate, whey protein, and chitosan.
2.1. Starches
2.2. Nonconventional Hydrocolloids: Untapped Possibilities and Relevance
3. Technological Considerations
3.1. Hydrocolloid Extraction and Processing Technologies
3.1.1. Enzymatic Extraction
3.1.2. Ultrasound-Assisted Extraction
3.1.3. Microwave-Assisted Extraction
3.2. Minor Components’ and Other Elements’ Effects
- Formation of toxic compounds
- Generation of free radicals
- Maillard reactions
- Impairing of amylolytic enzymes
- Changes in protein crosslinking.
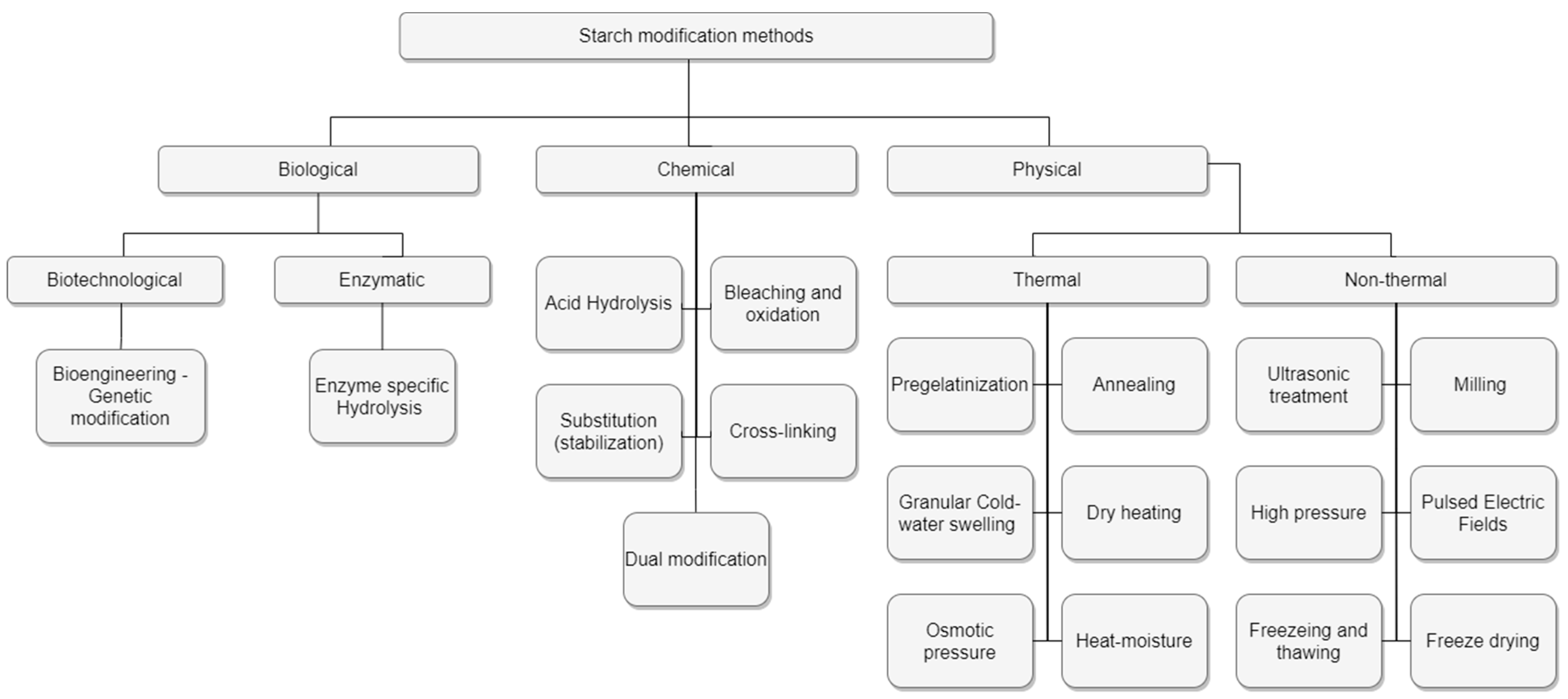
3.3. Hydrocolloid Modification
3.4. HC Interactions
4. Nutritional and Biofunctional Considerations
4.1. Bioactive Compound Protection
4.2. Bioactive Role of HCs
4.3. Special Considerations in Health Conditions
5. Future Considerations and Prospects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tadros, T. Food Rheology. In Encyclopedia of Colloid and Interface Science; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642206658. [Google Scholar]
- Baines, D.; Seal, R. Natural Food Additives, Ingredients and Flavourings; Woodhead Publishing Limited: Sawston, UK, 2012; ISBN 9788578110796. [Google Scholar]
- Laborde, D.; Martin, W.; Swinnen, J.; Vos, R. COVID-19 risks to global food security. Science 2020, 369, 500–502. [Google Scholar] [CrossRef] [PubMed]
- FAO. Enabling Environment Required to Promote Future Smart Food Production, Marketing and Consumption; Li, X., Siddique, K.H.M., Eds.; FAO: Rome, Italy, 2018; ISBN 9789251304952. [Google Scholar]
- Lairon, D. Biodiversity and Sustainable Nutrition with a Food-Based Approach; University Aix-Marseille: Marseille, France, 2012; ISBN 9789251073117. [Google Scholar]
- Benton, T.; Bieg, C.; Harwatt, H.; Pudassaini, R.; Wellesley, L. Food System Impacts on Biodiversity Loss Three Levers for Food; Chatham House: London, UK, 2021; ISBN 9783030023188. [Google Scholar]
- Narayanan, R.; Kumar, N.; Prajeesh, P. Bioresources for Food and Nutrition Security: The case of Wild Edibles of Western Ghats. In Bioresources and Bioprocess in Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 73–98. ISBN 9789811042843. [Google Scholar]
- Zhu, F. Underutilized and unconventional starches: Why should we care? Trends Food Sci. Technol. 2020, 100, 363–373. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids acting as emulsifying agents—How do they do it? Food Hydrocoll. 2018, 78, 2–14. [Google Scholar] [CrossRef]
- Razavi, S.M.A. Introduction to Emerging Natural Hydrocolloids; Wiley: Hoboken, NJ, USA, 2019; pp. 1–52. [Google Scholar] [CrossRef]
- Bisht, B.; Lohani, U.C.; Kumar, V.; Gururani, P.; Sinhmar, R. Edible hydrocolloids as sustainable substitute for non-biodegradable materials. Crit. Rev. Food Sci. Nutr. 2020, 1–33. [Google Scholar] [CrossRef]
- Fellow, P. Food Processing Technology: Principles and Practice, 4th ed.; Woodhead Publishing Limited: Duxford, UK, 2017; ISBN 9780081019078. [Google Scholar]
- Heertje, I. Structure and function of food products: A review. Food Struct. 2014, 1, 3–23. [Google Scholar] [CrossRef]
- Milani, J.M.; Golkar, A. Introductory Chapter: Some New Aspects of Colloidal Systems in Foods. In Some New Aspects of Colloidal Systems in Foods; InTechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Hesarinejad, M.A.; Shekarforoush, E.; Rezayian Attar, F.; Ghaderi, S. The dependency of rheological properties of Plantago lanceolata seed mucilage as a novel source of hydrocolloid on mono- and di-valent salts. Int. J. Biol. Macromol. 2020, 147, 1278–1284. [Google Scholar] [CrossRef]
- Kapoor, M.; Khandal, D.; Seshadri, G.; Aggarwal, S.; Khandal, R.K. Novel Hydrocolloids: Preparation & Applications—A Review. IJRRAS 2013, 16, 432–482. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Meyer, A.S. Chemistry, gelation, and enzymatic modification of seaweed food hydrocolloids. Trends Food Sci. Technol. 2021, 109, 608–621. [Google Scholar] [CrossRef]
- Hardy, Z.; Jideani, V.A. Foam-mat Drying Technology: A Review. Crit. Rev. Food Sci. Nutr. 2015, 57, 2560–2572. [Google Scholar] [CrossRef]
- Sangamithra, A.; Venkatachalam, S.; John, S.G.; Kuppuswamy, K. Foam Mat Drying of Food Materials: A Review. J. Food Process. Preserv. 2015, 39, 3165–3174. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9781845694142. [Google Scholar]
- Caballero, B.; Finglas, P.; Toldra, F. Encyclopedia of Food and Health, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780123849472. [Google Scholar]
- Orrego, C.E.; Salgado, N.; Botero, C. A Developments and trends in fruit bar production and characterization. Crit. Rev. Food Sci. Nutr. 2014, 54, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Guizani, N.; Al-Alawi, A.; Claereboudt, M.; Rahman, M.S. Instrumental texture profile analysis (TPA) of date fruits as a function of its physico-chemical properties. Ind. Crops Prod. 2013, 50, 866–873. [Google Scholar] [CrossRef]
- Vallejo, P.; Hernández, M.S.; Lares, M.; Herrera, A.; Fernández-Trujillo, J.P. Nutraceutical potential of fruit bars obtained from asaí (Euterpe precatoria) and copoazú (Theobroma grandiflorum). Acta Hortic. 2017, 1178, 135–142. [Google Scholar] [CrossRef]
- Balestra, F.; Petracci, M. Technofunctional Ingredients for Meat Products; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128148747. [Google Scholar]
- Gao, Z.; Fang, Y.; Cao, Y.; Liao, H.; Nishinari, K.; Phillips, G.O. Hydrocolloid-food component interactions. Food Hydrocoll. 2017, 68, 149–156. [Google Scholar] [CrossRef]
- Yemenicioğlu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A review of current and future food applications of natural hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1389–1406. [Google Scholar] [CrossRef]
- Andrieieva, S.; Dikhtyar, A.; Grinchenko, O.; Pyvovarov, Y.; Kolesnikova, M.; Omel’chenko, S.; Kotlyar, O. Development of technology of creams using hydrocolloids. EUREKA Life Sci. 2021, 34–42. [Google Scholar] [CrossRef]
- Vasile, F.E.; Judis, M.A.; Mazzobre, M.F. Prosopis alba exudate gum as novel excipient for fish oil encapsulation in polyelectrolyte bead system. Carbohydr. Polym. 2017, 166, 309–319. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Tollin, K.; Erz, A.; Vej, J. Developing New Functional Food and Nutraceutical Products; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128027806. [Google Scholar]
- Viebke, C.; Al-Assaf, S.; Phillips, G.O. Food hydrocolloids and health claims. Bioact. Carbohydr. Diet. Fibre 2014, 4, 101–114. [Google Scholar] [CrossRef]
- Goff, H.D.; Guo, Q. Chapter 1: The Role of Hydrocolloids in the Development of Food Structure. In Handbook of Food Structure Development; RSC Publishing: London, UK, 2019; pp. 1–28. ISBN 978-1-78801-615-5. [Google Scholar]
- Wüstenberg, T. Cellulose and Cellulose Derivatives in the Food Industry: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2015; ISBN 9783527324682. [Google Scholar]
- Jackson, C.; Clayden, S.; Reyes-Prieto, A. The Glaucophyta: The blue-green plants in a nutshell. Acta Soc. Bot. Pol. 2015, 84, 149–165. [Google Scholar] [CrossRef]
