Pilot Study for the Dietary Assessment of Xenobiotics Derived from Food Processing in an Adult Spanish Sample
Abstract
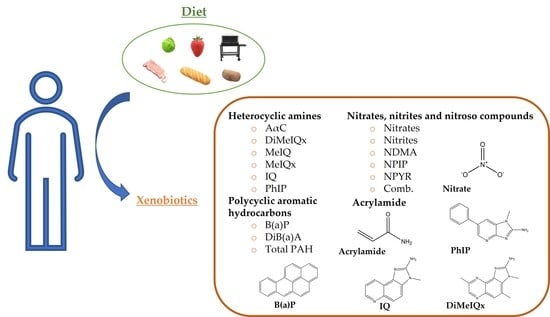
:1. Introduction
2. Subjects and Methods
2.1. Sample Recruitment and Study Design
2.2. General Characteristics and Food Frequency Questionnaire (FFQ)
2.3. Xenobiotic Estimation and Nutritional Analyses
2.4. Digestive Function Self-Assessment Questionnaire
2.5. Statistical Analyses
3. Results
3.1. Description of the Sample
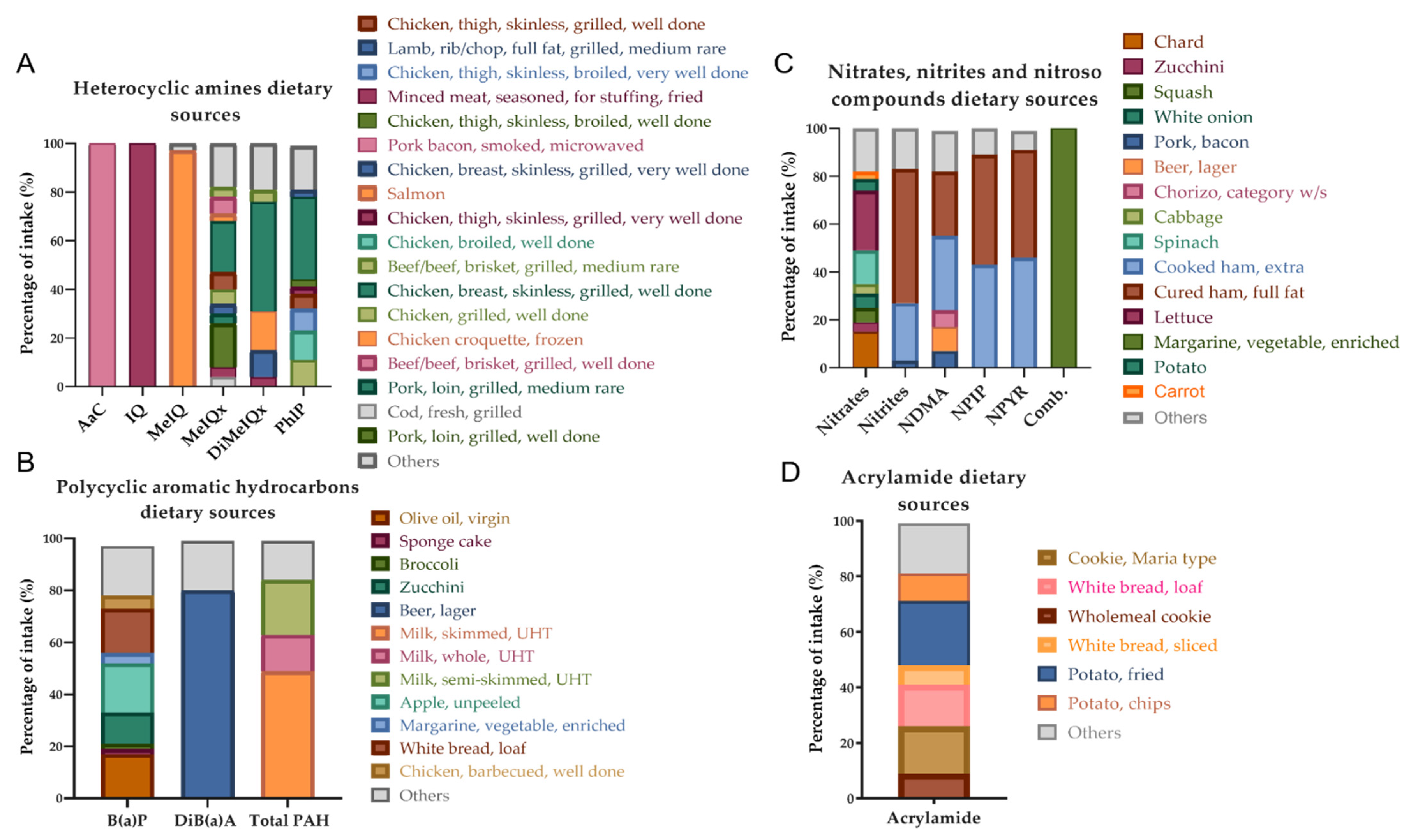
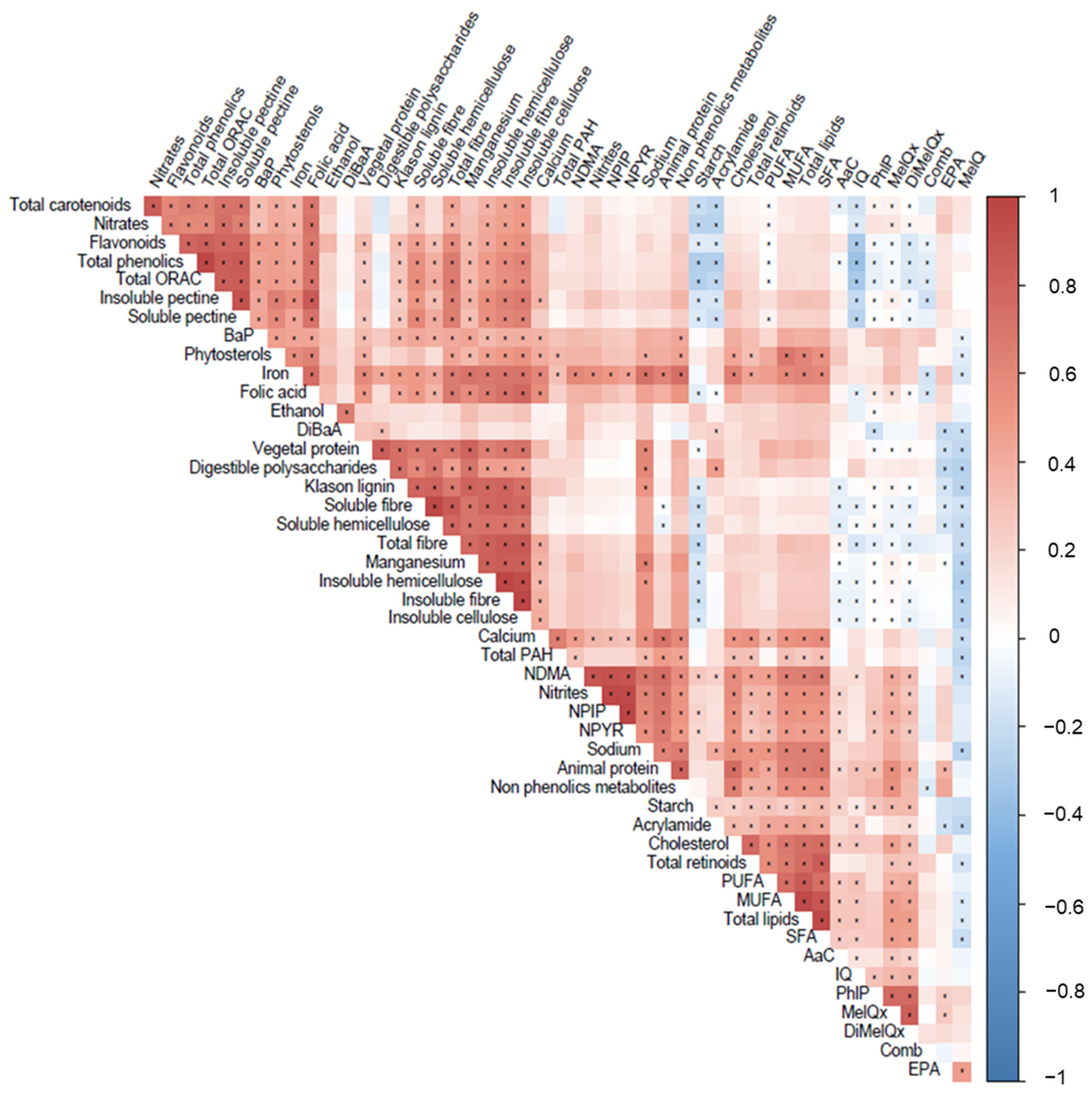
3.2. Xenobiotics: Doses and Dietary Origin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AESAN | Spanish Agency for Food Safety and Nutrition |
| AαC | Amino-alpha-carboline |
| B(a)P | Benzo (a) pyrene |
| BMI | Body mass index |
| CESNID | Centre for Higher Education in Nutrition and Dietetics |
| CHARRED | Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease |
| Comb. | Combined nitroso compounds |
| CRA | Colorectal adenoma |
| CRC | Colorectal cancer |
| DiB(a)A | Dibenzo (a) anthracene |
| DiMeIQx | 2-Amino-3,4,8 trimethylimidazo (4,5,f) quinoxaline |
| EFSA | European Food Safety Authority |
| EPIC European | Prospective Investigation into Cancer and Nutrition |
| FDA | U.S. Food and Drug Administration |
| FFQ | Food Frequency Questionnaire |
| HAs | Heterocyclic amines |
| IARC | International Agency for Research on Cancer |
| IQ | 2-Amino-3-methylimidazo (4,5,f) quinoline |
| MEC | Multiethnic cohort |
| MeIQ | 2-Amino-3.4 dimethylimidazo (4,5,f) quinoline |
| MeIQx | 2-Amino-3,8 dimethylimidazo (4,5,f) quinoxaline |
| MUFA | Monounsaturated fatty acid |
| NDMA | N-nitrosodimethylamine |
| NOAEL | No observed adverse effect level |
| NOCs | N-Nitroso compounds |
| NPIP | N-Nitrosopiperidine |
| NPYR | N-Nitrosopyrrolidine |
| OR | Odds ratio |
| ORAC | Oxygen radical absorbance capacity |
| PAHs | Polycyclic aromatic hydrocarbons |
