The Influence of Hypothermia Hibernation Combined with CO2 Anesthesia on Life and Storage Quality of Large Yellow Croaker (Pseudosciaena crocea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. Anesthesia and Hibernation Conditions of Large Yellow Croaker
2.2.1. Trial 1, CO2 Anesthesia
2.2.2. Trial 2, Hypothermia Hibernation
2.2.3. Trial 3, the Combined Storage Method of CO2 Anesthesia and Hypothermia Hibernation
2.3. Determination of Blood Components, Glucose Levels, and Total Serum Protein of the Large Yellow Croaker Using the Optimal Combined Method
2.4. Determination of Drip Loss
2.5. Determination of pH and Lactic Acid
2.6. Determination of Color Variations
2.7. Determination of Texture
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Effects of Anesthesia and Hibernation on the Live Large Yellow Croaker
3.2. Blood Components, Glucose Levels, and Total Serum Protein of the Large Yellow Croaker Stored Using the Optimal Combined Method
3.3. Drip Loss
3.4. pH and Lactic Acid
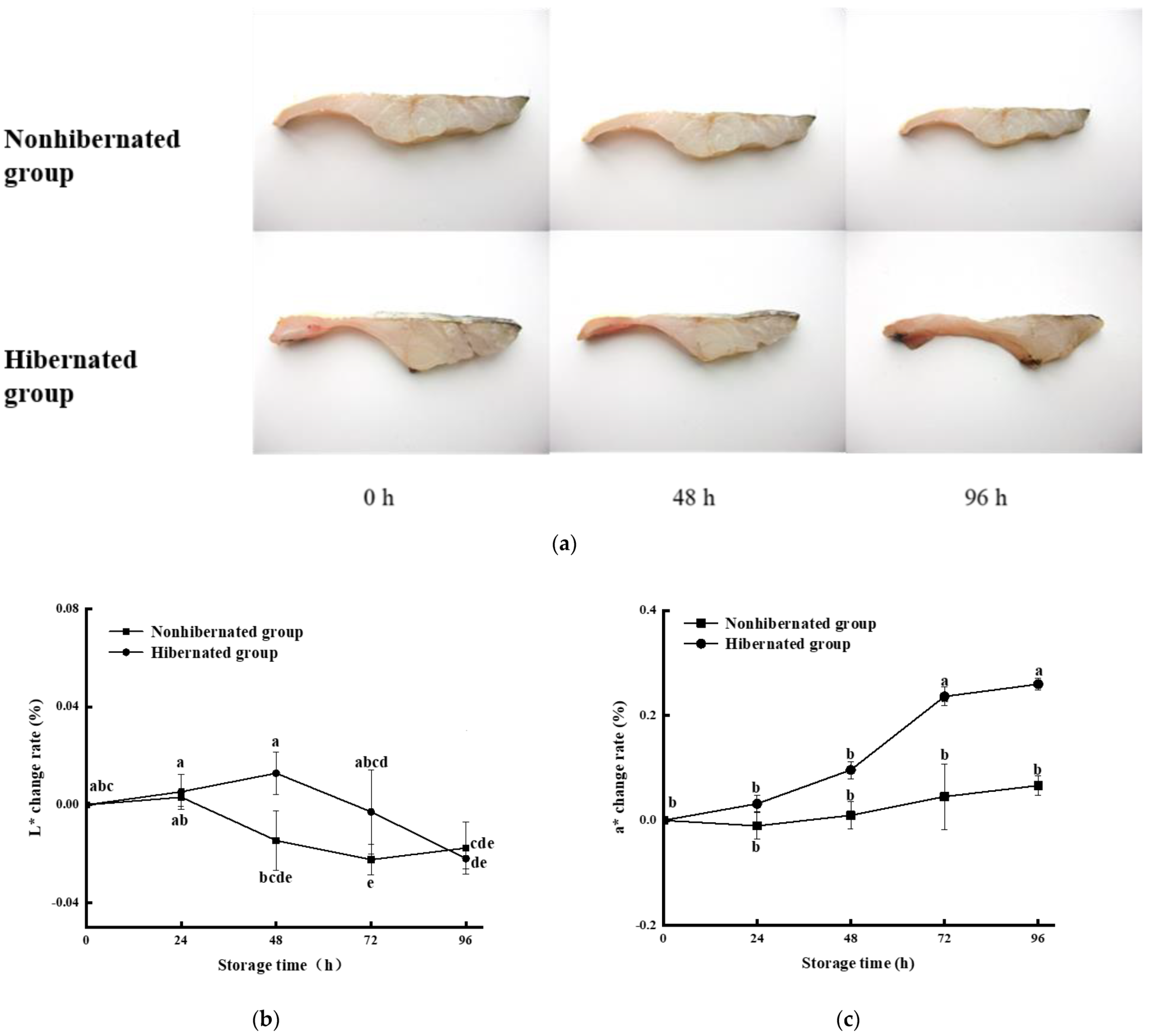
3.5. Color Variations
3.6. Texture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, T.F.; Benjakul, S.; Sanmartin, C.; Ying, X.G.; Ma, L.K.; Xiao, Z.S.; Yu, J.; Liu, G.Q.; Deng, S.G. Changes of volatile flavor compounds in large yellow croaker (Larimichthys crocea) during Storage, as evaluated by headspace gas chromatography–ion mobility spectrometry and principal component analysis. Foods 2021, 10, 2917. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Chen, J.R.; Chang, C.Y.; Tseng, Y.; Pan, B.S. Potential benefit of I-Tiao-Gung (Glycine tomentella) extract to enhance ornamental fish welfare during live transport. Aquaculture 2020, 534, 736304. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, W.S.; Yan, L.; Glamuzina, B.; Zhang, X. Development and evaluation of an intelligent traceability system for waterless live fish transportation. Food Control 2019, 95, 283–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z.; Xiao, X.; Zhang, X.; Li, D. MW-MTM: A mobile wireless monitoring and traceability management system for water-free live transport of aquatic products. J. Food Process Eng. 2017, 40, e12495. [Google Scholar] [CrossRef]
- Mi, H.B.; Qian, C.L.; Mao, L.C. Quality and biochemical properties of artificially hibernated crucian carp for waterless preservation. Fish Physiol. Biochem. 2012, 38, 1721–1728. [Google Scholar] [CrossRef]
- Salin, K.R. Live transportation of Macrobrachium rosenbergii (De Man) in chilled sawdust. Aquacult. Res. 2015, 36, 300–310. [Google Scholar] [CrossRef]
- Bai, C.; Xiong, G.; Xu, P.; Li, N.; Wang, J.; Liao, T. Effect of cold-anesthetization rate on blood biochemical parameters and muscle composition during live channel catfish Ictalurus punctatus waterless preservation. Fish Sci. 2020, 86, 1043–1053. [Google Scholar] [CrossRef]