- Larbey, C.; Mentzer, S.M.; Ligouis, B.; Wurz, S.; Jones, M.K. Cooked starchy food in hearths ca. 120 kya and 65 kya (MIS 5e and MIS 4) from Klasies River Cave, South Africa. J. Hum. Evol. 2019, 131, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Zhang, L.; Bian, X.; Cao, Q.; Wei, C. A-, B- and C-type starch granules coexist in root tuber of sweet potato. Food Hydrocoll. 2020, 98, 105279. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2013, 4, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Tetlow, I.J.; Emes, M.J. Starch biosynthesis in the developing endosperms of grasses and cereals. Agronomy 2017, 7, 81. [Google Scholar] [CrossRef]
- Pfister, B.; Zeeman, S.C. Formation of starch in plant cells. Cell. Mol. Life Sci. 2016, 73, 2781–2807. [Google Scholar] [CrossRef]
- Corgneau, M.; Gaiani, C.; Petit, J.; Nikolova, Y.; Banon, S.; Ritié-Pertusa, L.; Le, D.T.L.; Scher, J. Digestibility of common native starches with reference to starch granule size, shape and surface features towards guidelines for starch-containing food products. Int. J. Food Sci. Technol. 2019, 54, 2132–2140. [Google Scholar] [CrossRef]
- Guillén, S.; Oria, R.; Salvador, M.L. Impact of Cooking Temperature on in Vitro Starch Digestibility of Rice Varieties with Different Amylose Contents. Pol. J. Food Nutr. Sci. 2018, 68, 319–325. [Google Scholar] [CrossRef]
- Patindol, J.A.; Siebenmorgen, T.J.; Wang, Y.J. Impact of environmental factors on rice starch structure: A review. Starch/Staerke 2015, 67, 42–54. [Google Scholar] [CrossRef]
- Sjöö, M.; Nilsson, L. Starch in Food: Structure, Function and Applications; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier Science: Amsterdam, The Netherlands, 2017; ISBN 9780081008966. [Google Scholar]
- Joye, I.J. Starch. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 256–264. ISBN 9780081005965. [Google Scholar]
- Schmiele, M.; Sampaio, U.M.; Pedrosa Silva Clerici, M.T. Basic Principles: Composition and Properties of Starch. In Starches for Food Application; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–22. ISBN 978-0-12-809440-2. [Google Scholar]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CYTA—J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Brockington, S.F.; Yang, Y.; Gandia-Herrero, F.; Covshoff, S.; Hibberd, J.M.; Sage, R.F.; Wong, G.K.S.; Moore, M.J.; Smith, S.A. Lineage-specific gene radiations underlie the evolution of novel betalain pigmentation in Caryophyllales. New Phytol. 2015, 207, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Castel, V.; Rubiolo, A.C.; Carrara, C.R. Droplet size distribution, rheological behavior and stability of corn oil emulsions stabilized by a novel hydrocolloid (Brea gum) compared with gum arabic. Food Hydrocoll. 2017, 63, 170–177. [Google Scholar] [CrossRef]
- Sznaider, F.; Rojas, A.M.; Stortz, C.A.; Navarro, D.A. Chemical structure and rheological studies of arabinoglucuronoxylans from the Cercidium praecox exudate brea gum. Carbohydr. Polym. 2020, 228, 115388. [Google Scholar] [CrossRef]
- Taziki Shams-abadi, S.; Razavi, S.M.A. Cress seed gum improves rheological, textural and physicochemical properties of native wheat starch-sucrose mixture. Int. J. Biol. Macromol. 2021, 181, 945–955. [Google Scholar] [CrossRef]
- Razmkhah, S.; Mohammadifar, M.A.; Razavi, S.M.A.; Ale, M.T. Purification of cress seed (Lepidium sativum) gum: Physicochemical characterization and functional properties. Carbohydr. Polym. 2016, 141, 166–174. [Google Scholar] [CrossRef]
- Razmkhah, S.; Razavi, S.M.A.; Mohammadifar, M.A. Dilute solution, flow behavior, thixotropy and viscoelastic characterization of cress seed (Lepidium sativum) gum fractions. Food Hydrocoll. 2017, 63, 404–413. [Google Scholar] [CrossRef]
- Hedayati, S.; Niakousari, M.; Babajafari, S.; Mohammad, S. Ultrasound-assisted extraction of mucilaginous seed hydrocolloids: Physicochemical properties and food applications. Trends Food Sci. Technol. 2021, 118, 356–361. [Google Scholar] [CrossRef]
- Li, N.; Qi, G.; Sun, X.S.; Wang, D. Characterization of gum isolated from Camelina seed. Ind. Crops Prod. 2016, 83, 268–274. [Google Scholar] [CrossRef]
- Cao, X.; Li, N.; Qi, G.; Sun, X.S.; Bean, S.R.; Tilley, M.; Aramouni, F.M.; Wang, D. Optimization of camelina gum isolation from bran and protein extraction using decortication. J. Agric. Food Res. 2021, 6, 100223. [Google Scholar] [CrossRef]
- Anvari, M.; Tabarsa, M.; Cao, R.; You, S.; Joyner Melito, H.S.; Behnam, S.; Rezaei, M. Compositional characterization and rheological properties of an anionic gum from Alyssum homolocarpum seeds. Food Hydrocoll. 2016, 52, 766–773. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, L.; Wu, A.M. Effective extraction of Arabidopsis adherent seed mucilage by ultrasonic treatment. Sci. Rep. 2017, 7, 40672. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Koocheki, A.; Shahidi, F. Physical modification of Lepidium perfoliatum seed gum using cold atmospheric-pressure plasma treatment. Food Hydrocoll. 2021, 120, 106902. [Google Scholar] [CrossRef]
- Koocheki, A.; Taherian, A.R.; Razavi, S.M.A.; Bostan, A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll. 2009, 23, 2369–2379. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Fahmideh-Rad, E. Fenugreek seed gum: Biological properties, chemical modifications, and structural analysis—A review. Int. J. Biol. Macromol. 2019, 138, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Zhu, L.W.; Zhang, W.M.; Sun, R.C. Characterization of galactomannan gum from fenugreek (trigonella foenum-graecum) seeds and its rheological properties. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 1145–1154. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Physicochemical, thermal & rheological characteristics of a novel mucilage from Chia seed (Salvia hispanica). In Gums and Stabilisers for the Food Industry 18: Hydrocolloid Functionality for Affordable and Sustainable Global Food Solutions; Royal Society of Chemistry: London, UK, 2016; pp. 65–75. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Cui, S.W.; Guo, Q.; Ding, H. Some physicochemical properties of sage (Salvia macrosiphon) seedgum. Food Hydrocoll. 2014, 35, 453–462. [Google Scholar] [CrossRef]
- Pérez-Orozco, J.P.; Sánchez-Herrera, L.M.; Ortiz-Basurto, R.I. Effect of concentration, temperature, pH, co-solutes on the rheological properties of Hyptis suaveolens L. mucilage dispersions. Food Hydrocoll. 2019, 87, 297–306. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Alfaro, J.; Vargas, R.; Pacheco, J.; Araya, J.J. Evaluation of mucilages isolated from seeds of hyptis suaveolens, salvia hispanica and linum usitatissimum as pharmaceutical excipients in solid dose and liquid formulations. J. Excipients Food Chem. 2018, 9, 67–79. [Google Scholar]
- Salehi, F.; Kashaninejad, M. Static rheological study of Ocimum basilicum seed gum. Int. J. Food Eng. 2015, 11, 97–103. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed gum solution functional properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.; Ahmad, A.S.; Yin, Y.Y.; Yahya, N.; Ibrahim, N. Extraction, purification and characterization of durian (Durio zibethinus) seed gum. Food Hydrocoll. 2007, 21, 273–279. [Google Scholar] [CrossRef]
- Pacheco-Aguirre, J.A.; Ruiz-Sánchez, E.; Chel-Guerrero, L.; Corzo-Ríos, L.J.; Pérez-Gutiérrez, A.; Reyes-Ramírez, A.; Ballina-Gómez, H.S. Characterization of Guazuma ulmifolia Lam. seed gum and its effect on the activity of Metarhizium anisopliae (Metschn.) Sorokin on Bemisia tabaci Genn. Mex. J. Biotechnol. 2020, 34–48. [Google Scholar] [CrossRef]