| PANCAKE | Assessment of Nutrient Intake and Food Consumption Among Kids in Europe |
| PHEX | Phenol Explorer |
| PhIP | 2-Amino-1-methyl-6-phenylimidazo (4,5,b) pyridine |
| PUFA | Polyunsaturated fatty acid |
| PUMUO | University Program for Older Adults of the University of Oviedo |
| R24h | 24-h dietary recall |
| SEEDO | Spanish Society for the Study of Obesity |
| SFA | Saturated fatty acid |
| USDA | United States Department of Agriculture |
| WHO | World Health Organization |
References
- De Kok, T.M.C.M.; Van Maanen, J.M.S. Evaluation of fecal mutagenicity and colorectal cancer risk. Mutat. Res.-Rev. Mutat. Res. 2000, 463, 53–101. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.M.A.N.; Jooste, L.; Hano, C.; Aadil, R.M. Heterocyclic aromatic amines in meat: Formation, isolation, risk assessment, and inhibitory effect of plant extracts. Foods 2021, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Abnet, C.C.; Graubard, B.I.; Beane-Freeman, L.; Freedman, N.D.; Liao, L.; Dawsey, S.M.; Sinha, R. Anatomical subsite can modify the association between meat and meat compounds and risk of colorectal adenocarcinoma: Findings from three large US cohorts. Int. J. Cancer 2018, 143, 2261–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (IARC) Working Group. Red Meat and Processed Meat: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2018; Volume 114, pp. 1599–1600. ISBN 9789283201809. [Google Scholar]
- Chiavarini, M.; Bertarelli, G.; Minelli, L.; Fabiani, R. Dietary intake of meat cooking-related mutagens (HCAs) and risk of colorectal adenoma and cancer: A systematic review and meta-analysis. Nutrients 2017, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Le, N.T.; Silva Michels, F.A.; Song, M.; Zhang, X.; Bernstein, A.M.; Giovannucci, E.L.; Fuchs, C.S.; Ogino, S.; Chan, A.T.; Sinha, R.; et al. A prospective analysis of meat mutagens and colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Environ. Health Perspect. 