Lacticaseibacillus rhamnosus GG Survival and Quality Parameters in Kefir Produced from Kefir Grains and Natural Kefir Starter Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Kefir Samples
2.2. Manufacturing of Kefir Beverages
2.3. Physicochemical Analysis of Kefir: pH, Total Titratable Acidity (TTA), Syneresis
2.4. Enumeration of LGG
2.5. Sensory Analysis of Kefir
2.6. Determination of Apparent Viscosity and Gel Structure of Kefir
2.7. Statistical Analysis
3. Results and Discussion
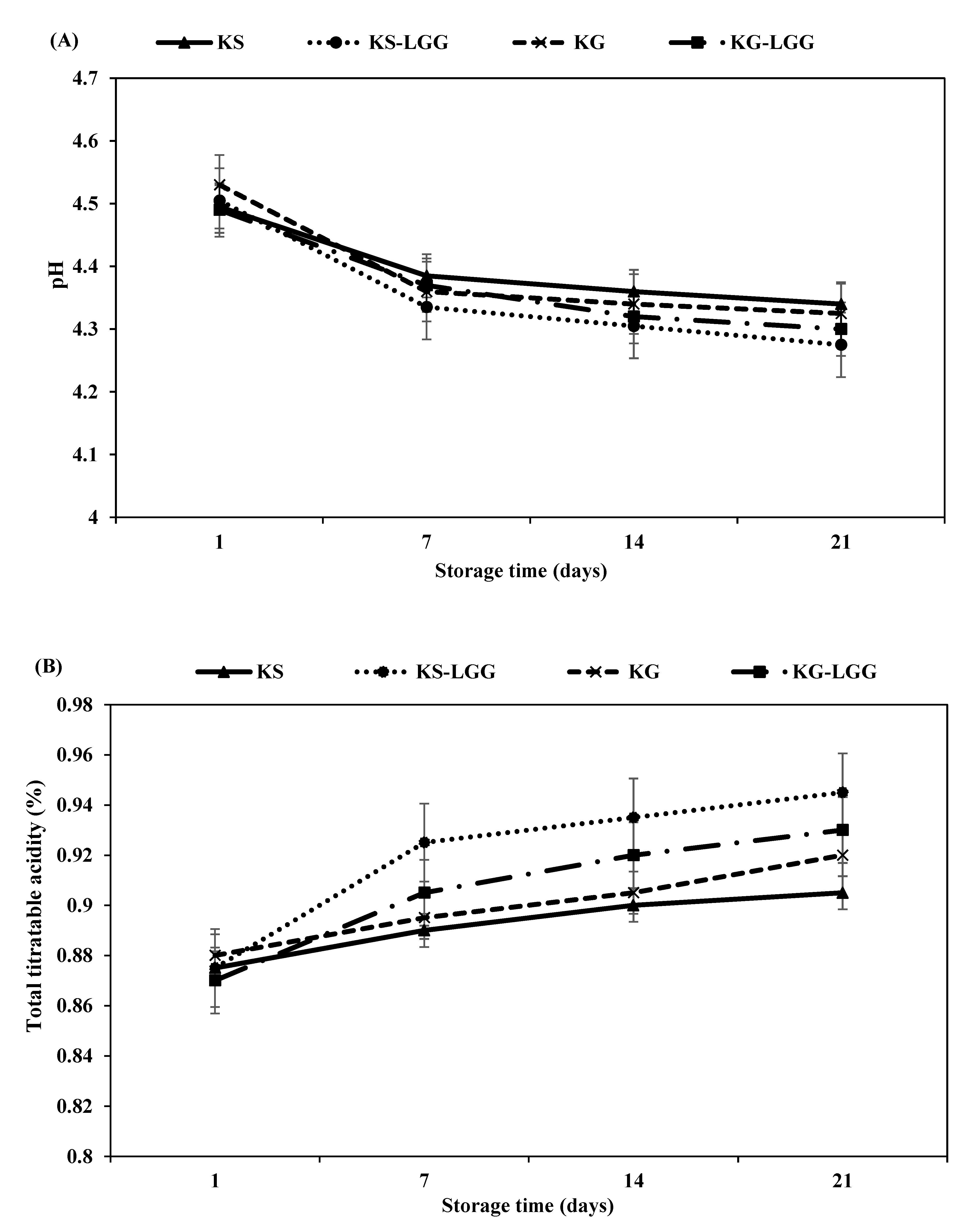
3.1. PH and TTA of Kefir
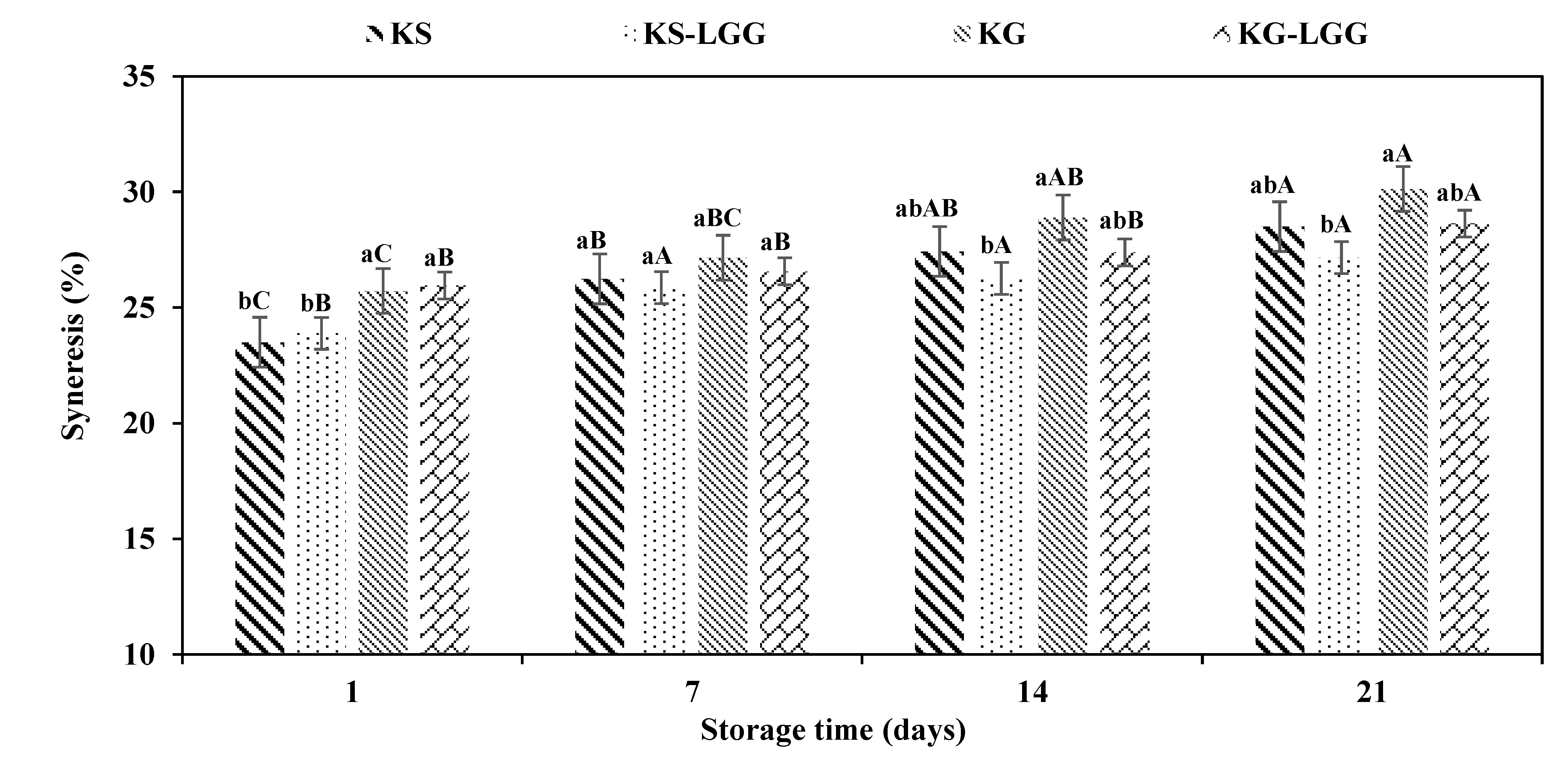
3.2. Syneresis of Kefir
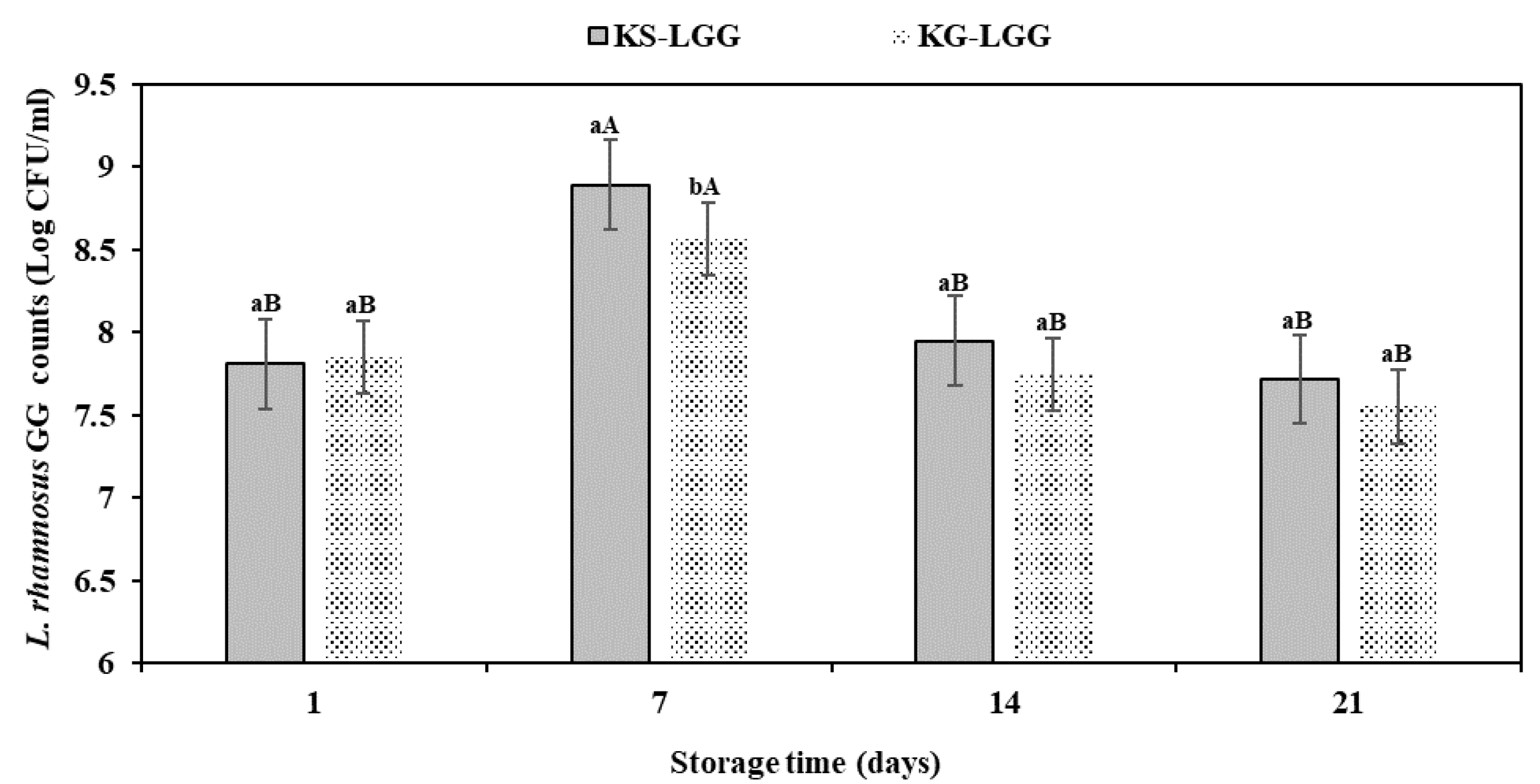
3.3. Viability of LGG during Kefir Storage
3.4. Sensory Acceptance of Kefir
3.5. Rheological Characteristics of Kefir
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Monteiro, D.; Faria, S.; Cruz, S.; Perreira, A.; Roque, A.C.; Tavares, P. Short communication: Effect of kefir grains on proteolysis of major milk proteins. J. Dairy Sci. 2010, 93, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azi, F.; Tu, C.; Meng, L.; Zhiyu, L.; Cherinet, M.T.; Ahmadullah, Z.; Dong, M. Metabolite dynamics and phytochemistry of a soy whey-based beverage bio-transformed by water kefir consortium. Food Chem. 2021, 16, 128–225. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods 2019, 58, 56–66. [Google Scholar] [CrossRef]
- Lopitz-Otsoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev. Iberoam Micol. 2006, 23, 67–74. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Neubauer, P. A big world in small grain: A review of natural milk kefir starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Vardjan, T.; Mohar Lorb, P.; Rogelj, I.; Čanžek, M.A. Characterization and stability of lactobacilli and yeast microbiota in kefir grains. J. Dairy Sci. 2013, 96, 2729–2736. [Google Scholar] [CrossRef] [Green Version]
- Gagliarini, N.; Diosma, G.; Garrote, G.L.; Abraham, A.G.; Piermaria, J. Whey protein-kefiran films as driver of probiotics to the gut. LWT 2019, 105, 321–328. [Google Scholar] [CrossRef]