- Yu, L.; Yakubov, G.E.; Zeng, W.; Xing, X.; Stenson, J.; Bulone, V.; Stokes, J.R. Multi-layer mucilage of Plantago ovata seeds: Rheological differences arise from variations in arabinoxylan side chains. Carbohydr. Polym. 2017, 165, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Chen, H.H.; Lin, H.X.; Xie, M.Y.; Nie, S.P. Structural features of alkaline extracted polysaccharide from the seeds of plantago asiatica L. and its rheological properties. Molecules 2016, 21, 1181. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Tabatabaei Yazdi, F.; Shahidi, F.; Hesarinejad, M.A.; Mortazavi, S.A.; Mohebbi, M. Plantago major seed mucilage: Optimization of extraction and some physicochemical and rheological aspects. Carbohydr. Polym. 2017, 155, 68–77. [Google Scholar] [CrossRef]
- Dehghan Sekachaei, A.; Sadeghi Mahoonak, A.; Ghorbani, M.; Kashaninejad, M.; Maghsoudlou, Y. Optimization of ultrasound-assisted extraction of quince seed gum through response surface methodology. J. Agric. Sci. Technol. 2017, 19, 323–333. [Google Scholar]
- Amarioarei, G.; Lungu, M.; Ciovicǎ, S. Molar mass characteristics of cherry tree exudate gums of different seasons. Cellul. Chem. Technol. 2012, 46, 583–588. [Google Scholar]
- Nkenmogne Kamdem, I.E.; Saidou, C.; Ngassoum, M.B.; Ndjouenkeu, R. Synergistic interactions in dilute aqueous solutions between alginate and tropical vegetal hydrocolloids. Heliyon 2020, 6, 4348. [Google Scholar] [CrossRef]
- Nwokocha, L.M.; Williams, P.A. Hydrodynamic and rheological properties of Irvingia gabonensis gum. Carbohydr. Polym. 2014, 114, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Edima, H.; Biloa, D.; Abossolo, S.; Mbofung, C. Optimization of the Extraction of Gum from Beilschmieda obscura. Br. J. Appl. Sci. Technol. 2014, 4, 4337–4355. [Google Scholar] [CrossRef]
- Martínez, S.E.Q.; Fuentes, E.E.T.; Zapateiro, L.A.G. Food hydrocolloids from butternut squash (Cucurbita moschata) peel: Rheological properties and their use in Carica papaya Jam. ACS Omega 2021, 6, 12114–12123. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, J.; Wei, Y.; Huang, L.; Li, F.; Zhang, Y.; Li, Q. Physicochemical Properties and Antioxidant Activity of Pumpkin Polysaccharide (Cucurbita moschata Duchesne ex Poiret) Modified by Subcritical Water. Foods 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S. Challenges towards characterization and applications of a novel hydrocolloid: Persian gum. Curr. Opin. Colloid Interface Sci. 2017, 28, 37–45. [Google Scholar] [CrossRef]
- Neverova, N.A.; Medvedeva, E.N.; Levchuk, A.A.; Babkin, V.A. The Research of Gmelin Larch (Larix gmelinii (Rupr.) Rupr.) Gum. Russ. J. Bioorganic Chem. 2018, 44, 912–915. [Google Scholar] [CrossRef]
- Vasile, F.E.; Judis, M.A.; Mazzobre, M.F. Moisture sorption properties and glass transition temperature of a non-conventional exudate gum (Prosopis alba) from northeast Argentine. Food Res. Int. 2020, 131, 109033. [Google Scholar] [CrossRef]
- Puvanesvari Gannasin, S.; Yogeshini, R.; Noranizan, M.A.; Kharidah, M. Functional and Preliminary Characterisation of Hydrocolloid from Tamarillo (Solanum betaceum Cav.) Puree. Molecules 2012, 17, 6869–6885. [Google Scholar] [CrossRef]
- Aftab, K.; Hameed, S.; Umbreen, H.; Ali, S.; Rizwan, M.; Alkahtani, S.; Abdel-Daim, M.M. Physicochemical and Functional Potential of Hydrocolloids Extracted from Some Solanaceae Plants. J. Chem. 2020, 2020, 3945. [Google Scholar] [CrossRef]
- Han, X.; Zhang, L.; Niu, D.; Nan, S.; Miao, X.; Hu, X.; Li, C.; Fu, H. Transcriptome and co-expression network analysis reveal molecular mechanisms of mucilage formation during seed development in Artemisia sphaerocephala. Carbohydr. Polym. 2021, 251, 117044. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L.; Lan, X.; Chen, Y.; Lu, C. Achene Mucilage Formation Process and Extrusion from Hydrated Pericarp of Mirabilis himalaica. Hortic. Plant J. 2021, in press. [Google Scholar] [CrossRef]
- Seitter, M.; Fleig, M.; Schmidt, H.; Hertel, C. Effect of exopolysaccharides produced by Lactobacillus sanfranciscensis on the processing properties of wheat doughs. Eur. Food Res. Technol. 2020, 246, 461–469. [Google Scholar] [CrossRef]
- Jakob, F.; Gebrande, C.; Bichler, R.M.; Vogel, R.F. Insights into the pH-dependent, extracellular sucrose utilization and concomitant levan formation by Gluconobacter albidus TMW 2.1191. Antonie Van Leeuwenhoek 2020, 113, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Bayu, A.; Warsito, M.F.; Putra, M.Y.; Karnjanakom, S.; Guan, G. Macroalgae-derived rare sugars: Applications and catalytic synthesis. Carbon Resour. Convers. 2021, 4, 150–163. [Google Scholar] [CrossRef]
- Sterner, M.; Gröndahl, F. Extraction of laminarin from Saccharina latissima seaweed using cross-flow filtration. J. Appl. Phycol. 2021, 33, 1825–1844. [Google Scholar] [CrossRef]
- Amin, H.H. Ulvan as A New Trend in Agriculture, Food Processing and Medicine. Asian J. Fish. Aquat. Res. 2020, 47–54. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from Undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef]
- Vilpoux, O.F.; Brito, V.H.; Cereda, M.P. Starch Extracted from Corms, Roots, Rhizomes, and Tubers for Food Application; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128094402. [Google Scholar]
- Savary, S.; Akter, S.; Almekinders, C.; Harris, J.; Korsten, L.; Rötter, R.; Waddington, S.; Watson, D. Mapping disruption and resilience mechanisms in food systems. Food Secur. 2020, 12, 695–717. [Google Scholar] [CrossRef]
- Orjuela-Baquero, N.M.; Hernández, M.S.; Carrillo, M.; Fernández-Trujillo, J.P. Diversity of roots and tubers cultivated in traditional chagras from the Colombian Amazon. Acta Hortic. 2016, 1118, 95–102. [Google Scholar] [CrossRef]
- Padulosi, S.; Thompson, J.; Rudebjer, P. Fighting Poverty, Hunger and Malnutrition with Neglected and Underutilized Species: Needs, Challenges and the Way Forward; CGSpace: Boston, MA, USA, 2013; ISBN 9789290439417. [Google Scholar]
- Sunderland, T.C.H. Food security: Why is biodiversity important? Int. For. Rev. 2011, 13, 265–274. [Google Scholar] [CrossRef]
- Giannuzzi, L. Food security in the time of Coronavirus (COVID-19). The Argentine case. Int. J. Food Nutr. Res. 2020, 4, 38. [Google Scholar] [CrossRef]
- United Nations. Policy Brief: The Impact of COVID-19 on Food Security and Nutrition; United Nations: New York, NY, USA, 2020; pp. 1–8. [Google Scholar]
- Montenegro, M. Banking on Wild Relatives to Feed the World. Gastron. J. Crit. Food Stud. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Medina, M.; López, L.; Buc, N. Bone tool and tuber processing: A multi-proxy approach at Boyo Paso 2, Argentina. Antiquity 2018, 92, 1040–1055. [Google Scholar] [CrossRef]
- Keats, S.; Wiggins, S.; Compton, J.; Vigneri, M. Food Price Transmission: Rising International Cereals Prices and Domestic Markets; ODI: London, UK, 2010; pp. 2007–2010. [Google Scholar]
- Gifuni, I.; Olivieri, G.; Krauss, I.R.; D’Errico, G.; Pollio, A.; Marzocchella, A. Microalgae as new sources of starch: Isolation and characterization of microalgal starch granules. Chem. Eng. Trans. 2017, 57, 1423–1428. [Google Scholar] [CrossRef]