2016, 124, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.E.; Lazarus, P.; Lesko, S.M.; Cross, A.J.; Sinha, R.; Laio, J.; Zhu, J.; Harper, G.; Muscat, J.E.; Hartman, T.J. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr. Cancer 2013, 65, 202–226. [Google Scholar] [CrossRef]
- World Cancer Research Fund International; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Colorectal Cancer; Continuous Update Project; World Cancer Research Fund International: London, UK, 2018; pp. 1–111. Available online: https://www.aicr.org/wp-content/uploads/2020/01/colorectal-cancer-2017-report.pdf (accessed on 10 January 2021).
- Jakszyn, P.; Agudo, A.; Ibãñez, R.; García-Closas, R.; Pera, G.; Amiano, P.; González, C.A. Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J. Nutr. 2004, 134, 2011–2014. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Jinap, S.; Ang, S.J.; Abdul-Hamid, A.; Hajeb, P.; Lioe, H.N.; Zaidul, I.S.M. Dietary exposure to heterocyclic amines in high-temperature cooked meat and fish in Malaysia. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 1060–1071. [Google Scholar] [CrossRef]
- Dybing, E.; Farmer, P.B.; Andersen, M.; Fennell, T.R.; Lalljie, S.P.D.; Müller, D.J.G.; Olin, S.; Petersen, B.J.; Schlatter, J.; Scholz, G.; et al. Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol. 2005, 43, 365–410. [Google Scholar] [CrossRef]
- Mucci, L.A.; Adami, H.O.; Wolk, A. Prospective study of dietary acrylamide and risk of colorectal cancer among women. Int. J. Cancer 2006, 118, 169–173. [Google Scholar] [CrossRef]
- Scientific Committee on Food Annex. Background Document to the Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food (Expressed on 4 December 2002); European Commission Health and Consumer Protection Directorate: Brussels, Belgium, 2002; pp. 1–84. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out153_en.pdf (accessed on 12 September 2021).