- Erdogan, F.S.; Ozarslan, S.; Guzel-Seydim, Z.B.; Kök Taş, T. The effect of kefir produced from natural kefir grains on the intestinal microbial populations and antioxidant capacities of Balb/c mice. Food Res. J. 2019, 115, 408–413. [Google Scholar] [CrossRef]
- Kök-Taş, T.; Seydim, A.C.; Özer, B.; Guzel-Seydim, Z.B. Effects of different fermentation parameters on quality characteristics of kefir. J. Dairy Sci. 2013, 96, 780–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarbati, A.; Ciani, M.; Canonico, L.; Galli, E.; Comitini, F. Exploitation of yeasts with probiotic traits for kefir production: Effectiveness of the microbial consortium. Fermentation 2022, 8, 9. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; Cruz, A.G.D. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Chugh, B.; Kamal-Eldin, A. Bioactive compounds produced by probiotics in food products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Mattila-Sandholm, T.; Blum, S.; Collins, J.K.; Crittenden, R.; de Vos, W.; Dunne, C.; Fondén, R.; Grenov, G.; Isolauri, E.; Kiely, B.; et al. Probiotics: Towards demonstrating efficacy. Trends Food Sci. Technol. 1999, 10, 393–399. [Google Scholar] [CrossRef]
- Vimercati, W.C.; Araújo, C.D.S.; Macedo, L.L.; Fonseca, H.C.; Guimarães, J.S.; Abreu, L.R.D.; Pinto, S.M. Physicochemical, rheological, microbiological and sensory properties of newly developed coffee flavored kefir. LWT 2020, 123, 109069. [Google Scholar] [CrossRef]
- Nyanzi, R.; Jooste, P.J.; Buys, E.M. Invited review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. Int. J. Dairy Sci. 2021, 104, 1–19. [Google Scholar] [CrossRef]
- Buran, İ.; Akal, C.; Ozturkoglu-Budak, S.; Yetisemiyen, A. Rheological, sensorial and volatile profiles of synbiotic kefirs produced from cow and goat milk containing varied probiotics in combination with fructooligosaccharide. LWT 2021, 148, 111591. [Google Scholar] [CrossRef]