- Putri, F.; Silalahi, M.D.S.; Rinanti, A. Starch producing in microalga biomass as a raw material for bioethanol. MATEC Web Conf. 2018, 197, 13018. [Google Scholar] [CrossRef]
- González-Reyes, E.; Méndez-Montealvo, G.; Solorza-Feria, J.; Toro-Vazquez, J.F.; Bello-Pérez, L.A. Rheological and thermal characterization of Okenia hypogaea (Schlech. & Cham.) starch. Carbohydr. Polym. 2003, 52, 297–310. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Juszczak, L.; Ziobro, R. Grass pea (Lathyrus sativus L.) starch as an alternative for cereal starches: Rheological properties and retrogradation susceptibility. J. Food Eng. 2008, 88, 528–534. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Pacheco-Vargas, G. Pulp and peel of unripe stenospermocarpic mango (Mangifera indica L. cv Ataulfo) as an alternative source of starch, polyphenols and dietary fibre. Food Res. Int. 2020, 138, 719. [Google Scholar] [CrossRef]
- Kringel, D.H.; Dias, A.R.G.; da Rosa Zavareze, E.; Gandra, E.A. Fruit Wastes as Promising Sources of Starch: Extraction, Properties, and Applications. Starch/Staerke 2020, 72, 200. [Google Scholar] [CrossRef]
- Sandoval Sandoval, J.L.; Fonseca Rodríguez, P.E.; Herrera Arévalo, A.O.; Pérez Sira, E.E.; Ricci, J.; Dufour, D. Development and Characterization of Edible Films from Chachafruto (Erythrina edulis Triana) Starch. Starch/Staerke 2021, 73, 269. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef] [PubMed]
- Bello-Pérez, L.A.; García-Suárez, F.J.; Méndez-Montealvo, G.; Do Nascimento, J.R.O.; Lajolo, F.M.; Cordenunsi, B.R. Isolation and characterization of starch from seeds of Araucaria brasiliensis: A novel starch for application in food industry. Starch/Staerke 2006, 58, 283–291. [Google Scholar] [CrossRef]
- Conforti, P.A.; Lupano, C.E. Starch characterisation of Araucaria angustifolia and Araucaria araucana Seeds. Starch/Staerke 2007, 59, 284–289. [Google Scholar] [CrossRef]
- Bellido-Valencia, O.; Huanca-Zúñiga, P.K.; Medina-Marroquín, L.A. Determination of the Morphology of the Starch Granules and the Optimum Internal Cooking Temperature of Four Andean Crops: Oca (Oxalis tuberosa Molina), Olluco (Ullucus tuberosus Loz), Isaño (Tropaeolum tuberosum Ruiz & Pavon) and Arracacha (Arracacia xant. Acta Univ. Cibiniensis. Ser. E Food Technol. 2017, 21, 33–42. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Rondán-Sanabria, G.G.; Finardi-Filho, F. The physical, chemical and functional characterization of starches from andean tubers: Oca (Oxalis tuberosa molina), olluco (Ullucus tuberosus caldas) and mashua (Tropaeolum tuberosum ruiz & pavón). Braz. J. Pharm. Sci. 2013, 49, 453–464. [Google Scholar] [CrossRef]
- Castanha, N.; Villar, J.; da Matta Junior, M.D.; Prudente dos Anjos, C.B.; Duarte Augusto, P.E. Structure and properties of starches from Arracacha (Arracacia xanthorriza) roots. Int. J. Biol. Macromol. 2018, 117, 1029–1038. [Google Scholar]
- Hernández-Medina, M.; Torruco-Uco, J.G.; Chel-Guerrero, L.; Betancur-Ancona, D. Caracterización fisicoquímica de almidones de tubérculos cultivados en Yucatán, México. Ciência Tecnol. Aliment. 2008, 28, 718–726. [Google Scholar] [CrossRef]
- Moorthy, S.N. Physicochemical and functional properties of tropical tuber starches: A review. Starch/Staerke 2002, 54, 559–592. [Google Scholar] [CrossRef]
- Rondán-Sanabria, G.G.; Finardi-Filho, F. Physical-chemical and functional properties of maca root starch (Lepidium meyenii Walpers). Food Chem. 2009, 114, 492–498. [Google Scholar] [CrossRef]
- Yaruro Cáceres, N.C.; Suarez Mahecha, H.; de Francisco, A.; Vásquez Mejia, S.M.; Diaz Moreno, C. Physicochemical, thermal, microstructural and paste properties comparison of four achira (Canna edulis sp.) starch ecotypes. Int. J. Gastron. Food Sci. 2021, 25, 380. [Google Scholar] [CrossRef]
- Zhang, T.; Li, K.; Ding, X.; Sui, Z.; Yang, Q.Q.; Shah, N.P.; Liu, G.; Corke, H. Starch properties of high and low amylose proso millet (Panicum miliaceum L.) genotypes are differentially affected by varying salt and pH. Food Chem. 2021, 337, 127784. [Google Scholar] [CrossRef] [PubMed]
- Montoya-López, J.; Quintero-Castaño, V.D.; Lucas-Aguirre, J.C. Characterization of starch and flour of gros michel banana fruit (Musa acuminata AAA). Acta Agron. 2015, 64, 12–22. [Google Scholar]
- Limbe, H.W.; Achmadi, S.S.; Faridah, D.N. Introducing Daluga (Cyrtosperma merkusii) starch from corms collected in Siau Island, North Sulawesi. IOP Conf. Ser. Earth Environ. Sci. 2019, 399, 012038. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, L.; Zhang, L.; Xu, B.; Wei, C. A new allomorph distribution of C-type starch from root tuber of Apios fortunei. Food Hydrocoll. 2017, 66, 334–342. [Google Scholar] [CrossRef]
- López, O.V.; Viña, S.Z.; Pachas, A.N.A.; Sisterna, M.N.; Rohatsch, P.H.; Mugridge, A.; Fassola, H.E.; García, M.A. Composition and food properties of Pachyrhizus ahipa roots and starch. Int. J. Food Sci. Technol. 2010, 45, 223–233. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Xu, F.; Zhu, K.; Huang, C. A novel underutilized starch resource—Lucuma nervosa A.DC seed and fruit. Food Hydrocoll. 2021, 120, 106934. [Google Scholar] [CrossRef]
- Ran, L.; Luo, J.; Wang, Y.; Zou, J.; Yao, H.; Zhang, R.; Chen, X.; Xiong, F. Structural and Physicochemical Properties of Starch Isolated from the Rhizome of Drynaria roosii: A Novel Source. Starch/Staerke 2021, 73, 19. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, M.; Kong, X.; Wu, D.; Zheng, Z.; Shu, X. A novel starch: Characterizations of starches separated from tea (Camellia sinensis (L.) O. Ktze) seed. Int. J. Biol. Macromol. 2019, 139, 1085–1091. [Google Scholar] [CrossRef]
- Thakur, Y.; Thory, R.; Sandhu, K.S.; Kaur, M.; Sinhmar, A.; Pathera, A.K. Effect of selected physical and chemical modifications on physicochemical, pasting, and morphological properties of underutilized starch from rice bean (Vigna umbellata). J. Food Sci. Technol. 2021, 58, 4785–4794. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, S.O.; Shin, M. Properties of novel starch isolated from Castanopsis cuspidate fruit grown in a subtropical zone of Korea. Food Sci. Biotechnol. 2010, 19, 63–68. [Google Scholar] [CrossRef]
- Zabot, G.L.; Silva, E.K.; Emerick, L.B.; Felisberto, M.H.F.; Clerici, M.T.P.S.; Meireles, M.A.A. Physicochemical, morphological, thermal and pasting properties of a novel native starch obtained from annatto seeds. Food Hydrocoll. 2019, 89, 321–329. [Google Scholar] [CrossRef]
- Sivamanib, S.; Archanaa, K.; Santhosh, R.; Sivarajasekar, N.; Prasad, N. Synthesis and characterization of starch nanoparticles from cassava Peel. J. Bioresour. Bioprod. 2018, 3, 161–165. [Google Scholar] [CrossRef]
- Hasanin, M.S. Simple, Economic, Ecofriendly Method to Extract Starch Nanoparticles from Potato Peel Waste for Biological Applications. Starch/Staerke 2021, 73, 55. [Google Scholar] [CrossRef]
- Makroo, H.A.; Naqash, S.; Saxena, J.; Sharma, S.; Majid, D.; Dar, B.N. Recovery and characteristics of starches from unconventional sources and their potential applications: A review. Appl. Food Res. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Li, H.; Gidley, M.; Dhital, S. High-Amylose Starches to Bridge the “Fiber Gap”: Development, Structure, and Nutritional Functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Lammerts van Bueren, E.T.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant Sci. 2017, 22, 842–856. [Google Scholar] [CrossRef]