- Nogacka, A.M.; Gómez-Martín, M.; Suárez, A.; González-Bernardo, O.; de los Reyes-Gavilán, C.G.; González, S. Xenobiotics formed during food processing: Their relation with the intestinal microbiota and colorectal cancer. Int. J. Mol. Sci. 2019, 20, 2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollberding, N.J.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L. Meat consumption, heterocyclic amines and colorectal cancer risk: The Multiethnic Cohort Study. Int. J. Cancer 2012, 131, 1125–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter-Gooder, P.K.; Lewis, N.M.; Heidal, K.B.; Eskridge, K.M. Validity and reliability of a quantitative Food Frequency Questionnaire measuring n-3 fatty acid intakes in cardiac patients in the midwest: A validation pilot study. J. Am. Diet. Assoc. 2006, 106, 1251–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra-Ruelas, É.; Bernal-Orozco, M.F.; Marcedo-Ojeda, G.; Márquez-Sandoval, Y.F.; Altamirano-Martínez, M.B.; Vizmanos, B. Validation of semiquantitative FFQ administered to adults: A systematic review. Public Health Nutr. 2020, 24, 3399–3418. [Google Scholar] [CrossRef] [PubMed]
- Ocké, M.; de Boer, E.; Brants, H.; van der Laan, J.; Niekerk, M.; van Rossum, C.; Temme, L.; Freisling, H.; Nicolas, G.; Casagrande, C.; et al. PANCAKE—Pilot study for the assessment of nutrient intake and food consumption among kids in Europe. EFSA Support. Publ. 2012, 9, 1–120. [Google Scholar] [CrossRef]
- Watson, E.O.; Heath, A.L.M.; Taylor, R.W.; Mills, V.C.; Barris, A.C.; Skidmore, P.M.L. Relative validity and reproducibility of an FFQ to determine nutrient intakes of New Zealand toddlers aged 12–24 months. Public Health Nutr. 2015, 18, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Santiago, S.; de la Pascual, O.V.; Romanos-Nanclares, A.; Rico-Campà, A.; Álvarez-Zallo, N.; Martínez-González, M.Á.; Martín-Calvo, N. Validity and reproducibility of a semi-quantitative food frequency questionnaire in Spanish preschoolers—The Sendo Project. Nutr. Hosp. 2020, 37, 672–684. [Google Scholar] [CrossRef]
- Sociedad Española para el Estudio de la Obesidad (SEEDO); Foz, M.; Barbany, M.; Remesar, X.; Carrillo, M.; Aranceta, J.; García-Luna, P.; Alemany, M.; Vázquez, C.; Palou, A.; et al. Consenso SEEDO’ 2000 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica. Med. Clin. 2000, 115, 587–597. [Google Scholar] [CrossRef]
- Jakszyn, P.; Ibáñez, R.; Pera, G.; Agudo, A.; García-Closas, R.; Amiano, P.; González, C.A. Food Content of Potential Carcinogens; Catalan Institute of Oncology: Barcelona, Spain, 2004. [Google Scholar]
- National Institutes of Health (NIH). CHARRED: Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease. Available online: https://dceg.cancer.gov/tools/design/charred (accessed on 21 May 2021).
- European Food Safety Authority (EFSA). Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J. 2012, 10, 2938–2976. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Survey Data on Acrylamide in Food; Food and Drug Administration: Silver Spring, MD, USA, 2015. Available online: https://www.fda.gov/food/chemical-contaminants-food/acrylamide (accessed on 3 March 2021).
- Farran, A.; Zamora, R.; Cervera, P. Tablas de Composición de Alimentos del Centro de Enseñanza Superior en Nutrición y Dietética (CESNID); McGraw-Hill: New York, NY, USA, 2003; ISBN 9789283201809. [Google Scholar]
- United States Department of Agriculture (USDA). Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 17 September 2021).
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010. [Google Scholar] [CrossRef]
- Marlett, J.; Cheung, T. Database and quick methods of assessing typical dietary fiber intakes using data for 228 commonly consumed foods. J. Am. Diet. Assoc. 1997, 1151, 1139–1148. [Google Scholar] [CrossRef]
- Drossman, D.A. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006, 130, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Hermann, S.; Linseisen, J. Heterocyclic aromatic amine intake increases colorectal adenoma risk: Findings from a prospective European cohort study. Am. J. Clin. Nutr. 2009, 89, 1418–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.M.; Miranda, A.M.; Santos, F.A.; Loureiro, A.P.M.; Fisberg, R.M.; Marchioni, D.M. High intake of heterocyclic amines from meat is associated with oxidative stress. Br. J. Nutr. 2015, 113, 1301–1307. [Google Scholar] [CrossRef] [Green Version]
- Rohrmann, S.; Zoller, D.; Hermann, S.; Linseisen, J. Intake of heterocyclic aromatic amines from meat in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort. Br. J. Nutr. 2007, 98, 1112–1115. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez, R.; Agudo, A.; Berenguer, A.; Jakszyn, P.; Tormo, M.J.; Sanchéz, M.J.; Quirós, J.R.; Pera, G.; Navarro, C.; Martinez, C.; et al. Dietary intake of polycyclic aromatic hydrocarbons in a Spanish population. J. Food Prot. 2005, 68, 2190–2195. [Google Scholar] [CrossRef]
- Falcó, G.; Domingo, J.L.; Llobet, J.M.; Teixidó, A.; Casas, C.; Müller, L. Polycyclic Aromatic Hydrocarbons in foods: Human exposure through the diet in Catalonia, Spain. J. Food Prot. 2003, 66, 2325–2331. [Google Scholar] [CrossRef]
- Loh, Y.H.; Jakszyn, P.; Luben, R.N.; Mulligan, A.A.; Mitrou, P.N.; Khaw, K.T. N-nitroso compounds and cancer incidence: The European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am. J. Clin. Nutr. 2011, 93, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Obón-Santacana, M.; Kaaks, R.; Slimani, N.; Lujan-Barroso, L.; Freisling, H.; Ferrari, P.; Dossus, L.; Chabbert-Buffet, N.; Baglietto, L.; Fortner, R.T.; et al. Dietary intake of acrylamide and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Br. J. Cancer 2014, 111, 987–997. [Google Scholar] [CrossRef]
- Wie, G.A.; Cho, Y.A.; Kang, H.H.; Ryu, K.A.; Yoo, M.K.; Kim, Y.A.; Jung, K.W.; Kim, J.; Lee, J.H.; Joung, H. Red meat consumption is associated with an increased overall cancer risk: A prospective cohort study in Korea. Br. J. Nutr. 2014, 112, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Zimmerli, B.; Rhyn, P.; Zoller, O.; Schlatter, J. Occurrence of heterocyclic aromatic amines in the Swiss diet: Analytical method, exposure estimation and risk assessment. Food Addit. Contam. 2001, 18, 533–551. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (EFSA ANS Panel); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific Opinion on the re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, e04786. [Google Scholar]
- Knize, M.; Felton, J.; Sinha, R.; Rothman, N. Collaborative Study; Biosciences Directorate of the University of California, Lawrence Livermore National Laboratory: Livermore, CA, USA, 2003; unpublished.