- Ross, L.G.; Sanchez, B.J.; Martinez, P.C.; Racotta, I.S.; Toledo Cuevas, M. Anaesthesia, sedation and transportation of juvenile Menidia estor (Jordan) using benzocaine and hypothermia. Aquacult. Res. 2007, 38, 909–917. [Google Scholar] [CrossRef]
- Pramod, P.K.; Ramachandran, A.; Sajeevan, T.P.; Thampy, S.; Pai, S.S. Comparative efficacy of MS-222 and benzocaine as anaesthetics under simulated transport conditions of a tropical ornamental fish Puntius filamentosus (Valenciennes). Aquacult. Res. 2010, 41, 309–314. [Google Scholar] [CrossRef]
- Kugino, K.; Tamaru, S.; Hisatomi, Y.; Sakaguchi, T. Long-duration carbon dioxide anesthesia of fish using ultra fine (Nano-Scale) bubbles. PLoS ONE 2016, 11, e0153542. [Google Scholar] [CrossRef]
- Chalon, J.; Martin, P.; Roberts, C.; Ramanathan, S.; Katz, R.; Turndorf, H. Anaesthetic Uptake by the goldfish: Effect of respiratory rate. Acta Anaesthesiol. Scand. 1983, 27, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, H.; Ueno, S.; Mizuno, H.; Ueda, T.; Fujikawa, H.; Nohara, T.; Fukada, C. Levels of CO2 in arterial blood of carp under carbon dioxide anesthesia. J. Nutr. Sci. Vitaminol. 1982, 28, 35. [Google Scholar] [CrossRef] [PubMed]
- Grttum, J.A.; Sigholt, T. Acute toxicity of carbon dioxide on European seabass (Dicentrarchus labrax): Mortality and effects on plasma ions. Comp. Biochem. Physiol. A Physiol. 1996, 115, 323–327. [Google Scholar] [CrossRef]
- Guan, W.L.; Zhao, M.M.; Liu, T.T.; Fan, X.; Chen, D.W. Cooling combined with hyperoxic CO2 anesthesia is effective in improving the air exposure duration of tilapia. Sci. Rep. 2017, 7, 14016. [Google Scholar] [CrossRef]
- Roth, B.; Slinde, E.; Arildsen, J. Pre or post mortem muscle activity in Atlantic salmon (Salmo salar). The effect on rigor mortis and the physical properties of flesh. Aquaculture 2006, 257, 504–510. [Google Scholar] [CrossRef]
- Marianna, P.; Di, C.B.; Philippe, M.; Huvet, A.; Quillien, V.; Milan, M.; Ferraresso, S.; Pegolo, S.; Patarnello, T.; Bargelloni, L. Understanding the mechanisms involved in the high sensitivity of Pecten maximus larvae to aeration. Aquaculture 2018, 497, 189–199. [Google Scholar]
- Zeng, L.; Ai, C.X.; Zheng, J.L.; Zhang, J.S.; Li, W.C. Cu pre-exposure alters antioxidant defense and energy metabolism in large yellow croaker Larimichthys crocea in response to severe hypoxia. Sci. Total Environ. 2019, 687, 702–711. [Google Scholar] [CrossRef]
- Gang, J.H.; Geewook, S. Efficacy of benzocaine as an anaesthetic for Crucian carp (Carassius carassius). Vet. Anaesth. Analg. 2010, 37, 132–135. [Google Scholar]
- He, H.J.; Wu, D.; Sun, D.W. Rapid and non-destructive determination of drip loss and pH distribution in farmed Atlantic salmon (Salmo salar) fillets using visible and near-infrared (Vis–Nir) hyperspectral imaging. Food Chem. 2014, 156, 394–401. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Devine, C. Ultrastrucvtural observations and pH-measurements on red and white muscle from Antarctic fish. Polar Biol. 1986, 6, 241–246. [Google Scholar] [CrossRef]