- Altay, F.; Gunasekaran, S. Influence of drying temperature, water content, and heating rate on gelatinization of corn starches. J. Agric. Food Chem. 2006, 54, 4235–4245. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Flores-Silva, P.C.; Bello-Perez, L.A. Cereal Starch Production for Food Applications. In Starches for Food Application; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 71–102. ISBN 9780128094402. [Google Scholar]
- Vieira da Silva, B.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Oliveira Bernardo, C.; Ramírez Ascheri, J.L.; Hidalgo Chávez, D.W.; Piler Carvalho, C.W. Ultrasound Assisted Extraction of Yam (Dioscorea bulbífera) Starch: Effect on Morphology and Functional Properties. Starch/Staerke 2018, 70, 185. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, Z.; Kolakowska, A. Chemical, Biological, and Functional Aspects of Food Lipids; Sikorski, Z., Kolakowska, A., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2011; Volume 2, ISBN 9788578110796. [Google Scholar]
- Nazarian-Firouzabadi, F.; Visser, R.G.F. Potato starch synthases: Functions and relationships. Biochem. Biophys. Rep. 2017, 10, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in chemical modifications of starches and their applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef]
- Gul, K.; Gan, R.; Sun, C.; Jiao, G.; Wu, D.; Li, H.; Kenaan, A.; Corke, H.; Fang, Y. Recent advances in the structure, synthesis, and applications of natural polymeric hydrogels. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Skrzypczak, P.; Gondek, E. Modification of kappa carrageenan by β-galactosidase as a new method to inhibit recrystallization of ice. Food Hydrocoll. 2016, 61, 31–35. [Google Scholar] [CrossRef]
- Averous, L.R.; Halley, P.J. From Genetic Engineering to Green Applications; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444537300. [Google Scholar]
- Giraldo-Gómez, G.I.; Rodríguez-Barona, S.; Sanabria-González, N.R. Preparation of instant green banana flour powders by an extrusion process. Powder Technol. 2019, 353, 437–443. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Eskandari, M.H.; Van der Meeren, P.; Hosseini, S.M.H. Study on hydrophobic modification of basil seed gum-based (BSG) films by octenyl succinate anhydride (OSA). Carbohydr. Polym. 2019, 219, 155–161. [Google Scholar] [CrossRef]
- Tang, H.; Gao, S.; Li, Y.; Dong, S. Modification mechanism of sesbania gum, and preparation, property, adsorption of dialdehyde cross-linked sesbania gum. Carbohydr. Polym. 2016, 149, 151–162. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A review: Interaction of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Bandral, J.D.; Gani, A.; Shams, R. Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. Int. J. Biol. Macromol. 2020, 165, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Lai, P.; Chen, I.H.; Liu, Y.F.; Wang, C.C.R. Effects of mucilage on the thermal and pasting properties of yam, taro, and sweet potato starches. LWT—Food Sci. Technol. 2010, 43, 849–855. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, K.X.; Lim, S.T.; Zhang, C.Q.; Wang, S.Y.; Liu, Q.Q.; Qian, J.Y. Morphology, pasting, and structural characteristics of potato starch/xanthan gum blend by critical melting and freeze-thawing treatment. Food Hydrocoll. 2021, 121, 107035. [Google Scholar] [CrossRef]
- Zhang, C.; Lim, S.T. Physical modification of various starches by partial gelatinization and freeze-thawing with xanthan gum. Food Hydrocoll. 2021, 111, 106210. [Google Scholar] [CrossRef]
- Miao, W.B.; Ning, Y.Y.; Huang, H.R.; Liu, H.M.; Cai, X.S.; De Wang, X. Effect of dry heat modification and the addition of Chinese quince seed gum on the physicochemical properties and structure of tigernut tuber starch. Arab. J. Chem. 2021, 14, 103407. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Wang, R.; Li, J.; Pan, W.; Zhang, X.; Xiao, W.; Wen, H.; Xie, J. Chestnut starch modification with dry heat treatment and addition of xanthan gum: Gelatinization, structural and functional properties. Food Hydrocoll. 2022, 124, 107205. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- McClements, D.J. Food hydrocolloids: Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Romero-Bastida, C.A.; Bello-Pérez, L.A.; García, M.A.; Martino, M.N.; Solorza-Feria, J.; Zaritzky, N.E. Physicochemical and microstructural characterization of films prepared by thermal and cold gelatinization from non-conventional sources of starches. Carbohydr. Polym. 2005, 60, 235–244. [Google Scholar] [CrossRef]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Beheshti, P.; Assadpoor, E. Rheological behavior and stability of d-limonene emulsions made by a novel hydrocolloid (Angum gum) compared with Arabic gum. J. Food Eng. 2012, 109, 1–8. [Google Scholar] [CrossRef]
- Sharma, D.R.; Kumar, S.; Kumar, V.; Thakur, A. Comprehensive review on nutraceutical significance of phytochemicals as functional food ingredients for human health management. J. Pharmacogn. Phytochem. 2019, 8, 385–395. [Google Scholar] [CrossRef]
- Malumba, P.; Doran, L.; Zanmenou, W.; Odjo, S.; Katanga, J.; Blecker, C.; Béra, F. Morphological, structural and functional properties of starch granules extracted from the tubers and seeds of Sphenostylis stenocarpa. Carbohydr. Polym. 2017, 178, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dhital, S.; Fu, X.; Huang, Q.; Liu, R.; Zhang, B.; He, X. Starch digestion in intact pulse cotyledon cells depends on the extent of thermal treatment. Food Chem. 2020, 315, 126268. [Google Scholar] [CrossRef]
- Varela, P.; Fiszman, S.M. Exploring consumers’ knowledge and perceptions of hydrocolloids used as food additives and ingredients. Food Hydrocoll. 2013, 30, 477–484. [Google Scholar] [CrossRef]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- Horstmann, S.; Lynch, K.; Arendt, E. Starch Characteristics Linked to Gluten-Free Products. Foods 2017, 6, 29. [Google Scholar] [CrossRef]
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2014, 53, 46–61. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Modrackova, N.; Makovska, M.; Mekadim, C.; Vlkova, E.; Tejnecky, V.; Bolechova, P.; Bunesova, V. Prebiotic potential of natural gums and starch for bifidobacteria of variable origins. Bioact. Carbohydrates Diet. Fibre 2019, 20, 100199. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Lu, W.; Nishinari, K.; Matsukawa, S.; Fang, Y. The future trends of food hydrocolloids. Food Hydrocoll. 2020, 103, 105713. [Google Scholar] [CrossRef]
- Pant, A.; Lee, A.Y.; Karyappa, R.; Lee, C.P.; An, J.; Hashimoto, M.; Tan, U.X.; Wong, G.; Chua, C.K.; Zhang, Y. 3D food printing of fresh vegetables using food hydrocolloids for dysphagic patients. Food Hydrocoll. 2021, 114, 106546. [Google Scholar] [CrossRef]
- Loh, H.C.; Seah, Y.K.; Looi, I. The COVID-19 Pandemic and Diet Change. Prog. Microbes Mol. Biol. 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Smag for livet—Nordean Fonden Taste for Life Center. Available online: https://www.smagforlivet.dk/materialer/videnskabelige-og-populærvidenskabelige-artikler-fra-smag-livet (accessed on 21 November 2021).