- Solyakov, A.; Skog, K. Screening for heterocyclic amines in chicken cooked in various ways. Food Chem. Toxicol. 2002, 40, 1205–1211. [Google Scholar] [CrossRef]
- Norat, T.; Bingham, S.; Ferrari, P.; Slimani, N.; Jenab, M.; Mazuir, M.; Overvad, K.; Olsen, A.; Tjønneland, A.; Clavel, F.; et al. Meat, fish, and colorectal cancer risk: The European Prospective Investigation into Cancer and Nutrition. J. Natl. Cancer Inst. 2005, 97, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of sodium nitrate (E 251) and potassium nitrate (E 252) as food additives. EFSA J. 2017, 15, e04787. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Acrilamida. Available online: http://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/subdetalle/acrilamida.htm%0A (accessed on 4 April 2021).
- DellaValle, C.T.; Xiao, Q.; Yang, G.; Ou Shu, X.; Aschebrook-Kilfoy, B.; Zheng, W.; Li, H.L.; Ji, B.-T.; Rothman, N.; Chow, W.-H.; et al. Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women’s Health Study. Int. J. Cancer 2014, 134, 2091–2926. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.P.; Zhao, J.; Green, R.; Sun, Z.; Roebothan, B.; Squires, J.; Buehler, S.; Dicks, E.; Zhao, J.; et al. Dietary N-nitroso compounds and risk of colorectal cancer: A case-control study in Newfoundland and Labrador and Ontario, Canada. Br. J. Nutr. 2014, 111, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Augustsson, K.; Skog, K.; Jägerstad, M.; Steineck, G. Assessment of the human exposure to heterocyclic amines. Carcinogenesis 1997, 18, 1931–1935. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization (FAO); World Health Organization (WHO). Food Additives: Evaluation of Certain Food Contaminants: Sixty-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization Technical Report Series; Food and Agriculture Organization: Rome, Italy, 2006; Volume 930, ISBN 9241209305. [Google Scholar]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Hidrocarburos Aromáticos Policíclicos (HAPs); Agencia Española de Seguridad Alimentaria y Nutrición: Madrid, Spain, 2020. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/HAPs_ficha_SEPT_2020.pdf (accessed on 20 May 2021).
- Lee, J.-S.; Han, J.-W.; Jung, M.; Lee, K.-W.; Chung, M.-S. Effects of thawing and frying methods on the formation of acrylamide and polycyclic aromatic hydrocarbons in chicken meat. Foods 2020, 9, 573. [Google Scholar] [CrossRef]

| Characteristics | Total (N = 70) | Gender | |
|---|---|---|---|
| Male (N = 25) | Female (N = 45) | ||
| Age (years) | 59 ± 12 | 62 ± 7 | 57 ± 14 |
| <57 | 24 (34%) | 5 (20%) | 19 (42%) |
| 57–65 | 18 (26%) | 9 (36%) | 9 (20%) |
| >66 | 28 (40%) | 11 (44%) | 17 (38%) |
| Energy intake (kcal/day) | 1885.87 ± 581.71 | 1935.28 ± 569.40 | 1858.42 ± 593.01 |
| Weight (kg) | 74.70 ± 16.02 | 84.48 ± 16.84 | 69.15 ± 12.67 * |
| Height (m) | 1.66 ± 0.08 | 1.74 ± 0.07 | 1.62 ± 0.05 * |
| BMI (kg/m2) | 26.90 ± 4.64 | 27.77 ± 4.67 | 26.41 ± 4.60 |
| Normal weight (18.5–24.9) | 25 (36%) | 7 (28%) | 18 (40%) |
| Overweight (25.0–29.9) | 32 (46%) | 13 (52%) | 19 (42%) |
| Obese (≥30.0) | 12 (17%) | 5 (20%) | 7 (16%) |
| Na | 1 (1%) | 0 (0%) | 1 (2%) |