- Oliveira, R.P.D.S.; Perego, P.; Oliveira, M.N.; Converti, A. Effect of inulin as a prebiotic to improve growth and counts of a probiotic cocktail in fermented skim milk. LWT 2011, 44, 520–523. [Google Scholar] [CrossRef]
- Valik, L.; Medvedova, A.; Lipatkova, D. Characterization of the growth of Lactobacillus rhamnosus GG. J. Food Nutr. Res. 2008, 47, 60–67. [Google Scholar]
- Innocente, N.; Biasutti, M.; Rita, F.; Brichese, R.; Comi, G.; Iacumin, L. Effect of indigenous Lactobacillus rhamnosus isolated from bovine milk on microbiological characteristics and aromatic profile of traditional yogurt. LWT 2016, 66, 158–164. [Google Scholar] [CrossRef]
- Galli, B.D.; Baptista, D.P.; Cavalheiro, F.G.; Negrão, F.; Eberlin, M.N.; Gigante, M.L. Peptide profile of Camembert-type cheese: Effect of heat treatment and adjunct culture Lactobacillus rhamnosus GG. Int. Food Res. J. 2019, 123, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Zobrist, S.; Donahue, C.; Edick, C.; Mansen, K.; Nadjari, M.H.Z.; Heerikhuisen, M.; Sybesma, W.; Molenaar, D.; Diallo, A.M.; et al. Naturally fermented milk from northern Senegal: Bacterial community composition and probiotic enrichment with Lactobacillus rhamnosus. Front. Microbiol. 2018, 9, 2218. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Godínez, J.; Gutiérrez-Rodríguez, J.F.; Contreras-López, E.; Rodríguez-Serrano, G.M.; Castañeda-Ovando, A.; Jaimez-Ordaz, J.; González-Olivares, L.G. Agave juice improves survival and proteolytic activity of Lactobacillus rhamnosus GG during ripening of semi-ripened mexican cheese. Food Sci. Technol. 2021, 2061, 6–11. [Google Scholar] [CrossRef]
- Rodgers, S. Preserving non-fermented refrigerated foods with microbial cultures—A review. Trends Food Sci. Technol. 2001, 12, 276–284. [Google Scholar] [CrossRef]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibáñez, F.C. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Hussain, N.; Li, R.; Takala, T.M.; Tariq, M.; Zaidi, A.H.; Saris, P.E.J. Generation of lactose- and protease-positive probiotic Lacticaseibacillus rhamnosus GG by conjugation with Lactococcus lactis NCDO 712. Appl. Environ. Microbiol. 2021, 87, e02957-20. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2005; p. 7. [Google Scholar]