- Almeida, E.L.; Marangoni, A.L.; Steel, C.J. Starches from non—conventional sources to improve the technological characteristics of pound cake. Ciência Rural 2013, 43, 2101–2108. [Google Scholar] [CrossRef]
- Rebelo Gomes, L.; Simões, C.D.; Silva, C. Demystifying thickener classes food additives though molecular gastronomy. Int. J. Gastron. Food Sci. 2020, 22, 262. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Hirashima, M. More Cooking Innovations. Novel Hydrocolloids for Special Dishes; CRC Press Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2019; ISBN 9781138084094. [Google Scholar]
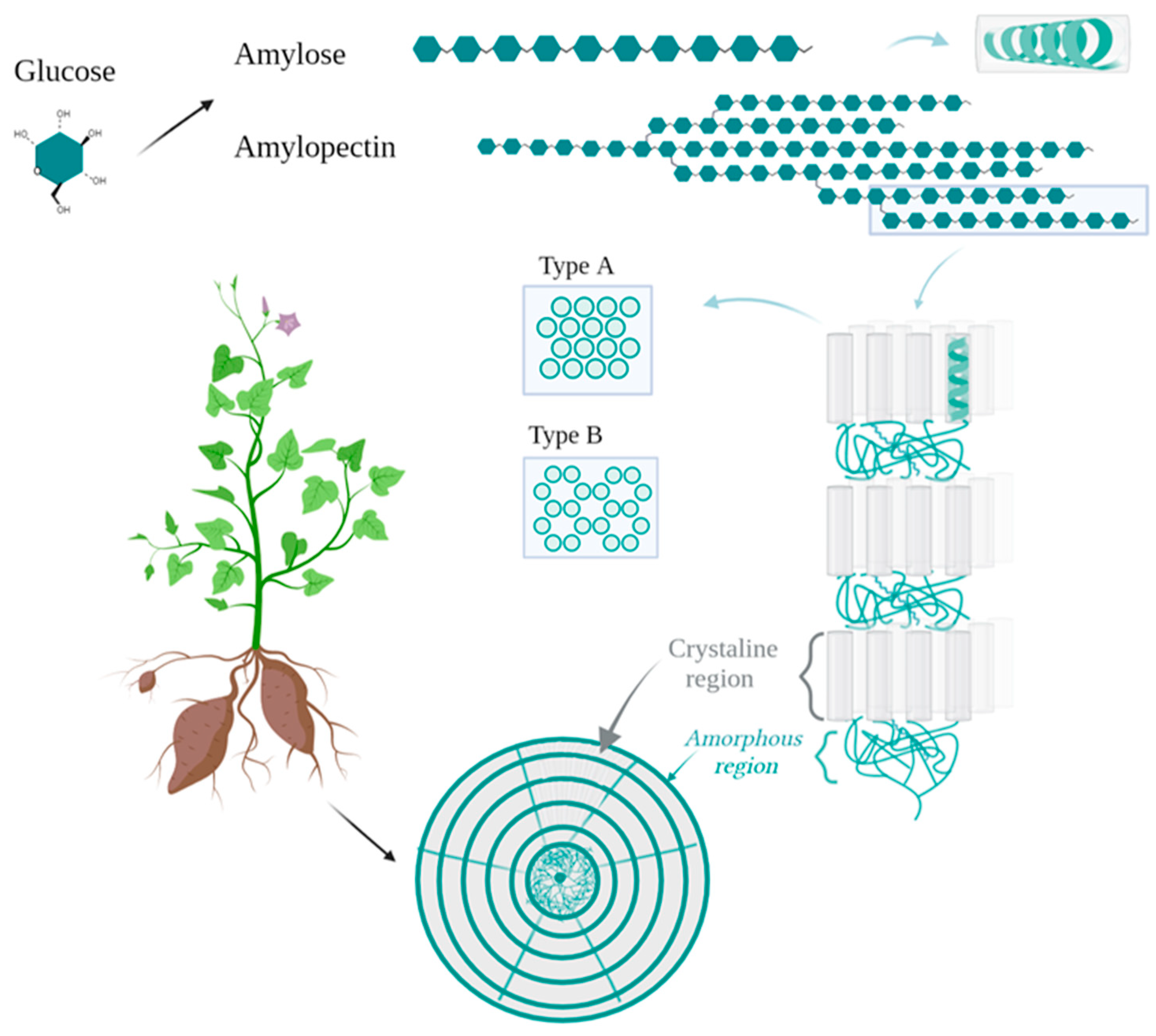
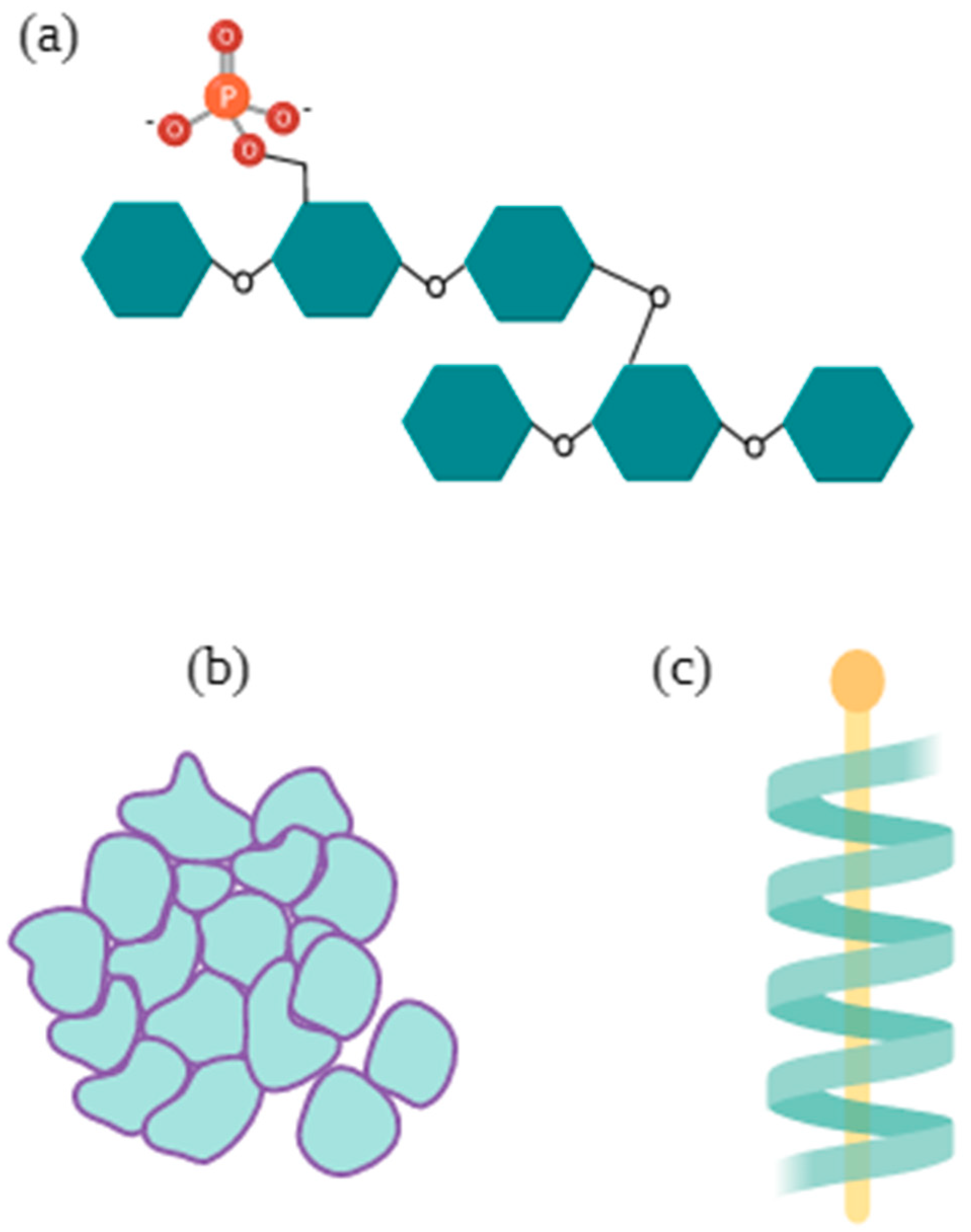
| Botanical Family | Species | Common Name 1 | Plant Structure | Extraction Yield | Molecular Weight | Composition | Rheological Behavior | Additional Remarks | References |
|---|---|---|---|---|---|---|---|---|---|
| Fabaceae | Cercidium praecox | Brea | seeds | 76% | 122 kDa | 75% carbohydrates, 9% proteins, 2.6% acetyl, 17% uronic acids | Pseudoplastic behavior with viscosity ≈ 110 Pa·s | Brea gum decreases corn oil droplets and polydispersity in emulsions, while increasing apparent viscosity and stability in a better way than gum arabic. | [51,52] |
| Brassicaceae | Lepidium sativum | Garden cress | 8.97 ± 0.12% | 1090 ± 8 kDa | Predominant monosaccharides: rhamnose 11.87 ± 0.41, arabinose 11.02 ± 0.16, xylose 9.06 ± 0.18 | “Non-Newtonian behavior with complex viscosity (At ʄ = 1 Hz) = 3.078 ± 0.015 Pa·s | Cress seed gum reduces native wheat starch gel retrogradation, gel hardness and syneresis. | [53,54,55] | |
| Brassicaceae | Camelina spp. | Camelina | 19.08% | 75.1% polysaccharides and 12.3% protein | Newtonian flow behavior at low shear rate (less than 0.011 s−1) with a viscosity of 62.80 Pa·s, with a concentration of 1%, it reaches 350 Pa·s, higher than k-carrageenan 100 Pa·s and HEC 80 Pa·s | Camelina gum serves as a food stabilizer, emulsifier, and gelling agent with higher viscosity, storage, and viscous moduli than conventional components. | [56,57,58] | ||
| Alyssum homolocarpum | Qodume Shirazi | 9.87% | 122.5 × 106 g mol−1 | Anionic rhamnogalactan consisting of 61% carbohydrates, 17.9% proteins, and 10.9% uronic acids | Pseudoplastic behavior with zero shear viscosity = 19.24 Pa· s and infinite shear viscosity of 0.0013 Pa· s when the concentration is 1% tested at 25 °C. | The multiple negatively charged carboxyl groups on A. homolocarpum gum, along with its the low sensitivity temperature changes in dispersions, makes it into a useful stabilizer for foods requiring thermal processing. | [56,59] | ||
| Arabidpsis thaliana | Mouse ear cress | Increased up to 6-fold in ultrasonic assisted extraction versus chemical agents. | Primarily rhamnogalacturonan I, besides of cellulose, galactan, xylan, arabinan, and homogalacturonan | Arabidopsis mucilage comprises a water-soluble, nonadherent external layer, and an internal adherent one with similar sugars to commercial HCs, increasing its potential as a food thickener | [56,60] | ||||
| Lepidium perfolatum | Clasping pepperweed | 17.63% | 200 g/mol | Protein content of 2.84%, color hue angle coordinate 60.5°, and emulsion stability of 88.96% | Non-Newtonian behavior with viscosity = 463.07 mPa·s and peak at 519.8 mPa when extracted at 45 °C for 1.5 h at pH 8. | L. perfolatum shows great potential for commercial competitiveness, as its extraction yield is greater than other nonconventional gums such as yanan, flaxseed, malva nut, mesquite seed, fenugreek, or Opuntia mucilage | [56,61,62] | ||
| Fabaceae | Trigonella foenum-graecum | Fenugreek | Up to 18.54% when ultrasound assistance is performed | 3.23 × 105 g mol−1 | Galactomannan (73.6%) with 5.5% protein, 0.5% ash, and 10.2% moisture on regular conditions, and 85.89% carbohydrate, 0.85% (db) protein, 5.35% (db) ash, and 7.03% (wb) moisture when extracted with ultrasound aid. | Pseudoplastic behavior with viscosity ≈ 286 mPa·s (30 °C, 170 s−1) up to 8.5414 Pa·s (at 1%) | Fenugreek in food formulations is known for its attractive flavor and color, and the gum a uniform, smooth surface that could be of great use in edible films | [56,63,64] | |
| Lamiaceae | Salvia hispanica | Chía | 10.90% | 800–2000 kDa | Anionic heteropolysaccharide, whose defatted seed gum composition was 10.90% lipids, 18.99% fiber, 33.26% protein, 8.28% ash and 8.95% water, while regular chia gum was 26.24% lipids, 28.96% fiber, 25.07% protein, 5.48% ash, and 9.32% moisture | Non-Newtonian behavior with an intrinsic viscosity ∼ 16 dLg−1 | Due to its dietary fiber, flavor-retaining fat contents, and hemicellulose absence, chia gum could play an important role in food processing as a thickener for sensory improvement | [56,65,66] | |
| Salvia macrosiphon | Marmareshk | 7.04–12.20% | ≈4 × 105 Da | Galactomannan with a 1.78–1.93:1 mannose to galactose ratio, with a composition of 69.01% carbohydrates, 2.08% protein, 11.24% moisture, 9.20% ash, and 30.2% uronic acids | High extraction yield in respect to other gums makes it an appealing new HC, of similar composition, conformation and rheology to conventional guar and xanthan gums, useful in food and pharmaceutical applications | [56,67] | |||
| Hyptis suaveolens | Chan–pignut | 3.4 ± 0.4% | Total polysaccharides in mucilage = 220 kDa | Non-Newtonian behavior of zero-shear rate viscosity at 0.75% concentration of 1139.79 ± 81.11 Pa·s | Dispersions of 0.25% or greater concentrations show an intermediate behavior between a weak and elastic gel. Interactions of the H. suaveolens mucilage with NaCl and sucrose on a pseudoplastic behavior show possible sweet and salty food applications, as a stabilizing, thickening, and gelling agent | [56,68,69] | |||
| Ocimum basilicum | Basil | 11.46–15.42% | Crude gum had 7.39% moisture, 2.01% protein, 11.55% lipids, 5.89% ash, and 74.19% carbohydrates, while purified gum presented 5.79% moisture, 1.56% protein, 9.71% lipids, 3.32% ash, and 79.62% carbohydrates | Non-Newtonian fluid of 0.230 ± 0.023 Pa·s viscosity under Herschel–Bulkley model | Seeds geographical origin influenced extraction yield of the gum | [56,62,70] | |||