- Olsen, S.H.; Sorensen, N.K.; Stormo, S.K.; Elvevoll, E.O. Effect of slaughter methods on blood spotting and residual blood in fillets of Atlantic salmon (Salmo salar). Aquaculture 2006, 258, 462–469. [Google Scholar] [CrossRef]
- Adeyemi, K.D.; Sabow, A.B.; Shittu, R.M.; Karim, R.; Karsani, S.A.; Sazili, A.Q. Impact of chill storage on antioxidant status, lipid and protein oxidation, color, drip loss and fatty acids of semimembranosus muscle in goats. CyTA-J. Food 2016, 14, 405–414. [Google Scholar] [CrossRef]
- Jiang, T.; Yuan, P.; Hirasaka, K.; Hamada, Y.; Hara, K.; Tachibana, K.; Taniyama, S. The effect of blood deposition on the degradation of the connective tissue of the yellowtail Seriola quinqueradiata during storage. Fish Sci. 2019, 85, 1099–1107. [Google Scholar] [CrossRef]
- Lu, D.L.; Ma, Q.; Wang, J.; Li, L.Y.; Han, S.L.; Limbu, S.M.; Li, D.L.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy. J. Physiol. 2019, 597, 1585–1603. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, T.; Shen, J. Effects of cold acclimation and storage temperature on crucian carp (Carassius auratus gibelio) in a waterless preservation. Fish Physiol. Biochem. 2014, 40, 973–982. [Google Scholar]
- Sandblom, E.; Seth, H.; Sundh, H.; Sundell, K.; Axelsson, M.; Kiessling, A. Stress responses in Arctic char (Salvelinus alpinus L.) during hyperoxic carbon dioxide immobilization relevant to aquaculture. Aquaculture 2013, 414–415, 254–259. [Google Scholar] [CrossRef]
- Kolanczyk, R.C.; Fitzsimmons, P.N.; McKim, J.M.; Erickson, R.J.; Schmieder, P.K. Effects of anesthesia (tricaine methanesulfonate, MS222) on liver biotransformation in rainbow trout (Oncorhynchus mykiss). Aquatic. Toxicol. 2003, 64, 177–184. [Google Scholar] [CrossRef]
- Haard, N.F. Control of chemical composition and food quality attributes of cultured fish. Food Res. Int. 1992, 25, 289–307. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Fraser, E.J.; Li, W.Z.; Fang, Y.X.; Taylor, R.R.; Rogers, J.; Brass, A.; Cossins, A.R. Coping with cold: An integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc. Natl. Acad. Sci. USA 2004, 101, 16970–16975. [Google Scholar] [CrossRef]
- Yl-Ajos, M.; Ruusunen, M.; Puolanne, E. The significance of the activity of glycogen debranching enzyme in glycolysis in porcine and bovine muscles. Meat Sci. 2006, 72, 532–538. [Google Scholar] [CrossRef][Green Version]
- Lakshmanan, R.; Parkinson, J.A.; Piggott, J.R. High-pressure processing and water-holding capacity of fresh and cold-smoked salmon (Salmo salar). LWT-Food Sci. Technol. 2007, 40, 544–551. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, S.S.; Wang, S.Q.; Tan, M.Q.; Zhu, B.W. Influence of refrigerated storage on water status, protein oxidation, microstructure, and physicochemical qualities of Atlantic mackerel (Scomber scombrus). Foods 2021, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, W.; Li, J.; Zhang, X.; Zhu, J.; Li, X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control 2012, 25, 101–106. [Google Scholar] [CrossRef]