| Smoking status | |||
| Current smoker | 7 (10%) | 3 (12%) | 4 (9%) |
| Former smoker | 27 (39%) | 14 (56%) | 13 (29%) * |
| Never smoker | 36 (51%) | 8 (32%) | 28 (62%) * |
| Exercise (hours/week) | 1.13 ± 1.93 | 1.80 ± 2.20 | 0.76 ± 1.68 * |
| Sleeping (hours/day) | 6.93 ± 1.11 | 6.80 ± 1.08 | 7.00 ± 1.13 |
| Family CRC history | |||
| Presence | 11 (16%) | 5 (20%) | 6 (13%) |
| Absence | 52 (74%) | 18 (72%) | 34 (76%) |
| Na | 6 (9%) | 2 (8%) | 4 (9%) |
| Previous pathologies | |||
| Hypertension | 11 (16%) | 7 (28%) | 4 (9%) * |
| Diabetes | 6 (9%) | 3 (12%) | 3 (7%) |
| Obesity | 28 (40%) | 12 (48%) | 16 (36%) |
| Asthma and/or allergies | 12 (17%) | 4 (16%) | 8 (18%) |
| None | 14 (20%) | 4 (16%) | 10 (22%) |
| Intestinal pathologies | |||
| Diarrhea | 1 (1%) | 0 (0%) | 1 (2%) |
| Constipation | 9 (13%) | 1 (4%) | 8 (18%) |
| Hemorrhoids | 29 (41%) | 6 (24%) | 23 (51%) * |
| Fissures | 2 (3%) | 1 (4%) | 1 (2%) |
| None | 30 (43%) | 17 (68%) | 13 (29%) * |
| Bleeding frequency | |||
| Daily | 1 (1%) | 1 (4%) | 0 (0%) |
| At least once a week | 0 (0%) | 0 (0%) | 0 (0%) |
| Occasionally | 24 (34%) | 8 (32%) | 16 (36%) |
| Never | 45 (64%) | 16 (64%) | 29 (64%) |
| Rome III Criteria | |||
| No discomfort | 49 ± 28 | 50 ± 24 | 49 ± 30 |
| Mild discomfort | 31 ± 21 | 33 ± 19 | 29 ± 22 |
| Moderate discomfort | 11 ± 12 | 8 ± 10 | 12 ± 14 |
| Severe discomfort | 1 ± 7 | 1 ± 2 | 2 ± 9 |
| Very severe discomfort | 1 ± 8 | 0 ± 2 | 2 ± 10 |
| Na | 7 ± 20 | 8 ± 23 | 7 ± 19 |
| Stool frequency a | 7 ± 2 | 7 ± 2 | 7 ± 3 |
| Stool consistency | |||
| Liquid | 0 (0%) | 0 (0%) | 0 (0%) |
| Soft | 42 (60%) | 15 (60%) | 27 (60%) |
| Hard | 27 (39%) | 10 (40%) | 17 (38%) |
| Food Groups Intake (g/Day) | Total (N = 70) | Gender | |
|---|---|---|---|
| Male (N = 25) | Female (N = 45) | ||
| Cereals and cereals products | 195.09 ± 138.37 | 185.08 ± 106.56 | 200.66 ± 154.09 |
| Whole grain cereals | 57.69 ± 118.62 | 23.31 ± 41.01 | 76.78 ± 141.78 |
| Milk and dairy products | 392.43 ± 236.26 | 323.14 ± 216.16 | 425.92 ± 242.56 |
| Meat and meat products | 147.47 ± 89.62 | 146.89 ± 72.32 | 147.79 ± 98.70 |
| White meat | 48.77 ± 37.88 | 48.05 ± 39.05 | 49.16 ± 37.66 |
| Red meat | 42.17 ± 30.04 | 47.13 ± 33.94 | 39.42 ± 27.66 |
| Processed meat | 58.90 ± 52.99 | 54.00 ± 28.47 | 61.62 ± 62.77 |
| Eggs | 43.51 ± 29.53 | 49.23 ± 33.74 | 40.33 ± 26.79 |
| Fish | 61.83 ± 36.99 | 63.46 ± 30.00 | 60.93 ± 40.66 |
| Seafood | 22.82 ± 19.64 | 22.92 ± 19.16 | 22.77 ± 20.12 |
| Oils and fats | 16.18 ± 8.57 | 18.05 ± 9.09 | 15.15 ± 8.19 |
| Vegetables | 308.53 ± 179.13 | 262.94 ± 153.23 | 333.86 ± 188.88 |
| Legumes | 42.61 ± 76.11 | 49.79 ± 77.89 | 38.62 ± 75.70 |
| Potatoes and tubers | 50.38 ± 31.75 | 60.50 ± 32.11 | 44.76 ± 30.46 * |
| Fruits | 130.68 ± 90.87 | 156.27 ± 126.20 | 116.47 ± 60.69 |
| Nuts and seeds | 13.29 ± 17.60 | 9.12 ± 9.00 | 15.61 ± 20.65 |
| Sugar and sweets | 7.45 ± 10.11 | 9.93 ± 12.44 | 6.07 ± 8.39 |
| Snacks | 2.09 ± 4.45 | 3.16 ± 4.55 | 1.49 ± 4.32 |
| Sauces and condiments | 8.17 ± 7.17 | 8.04 ± 5.25 | 8.24 ± 8.10 |
| Other foods | 10.20 ± 14.37 | 14.84 ± 19.64 | 7.62 ± 9.72 * |