| Linaceae | Linum usitatissimum | Flaxseed | 10.97–12.73% | Commercial flax gums report composition of up to 41% lipids, 28% total dietary fiber, 20% protein, 7.7% moisture, and 3.4% ash | Viscosities of gums extracted at 70 °C and 98 °C were 96.7 vs. 78.8 mPa·s, respectively | Extraction conditions show a higher yield with improved emulsification properties and enhanced emulsion stability when higher temperatures where used, but color is a hindrance of the conditions. | [56,71] | ||
| Malvaceae | Durio zibethinus | Durian | 18% for crude seed gum (light brown), 1.2% for air-dried pure gum and 0.5% for freeze-dried | Pure gum consisted of 17.9% moisture, 29.8% ash, with presence of L-rhamnose, glucose and D-galactose in a 3:9:1 ratio. | Durian gum had a viscosity 65 mPa·s at 1% concentrations, with 29.8°C temperatures at neutrality, but increased slightly with pH from 9 to 10 | Although viscosity of the gum was higher at neutral pH, and 1% dispersions have a similar pH to guar gum, durian gums showed a fair stability along a pH range from 2 to 10. A highlight in the gum’s composition is its high zinc content in comparison to other HCs | [56,72] | ||
| Guazuma ulmifolia | Mutamba or bay cedar | 67.1 g/kg of seeds | G. ulmifolia gum consisted of 87.19% total carbohydrates, 10.47% ash, 7.10% fat, 2.93% crude fiber, and 0.89% protein. | Non-Newtonian behavior with a consistency index of 5.11 ± 0.28 Pa·s at 25 °C with concentrations of 1% of G. ulmifolia gum | The gum shows good water dispersion and good stability at high temperatures, and is a good carrier and adhesive agent for biological control of pests. | [56,73] | |||
| Plantaginaceae | Plantago ovate | Isfarzeh or psyllium | 4.5–9.2% depending on water temperature for extraction and KOH presence | 950–1100 kDa | Psyllium gum and its different fractions predominantly consisted of xylose, (58.2–73.7 mol %), rhamnose (15.1 mol %), arabinose (12.3–24.2 mol %), galactose (3.7–3.8 mol %), and galacturonic acid (0.4–9.7 mol %) | Non-Newtonian behavior with intrinsic viscosity of 3.1–7.4 dL/g in different fractions obtained by higher temperatures or KOH aids. | Secretes three layers of fluid mucilage with similar molecular weight and composition; besides having different possible applications due to water solubility, offers possibilities for biomimetic engineering. | [56,74] | |
| Plantago asiatica | Chinese plantain | 13.90% | 3.8 × 10−6 kDa | P. asiatica gum consisted of 82.84% sugar, 0.68% protein, and 20.50% uronic acid and had a monosaccharide composition in a molar ratio of 63.95 Xyl, 15.41 Ara, 2.58 Gal, 1.29 Glc, and 1.00 Rha. | Intrinsic viscosity of the polysaccharide was 5.81 dL/g | Acidic arabinoxylan obtained from P. asiatica has a pseudoplastic behavior of weak gelling property that improved with sodium and calcium ions. | [56,75] | ||
| Plantago major | Barhang or greater plantain | 15.18% | 1.2 × 106 Da. | P. major gum was composed of 82.85% carbohydrates, 6.80% ash, 6.66% protein, 3.69% moisture, and trace amounts of fat. | Intrinsic viscosity in deionized water at 25 °C was 14.24 ± 0.61 dL/g | In addition to being a high-yield, economically interesting gum for stabilizing foods, P. major showed appealing bioactive characteristics in terms of total phenol contents, total flavonoid content, and antioxidant activity in amounts of 76.79 mg GAE/g dry, 97.8 mg g−1 and 915.54 g/mL, respectively | [56,76] | ||
| Rosaceae | Cydonia oblonga | Quince | 16.29% | C. oblonga gum consisted of 71.6% carbohydrates, 12.59% ash, 3.16% fat, 2.81% protein, and 9.84% moisture. | Apparent viscosity of the gum was approximately 52.4 mPa·s | Ultrasound-assisted extraction significantly increases purity, extraction yield, and viscosity of C. oblonga gums | [56,77] | ||
| Prunus cerarus | Dwarf or sour cherry | Exudate | 0.56 × 105 g/mol | Arabinogalactan including arabinose, xylose, galactose, glucopyranosyluronic acid, rhamnose, and mannose. | Intrinsic viscosity of P. cerasus and related P. avium were 3.84 and 7.08 dl/g, respectively | Harvest season and species strongly affect molar mass distribution and polydispersity of Prunus gums, but similar magnitudes of molecular weight to arabic, karaya, and tragacanth exudate gums suggests a possible industrial application. | [56,78] | ||
| Annonaceae | Annona crassiflora | Araticum | peel | [56] | |||||
| Irvingiaceae | Irvingia gabonensis | African mango | seeds | 79% mass recovery | 1.5 × 106 g/mol | Polysaccharide prevailing fraction made by 61.72% galactose, 18.8% arabinose, 8.7% rhamnose, 9.1% galacturonic acid, 1.1% glucose, and 0.5% glucuronic acid. | Intrinsic viscosity at infinite ionic strength was 4.9 dL/g | The arabinogalactan contains a small portion of neutral sugars and uronic acids that give rise to polyelectric properties that confer stabilizing properties to the gum. | [79,80] |
| Lauraceae | Beilschmiedia obscura | Khan | 6.60% | Apparent viscosity ≈ 0.70 ± 0.06 dL/g | [79,81] | ||||
| Malvaceae | Triumfetta cordifolia | Nkui | Bark | Apparent viscosity ≈ 18.29 ± 0.64 dL/g | [79] | ||||
| Corchorus olitorius | Lalo–nkeling nkeling | leaves | Apparent viscosity ≈ 2.11 ± 0.39 dL/g | ||||||
| Adansonia digitata | Baobab–bocco | Apparent viscosity ≈ 1.64 ± 0.06 dL/g | |||||||
| Pedaliaceae | Ceratotheca sesamoides | Gougdo or false sesame | Apparent viscosity ≈ 3.04 ± 0.39 dL/g | ||||||
| Phyllantaceae | Bridelia thermifolia | Kelly | bark | Apparent viscosity ≈ 2.47 ± 0.17 dL/g | |||||
| Cucurbitaceae | Cucurbita moschata | Butternut squash | peel | 10% | 26–96 kDa prevailing higher molecular weight of C. moschata pectin | Predominant composition in neutral sugars as: glucose, rhamnose, galactose, arabinose, and mannose | Gum’s intrinsic viscosity from 0.085 to 0.027 L/g when temperature rises, and pectin around 4.5 dL/g | [82,83] | |
| Rosaceae | Prunus spp. (P. scoparia, P. dulcis, P. armeniaca, P. spinosa, P. caroliniana, P. laurocerasus, P. virginiana, P. persica) | Persian gum (from wild almond), cherries, plums, peaches. | Exudates | [84] | |||||
| Pinaceae | Larix gmelinii | Gmelin Larch wood | exudates | P. alba gum was useful as a polyelectrolyte stabilizing alginate–calcium–chitosan beads, as a fish oil excipient. | [30,85] | ||||