- Eduardo, E.; Luís, G.; Jaime, A. Effects of vacuum and modified atmosphere packaging on the quality and shelf-life of gray triggerfish (Balistes capriscus) fillets. Foods 2021, 10, 250. [Google Scholar]
- Cai, L.; Wu, X.; Li, X.; Zhong, K.; Li, Y.; Li, J. Effects of different freezing treatments on physicochemical responses and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT-Food Sci. Technol. 2021, 59, 122–129. [Google Scholar] [CrossRef]
- Sungsri, I.R.; Benjakul, S.; Kijroongrojana, K. Pink discoloration and quality changes of squid (Loligo formosana) during iced storage. LWT-Food Sci. Technol. 2011, 44, 206–213. [Google Scholar] [CrossRef]
- Aranda, R.; He, C.; Worley, C.E.; Levin, E.J.; Li, R.; Olson, J.S.; Phillips, G.N.; Richards, M.P. Structural analysis of fish versus mammalian hemoglobins: Effect of the heme pocket environment on autooxidation and hemin loss. Proteins 2009, 75, 217–230. [Google Scholar] [CrossRef]
- Jiang, T.; Miyazaki, R.; Hirasaka, K.; Yuan, P.X.; Yoshida, A.; Hara, K.; Tachibana, K.; Taniyama, S. Effect of blood deposition phenomenon on flesh quality of yellowtail (Seriola quinqueradiata) during storage. J. Texture Stud. 2019, 50, 325–331. [Google Scholar] [CrossRef]
- Wulf, D.M.; Wise, J.W. Measuring muscle color on beef carcasses using the L*a*b* color space. J. Anim. Sci. 1999, 77, 2418–2427. [Google Scholar] [CrossRef]
- Vijayan, M.M.; Leatherland, J.F. Effect of stocking density on the growth and stress-response in brook charr, Salvelinus fontinalis. Aquaculture 1998, 75, 159–170. [Google Scholar] [CrossRef]
- Stien, L.H.; Hirmas, E.; Bjørnevik, M.; Karlsen, O.; Nortvedt, R.; Rora, A.M.B.; Sunde, J.; Kiessling, A. The effects of stress and storage temperature on the colour and texture of pre-rigor filleted farmed cod (Gadus morhua L.). Aquacult. Res. 2010, 36, 1197–1206. [Google Scholar] [CrossRef]
- Yang, X.Y.; Sebranek, J.G.; Luo, X.; Zhang, W.G.; Zhang, M.M.; Xu, B.C.; Zhang, Y.M.; Liang, R.R. Effects of calcium salts on the physicochemical quality of cured beef sausages during manufacturing and storage: A potential calcium application for sausages with alginate casings. Foods 2021, 10, 2783. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.G.; Fjaera, S.O.; Skjervold, P.O. Salmon fillet texture is determined by myofiber-myofiber and myofiber-myocommata attachment. J. Food Sci. 2010, 67, 2067–2071. [Google Scholar] [CrossRef]
- Hernández, M.D.; López, M.B.; Álvarez, A.; Ferrandini, E.; García García, B.; Garrido, M.D. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- Johnston, I.A.; Li, X.; Vieira, V.; Nickell, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculture 2006, 256, 323–336. [Google Scholar] [CrossRef]