| Nonalcoholic beverages (mL/day) | 225.86 ± 231.79 | 283.30 ± 325.24 | 193.96 ± 153.74 |
| Alcoholic beverages (mL/day) | 133.42 ± 171.11 | 191.02 ± 175.93 | 101.42 ± 161.55 * |
| Xenobiotics | Value (N = 70) | Type of Study | |||||
|---|---|---|---|---|---|---|---|
| Reference Value | Sample Size (Gender) | Age (Years) | Health Status | Country | Reference | ||
| Heterocyclic amines (ng/day) | |||||||
| MeIQx | 29.48 ± 27.85 | 16.8 (±29.7) | n = 3.699 (MF) | 35–65 | Healthy | DE | [31] a |
| 102.7 | n = 561 (MF) | >20 | Na | BR | [32] b | ||
| DiMeIQx | 8.18 ± 7.96 | 3.0 (±4.5) | n = 3.699 (MF) | 35–65 | Healthy | DE | [31] a |
| 9.8 | n = 561 (MF) | >20 | Na | BR | [32] b | ||
| PhIP | 187.59 ± 257.04 | 41.0 (±117.5) | n = 3.699 (MF) | 35–65 | Healthy | DE | [31] a |
| 324.3 | n = 561 (MF) | >20 | Na | BR | [32] b | ||
| Total HAs | 226.99 ± 285.50 | 69.4 | n = 21.462 (MF) | 35–65 | Na | DE | [33] a |
| 436.8 | n = 561 (MF) | >20 | Na | BR | [32] b | ||
| Polycyclic aromatic hydrocarbons (µg/day) | |||||||
| B(a)P | 0.03 ± 0.03 | 0.14 (±0.07) | n = 40.690 (MF) | 35–64 | Na | SP | [34] a |
| DiB(a)A | 0.07 ± 0.10 | 0.06 | n = 3.890.240 (M) | 20–65 | Na | SP | [35] c |
| Total PAHs | 5.04 ± 3.84 | 8.57 (±2.69) | n = 40.690 (MF) | 35–64 | Na | SP | [34] a |
| Nitrates, nitrites, and nitroso compounds | |||||||
| Nitrites (mg/day) | 3.14 ± 2.90 | 1.48 (±0.51) | n = 20.095 (MF) | 40–79 | Healthy | UK | [36] a |
| NDMA (µg/day) | 0.17 ± 0.14 | 0.06 (±0.05) | n = 20.095 (MF) | 40–79 | Healthy | UK | [36] a |
| NPIP (µg/day) | 0.09 ± 0.09 | 72.3 (±19.2) d | n = 20.095 (MF) | 40–79 | Healthy | UK | [36] a |
| NPYR (µg/day) | 0.15 ± 0.16 | ||||||
| Comb. (ng/day) | 1.71 ± 5.10 | ||||||
| Acrylamide (µg/day) | 15.12 ± 11.60 | 20.6 (±12.1) | n = 22.783 (F) | 29–69 | Cases & healthy | SP | [37] a |
| Mean Daily Intake | Heterocyclic Amines (ng/Day) | Polycyclic Aromatic Hydrocarbons (μg/Day) | Nitroso Compounds (μg/Day) | Acrylamide (μg/Day) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AαC | IQ | MeIQ | MeIQx | DiMeIQx | PhIP | B(a)P | DiB(a)A | Total PAHs | Nitrates (mg/Day) | Nitrites (mg/Day) | NDMA | NPIP | NPYR | Comb (ng/Day) | Acrylamide | |
| BMI (kg/m2) | ||||||||||||||||
| Normal weight | 0.01 | 0.15 | 1.79 | 27.92 | 7.88 | 159.69 | 0.03 | 0.06 | 3.89 | 118.39 | 2.85 | 0.16 | 0.08 | 0.14 | 2.80 | 11.96 |
| Overweight | 0.03 | 0.11 | 1.48 | 27.24 | 7.26 | 152.55 | 0.03 | 0.07 | 4.88 | 123.03 | 3.43 | 0.18 | 0.10 | 0.16 | 1.25 | 16.36 |
| Obese | 0.00 | 0.18 | 1.45 | 37.48 | 9.97 | 330.26 | 0.04 | 0.07 | 7.95 * | 153.24 | 2.91 | 0.16 | 0.08 | 0.13 | 0.83 | 16.17 |
| Smoking status | ||||||||||||||||
| Current smoker | 0.01 | 0.15 | 1.95 | 35.17 | 10.32 | 177.52 | 0.04 | 0.04 | 4.35 | 110.49 | 2.41 | 0.13 | 0.07 | 0.12 | 1.43 | 21.37 |
| Former smoker | 0.01 | 0.14 | 1.46 | 25.88 | 7.57 | 226.07 | 0.03 | 0.09 | 4.82 | 130.36 | 2.73 | 0.14 | 0.07 | 0.11 | 1.11 | 12.16 |
| Never smoker | 0.03 | 0.14 | 1.60 | 31.09 | 8.21 | 160.69 | 0.03 | 0.05 | 5.35 | 125.77 | 3.58 | 0.19 | 0.11 | 0.18 | 2.22 | 16.13 |
| Exercise | ||||||||||||||||
| Active | 0.01 | 0.13 | 1.54 | 29.17 | 9.05 | 210.78 | 0.03 | 0.06 | 3.65 | 110.16 | 2.31 | 0.14 | 0.06 | 0.09 | 1.20 | 15.09 |