| Fabaceae | Prosopis alba | White carob | exudate | [86] | |||||
| Solanaceae | Solanum betaceum | Tamarillo | puree | [87] | |||||
| Solanaceae | Solanum nigrum | Black nightshade | fruit | [88] | |||||
| Solanum surattense | Yellow berried nightshade | fruit | |||||||
| Solanum indicum | Poison berry | fruit |
| Geographical Origin | Taxonomy | Plant Structure | Amylose Content (%) | Granule Characteristics | Thermal Properties | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Spp. | Common Name | Morphology | Size (μm) | ΔH (J/g) | To (°C) | Tp (°C) | Tc (°C) | ||||
| Fresh and saline environments | Chlorellaceae (Chlorophyta Phyllum) | Chlorella sorokiniana | Microalgae | Whole biomass | 17 | Ovals and spheres | 1.5 | 5.49 | 24 | 69 | 97 | [108] |
| South America (Andean Region) | Fabaceae | Erythrina edulis | Chachafruto, basul | Seed | 14.14 | Oval, spherical, truncated | 40 | 18.54 | 65.26 | 70.48 | 75.79 | [114] |
| Central Andean Region | Amaranthaceae | Chenopodium quinoa | Quinoa | Seed | 15.7 | Semi–spherical cumulus | Submicrons | 49.69 | 60.41 | 71.14 | [115] | |
| Mediterranean | Fabaceae | Lathyrus sativus | Grass pea (cv. Derek, Krab) | Seed | 35.2–35.8 | Oval–ellipsoid | 6–40 | 12.6 | 60.2–61.3 | 67.5–68.5 | 74.2–74.6 | [111] |
| Mexico | Nyctaginaceae | Okenia hypogaea | Okenia | Seed | 26.1 | Round–oval | 1–3 | 11.94 | 71.3 | [110] | ||
| Gondwana/South hemisphere | Araucariaceae (Conifer) | Araucaria brasiliensis | Paraná, Brazil pine | Seed | 22.4–25 | Round–oval with a central hilum | 10–25 | 8 | 63.6 | 63.4–68.5 | 75.8 | [116,117] |
| Gondwana/South hemisphere | Araucariaceae (Conifer) | Araucaria araucana | Pewen, Chilean pine | Seed | 17.3 | Round–oval with a central hilum | 8.4 | 61 | 66.6 | 73.5 | [117] | |
| Central Andean Region | Oxalidaceae | Oxalis tuberosa | Oca, ibia | Stem tuber | 27.6 | Primarily cylindrical, ellipsoid, and oval | 6.99–38.2 | 9.66 | 50.26 | 55.17 | 63.91 | [118,119] |
| Central Andean Region | Bassellaceae | Ullucus tuberosus | Olluco, chugua, ruba | Tuber | 26.49 | Irregular, primarily ellipsoid, oval, conical and prismatic | 4.48–32.64 | 10.23 | 52.81 | 58.93 | 67.88 | |
| Central Andean Region | Tropaeolaceae | Tropaeolum tuberosum | Isaño, mashua, cubio | Tuber | 27.44 | Oval, spherical, and truncated | 4.45–22.9 | 9.78 | 51.85 | 56.92 | 65.22 | |
| Central Andean Region | Apiaceae | Arracacia xanthorrhiza | Arracacha | Taproot | 35.7–39 | Round, smaller polygonal or truncated | 5.36–23.80 | 6.1–8.8 | 53–54.9 | 57.8–59.1 | 70.4–73.9 | [118,120] |
| Mexico | Araceae | Xanthosoma yucatanensis | Makal, malanga | Rhizome | 23.6 | Round | 12.4 | 14.9 | 72.6 | 78.5 | 84.2 | [121] |
| Central America | Araceae | Xanthosoma sagittifolium | Arrowleaf elephant ear, taro, bore | Corm | 16–24 | Round | 2.8–50 | 4–15 | 66–83 | 69–85 | 79–90 | [122] |
| South America | Brassicaceae | Lepidium meyenii | Maca | Taproot | 20.5 | Oval and irregular | 5.8–14.9 | 6.22 | 45.7 | 47.7 | 51.16 | [123] |
| South and Central America | Cannaceae | Canna edulis | Achira, edible canna | Tuber | 21.24–31.71 | Disk–oval | 24.4–102.53 | 10.62–13.55 | 61.16–63.06 | 63.34–65.64 | 67.87–71.09 | [124] |
| Central Asia | Poaceae | Panicum miliaceum | Proso (broomcorn) millet | Seed | 0.75–30.7 | Round (smaller), polygonal (larger) | 1.66–11.66 | 15.6–28.1 | 68.4–75.6 | 72.1–79.6 | 68–83.5 | [125] |
| Southern Asia | Musaceae | Musa acuminata | Gros Michel banana | Fruit | Ellipsoidal | 16.6–48.53 | 44.62 | 33.59 | 48.36 | 64.37 | [126] | |
| Melanesia | Araceae | Cyrtosperma merkusii | Giant swamp taro | Corm | 24.97 | Round and spherical | 12.5 | [127] | ||||
| Eastern China and Japan | Fabaceae | Apios fortunei | Hodo—potato bean | Root tuber | Spherical, polygonal, ellipsoidal | 5–30 | 62.1 | 68.3–75.4 | 83.9 | [128] | ||
| Andean Region | Fabaceae | Pachirrhizus ahipa | Andean yam bean | Tuberous root | 13.71 | Round and polygonal | 7.95 | 9.1 | 65.19 | 69.13 | [129] | |
| Central America | Sapotaceae | Pouteria campechiana | Cupcake fruit, canistel | Fruit and seed | 16.63–33.65 | Oval to bell–shaped | 4.92–30.15 | 8.43–11.06 | 59.75–67.30 | 65.97–73.34 | 77.79–82.92 | [130] |
| Eastern Asia | Polipodyaceae (Fern) | Drynaria roosii | Gu-sui-bu | Rhizome | 30.01 | Elliptical, spherical, irregular | 15.15 | [131] | ||||
| Eastern Asia | Theaceae | Camellia sinensis | Tea | Seed | 27.06–33.17 | Flat spherical or oval | 9 | 12.8–12.94 | 60.84–68.56 | 64.99–76.03 | 70.47–82.74 | [132] |
| Indo-China | Fabaceae | Vigna umbellata | Rice bean | Seed | 27.29 | Round (smaller), Oval, and elliptical (larger) | 659.8 nm | N/A | [133] | |||
| Southern Japan and Korea | Fagaceae | Castanopsis cuspidate | Japanese chinquapin | Fruit | 56.1 | Ellipsoid to polygonal or angulate | 8.13–20 | 14.1 | 56 | 61.3 | 72.4 | [134] |
| Mexican cross from Philippines cultivar | Anacardiaceae | Mangifera indica cv. Ataulfo | Mango | Stenospermocarpic unripe fruit | 10–15 | [112] | ||||||
| Tropical Americas | Bixaceae | Bixa orellana | Annato | Byproduct seed | 27.8 | Oval, flake-like | 17.2 | 64.7 | [135] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-López, S.V.; Zuluaga-Domínguez, C.M.; Fernández-Trujillo, J.P.; Hernández-Gómez, M.S. Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications. Foods 2022, 11, 401. https://doi.org/10.3390/foods11030401
Medina-López SV, Zuluaga-Domínguez CM, Fernández-Trujillo JP, Hernández-Gómez MS. Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications. Foods. 2022; 11(3):401. https://doi.org/10.3390/foods11030401
Chicago/Turabian StyleMedina-López, Sandra Viviana, Carlos Mario Zuluaga-Domínguez, Juan Pablo Fernández-Trujillo, and María Soledad Hernández-Gómez. 2022. "Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications" Foods 11, no. 3: 401. https://doi.org/10.3390/foods11030401
APA StyleMedina-López, S. V., Zuluaga-Domínguez, C. M., Fernández-Trujillo, J. P., & Hernández-Gómez, M. S. (2022). Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications. Foods, 11(3), 401. https://doi.org/10.3390/foods11030401