- Aryana, K.J.A.R. Folic acid fortified fat-free plain set yoghurt. Int. J. Dairy Technol. 2003, 56, 219–222. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Hussain, N.A.; Niamah, A.K. Antibacterial spectrum of produced reuterin from new isolates of Lactobacillus reuteri. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 134–139. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Yousefvand, A.; Ahmadi Gavlighi, H.; Zarei, M.; Farhangnia, P. Developing novel synbiotic low-fat yogurt with fucoxylogalacturonan from tragacanth gum: Investigation of quality parameters and Lactobacillus casei survival. Food Sci. Nutr. 2020, 8, 4491–4504. [Google Scholar] [CrossRef]
- Karaca, O.B.; Güzeler, N.; Tangüler, H.; Yaşar, K.; Akın, M.B. Effects of apricot fibre on the physicochemical characteristics, the sensory properties and bacterial viability of nonfat probiotic yoghurts. Foods 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Ghosh, B.C. Quality characteristics of kefir as a carrier for probiotic Lactobacillus rhamnosus GG. Int. J. Dairy Technol. 2019, 70, 384–391. [Google Scholar] [CrossRef]
- Collar, C. Review: Biochemical and technological assessment of the metabolism of pure and mixed cultures of yeast and lactic acid bacteria in breadmaking. Int. Food Sci. Technol. 1996, 2, 349–367. [Google Scholar] [CrossRef]
- Kesenkas, H.; Ddnkcd, N.; Seckdn, K.; Kinik, Ö.; Gonc, S.; Ergonul, P.G.; Kavas, G.; Gökhan, A. Physicochemical, microbiological and sensory characteristics of Soymilk Kefir Harun. Afr. J. Microbiol. Res. 2011, 22, 3737–3746. [Google Scholar]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Montanuci, F.D.; Pimentel, T.C.; Garcia, S.; Prudencio, S.H. Effect of starter culture and inulin addition on microbial viability, texture, and chemical characteristics of whole or skim milk kefir. Food Sci. Technol. 2012, 32, 580–865. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, B.A.; Öner, M.D. Production and evaluation of yogurt with concentrated grape juice. J. Food Sci. 1999, 64, 530–532. [Google Scholar] [CrossRef]