| Sedentary | 0.02 | 0.14 | 1.60 | 29.66 | 7.69 | 174.71 | 0.03 | 0.07 | 5.82 * | 134.82 | 3.59 | 0.19 | 0.10 * | 0.18 * | 2.00 | 15.15 |
| Sleeping | ||||||||||||||||
| ≥7 h/day | 0.02 | 0.13 | 1.47 | 27.88 | 7.24 | 185.21 | 0.03 | 0.06 | 4.61 | 130.64 | 2.65 | 0.14 | 0.07 | 0.12 | 1.20 | 13.38 |
| <7 h/day | 0.02 | 0.15 | 1.86 | 33.51 | 10.51 | 193.55 | 0.03 | 0.09 | 6.14 | 114.43 | 4.35 * | 0.23 * | 0.13 * | 0.21 * | 3.00 | 19.50 * |
| Intestinal pathologies | ||||||||||||||||
| Constipation | 0.00 | 0.19 | 2.17 | 38.64 | 11.05 | 261.38 | 0.05 | 0.02 | 8.33 | 94.33 | 3.24 | 0.14 | 0.09 | 0.15 | 1.11 | 9.83 |
| Regular transit | 0.02 | 0.13 | 1.49 | 28.13 | 7.75 | 176.71 | 0.03 | 0.07 | 4.56 * | 130.68 | 3.12 | 0.17 | 0.09 | 0.15 | 1.80 | 15.91 |
| Hemorrhoids | 0.03 | 0.15 | 1.47 | 33.13 | 9.70 | 195.17 | 0.03 | 0.07 | 4.83 | 126.60 | 4.01 | 0.22 | 0.12 | 0.20 | 0.34 | 16.67 |
| No hemorrhoids | 0.01 | 0.13 | 1.66 | 26.90 | 7.10 | 182.23 | 0.03 | 0.06 | 5.19 | 125.59 | 2.52 * | 0.13 * | 0.06 * | 0.11 * | 2.68 | 14.03 |
| Fissures | 0.05 | 0.40 | 2.73 | 36.88 | 10.96 | 469.58 | 0.02 | 0.04 | 2.72 | 120.76 | 7.87 | 0.37 | 0.24 | 0.39 | 5.00 | 16.54 |
| No fissures | 0.02 | 0.13 * | 1.55 | 29.27 | 8.09 | 179.30 | 0.03 | 0.07 | 5.11 | 126.17 | 3.00 * | 0.16 * | 0.08 * | 0.14 * | 1.62 | 15.08 |
| Bleeding | ||||||||||||||||
| Ever | 0.03 | 0.14 | 1.43 | 28.74 | 8.94 | 180.16 | 0.03 | 0.09 | 4.76 | 124.45 | 3.96 | 0.22 | 0.12 | 0.20 | 0.80 | 15.21 |
| Never | 0.01 | 0.14 | 1.66 | 29.90 | 7.75 | 191.72 | 0.03 | 0.05 | 5.20 | 126.88 | 2.67 | 0.14 * | 0.07 | 0.12 | 2.22 | 15.08 |
| Rome III Criteria | ||||||||||||||||
| Moderate or greater a | 0.00 | 0.20 | 1.89 | 36.92 | 19.70 | 258.23 | 0.03 | 0.01 | 10.56 | 82.01 | 0.54 | 0.03 | 0.01 | 0.02 | 5.00 | 10.56 |
| Never or mild b | 0.02 | 0.14 | 1.57 | 29.27 | 7.84 * | 185.51 | 0.03 | 0.07 | 4.88 * | 127.31 | 3.21 | 0.17 | 0.09 | 0.15 | 1.62 | 15.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapico, A.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de los Reyes-Gavilán, C.G.; González, S. Pilot Study for the Dietary Assessment of Xenobiotics Derived from Food Processing in an Adult Spanish Sample. Foods 2022, 11, 470. https://doi.org/10.3390/foods11030470
Zapico A, Ruiz-Saavedra S, Gómez-Martín M, de los Reyes-Gavilán CG, González S. Pilot Study for the Dietary Assessment of Xenobiotics Derived from Food Processing in an Adult Spanish Sample. Foods. 2022; 11(3):470. https://doi.org/10.3390/foods11030470
Chicago/Turabian StyleZapico, Aida, Sergio Ruiz-Saavedra, María Gómez-Martín, Clara G. de los Reyes-Gavilán, and Sonia González. 2022. "Pilot Study for the Dietary Assessment of Xenobiotics Derived from Food Processing in an Adult Spanish Sample" Foods 11, no. 3: 470. https://doi.org/10.3390/foods11030470
APA StyleZapico, A., Ruiz-Saavedra, S., Gómez-Martín, M., de los Reyes-Gavilán, C. G., & González, S. (2022). Pilot Study for the Dietary Assessment of Xenobiotics Derived from Food Processing in an Adult Spanish Sample. Foods, 11(3), 470. https://doi.org/10.3390/foods11030470