- Stoller, G.M.; Zerby, H.N.; Moeller, S.J.; Baas, T.J.; Johnson, C.; Watkins, L.E. The effect of feeding ractopamine (Paylean) on muscle quality and sensory characteristics in three diverse genetic lines of swine. J. Anim. Sci. 2003, 81, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of iced storage of bigeye snapper (Priacanthus tayenus) on the chemical composition, properties and acceptability of Som-fug, a fermented Thai fish mince. Food Chem. 2007, 102, 270–280. [Google Scholar] [CrossRef]




| CO2 anesthesia | CO2 Aeration Rate (ppm/s) | Survival/Total (Survival Rate) | Survival Time (h) |
| 2.0 | 10/11 (90.90%) | 3.5 | |
| 7.0 | 7/12 (58.33%) | 2.5 | |
| 12.0 | 4/11 (36.36%) | 1.5 | |
| 17.0 | 1/10 (10.00%) | 1.0 | |
| 22.0 | 0/13 (0.00%) | 0.2 | |
| Hibernation | Cooling Rate (°C/h) | Survival/Total (Survival Rate) | Survival Time (h) |
| 1.0 | 9/10 (90.00%) | 30.0 | |
| 3.0 | 6/11 (54.54%) | 26.0 | |
| 5.0 | 3/9 (33.33%) | 11.0 | |
| 7.0 | 1/12 (8.33%) | 1.0 | |
| 9.0 | 0/12 (0.00%) | 0.0 |
| Hibernation combined with CO2 aeration rate of 2 ppm/s | Cooling Rate (°C/h) | Survival/Total (Survival Rate) | Survival Time (h) |
| 1.0 | 9/10 (90.00%) | 36.0 | |
| 3.0 | 9/10 (90.00%) | 36.0 | |
| 5.0 | 5/10 (50.00%) | 15.0 |
| Stage | LYM (109/L) | RBC (1012/L) | HGB (g/L) | HCT (%) | MCV (fL) | MCH (pg) |
|---|---|---|---|---|---|---|
| Before hibernation | 2.03 ± 0.11 c | 0.79 ± 0.02 b | 17.08 ± 0.76 b | 13.69 ± 0.10 b | 173.34 ± 0.23 a | 21.61 ± 0.52 a |
| During hibernation | 3.10 ± 0.04 b | 0.93 ± 0.04 a | 19.89 ± 0.16 a | 14.83 ± 0.19 a | 159.15 ± 0.50 b | 21.36 ± 1.09 a |
| After recovery | 3.97 ± 0.07 a | 0.87 ± 0.06 ab | 19.84 ± 0.44 a | 14.45 ± 0.33 a | 165.90 ± 0.73 ab | 21.87 ± 0.93 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, N.; Gao, Y.; Wang, Y.; Deng, S.; Yuan, P.; Jiang, T.; Zheng, W. The Influence of Hypothermia Hibernation Combined with CO2 Anesthesia on Life and Storage Quality of Large Yellow Croaker (Pseudosciaena crocea). Foods 2022, 11, 514. https://doi.org/10.3390/foods11040514
Tan N, Gao Y, Wang Y, Deng S, Yuan P, Jiang T, Zheng W. The Influence of Hypothermia Hibernation Combined with CO2 Anesthesia on Life and Storage Quality of Large Yellow Croaker (Pseudosciaena crocea). Foods. 2022; 11(4):514. https://doi.org/10.3390/foods11040514
Chicago/Turabian StyleTan, Nanfeng, Yuanpei Gao, Yueke Wang, Shanggui Deng, Pengxiang Yuan, Tong Jiang, and Wanyuan Zheng. 2022. "The Influence of Hypothermia Hibernation Combined with CO2 Anesthesia on Life and Storage Quality of Large Yellow Croaker (Pseudosciaena crocea)" Foods 11, no. 4: 514. https://doi.org/10.3390/foods11040514
APA StyleTan, N., Gao, Y., Wang, Y., Deng, S., Yuan, P., Jiang, T., & Zheng, W. (2022). The Influence of Hypothermia Hibernation Combined with CO2 Anesthesia on Life and Storage Quality of Large Yellow Croaker (Pseudosciaena crocea). Foods, 11(4), 514. https://doi.org/10.3390/foods11040514