- Vogado, C.D.O.; Leandro, E.D.S.; Zandonadi, R.P.D.; Alencar, E.R.; Ginani, V.C.; Nakano, E.Y.; Habú, S.; Aguiar, P.A. Enrichment of probiotic fermented milk with green banana pulp: Characterization microbiological, physicochemical and sensory. Nutrients 2018, 10, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yerlikaya, O.; Akpinar, A.; Saygili, D. Analysis of some physicochemical, rheological, sensorial properties, and probiotic viability of fermented milks containing Enterococcus faecium and Enterococcus durans strains. J. Food Process. Preserv. 2020, 44, e14553. [Google Scholar] [CrossRef]
- Delgadillo, J.O.V.; Lara, M.D.J.L.; Santillan, C.K.L.; Sampieri, C.B.; Micloth, L.D.C.L. Physicochemical and rheological characterization of an acidic milk product: Kefir concentration effect. J. Food Eng. 2017, 7, 86–92. [Google Scholar]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohyd. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Vareltzis, P.; Adamopoulos, K.; Stavrakakis, E.; Stefanakis, A.; Goula, A.M. Approaches to minimise yoghurt syneresis in simulated tzatziki sauce preparation. Int. J. Dairy Technol. 2016, 69, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Branco, G.F.; Cruz, A.G.; de Assis Fonseca Faria, J.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. 2010, 9, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Witthuhn, R.C.; Schoeman, T.; Britz, T.J. Isolation and characterization of the microbial population of different South African kefir grains. Int. J. Dairy Technol. 2004, 57, 33–37. [Google Scholar] [CrossRef]
- Saxelin, M. Lactobacillus GG—A human probiotic strain with thorough clinical documentation. Food Rev. Int. 1997, 13, 293–313. [Google Scholar] [CrossRef]
- Savijoki, K.; Nyman, T.A.; Kainulainen, V.; Miettinen, I.; Siljamäki, P.; Fallarero, A.; Sandholm, J.; Satokari, R.; Varmanen, P. Growth mode and carbon source impact the surfaceome dynamics of Lactobacillus rhamnosus GG. Front. Microbiol. 2019, 10, 1272. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.N.; Sodini, I.; Remeuf, F.; Corrieu, G. Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int. Dairy J. 2001, 11, 935–942. [Google Scholar] [CrossRef]
- Oliveira, R.P.D.S.; Perego, P.; Converti, A.; Oliveira, M.N. The effect of inulin as a prebiotic on the production of probiotic fibre-enriched fermented milk. Int. J. Dairy Technol. 2009, 62, 195–203. [Google Scholar] [CrossRef]
- Oliveira, R.P.D.S.; Perego, P.; Oliveira, M.N.; Converti, A. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT 2012, 47, 358–363. [Google Scholar] [CrossRef]
- Leite, A.M.D.O.; Miguel, M.A.L.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.F. Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Braz. J. Microbiol. 2013, 349, 341–349. [Google Scholar] [CrossRef]
- Hekmat, S.; Reid, G. Sensory properties of probiotic yogurt is comparable to standard yogurt. Nutr. Res. 2006, 26, 163–166. [Google Scholar] [CrossRef]
- Østlie, H.M.; Helland, M.H.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria in milk. Int. J. Food Microbiol. 2003, 15, 17–27. [Google Scholar] [CrossRef]
- Østlie, H.M.; Treimo, J.; Narvhus, J.A. Effect of temperature on growth and metabolism of probiotic bacteria in milk. Int. Dairy J. 2005, 15, 989–997. [Google Scholar] [CrossRef]
- Kiekens, S.; Vandenheuvel, D.; Broeckx, G.; Claes, I.; Allonsius, C.; De Boeck, I.; Thys, S.; Timmermans, J.P.; Kiekens, F.; Lebeer, S. Impact of spray-drying on the pili of Lactobacillus rhamnosus GG. Microb. Biotechnol. 2019, 12, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Galli, B.D.; Baptista, D.P.; Cavalheiro, F.G.; Gigante, M.L. Lactobacillus rhamnosus GG improves the sensorial profile of Camembert-type cheese: An approach through flash-profile and CATA. LWT 2019, 107, 72–78. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface Sci. 2009, 147–148, 214–227. [Google Scholar] [CrossRef]
- Barukčić, I.; Gracin, L.; Jambrak, A.R.; Božanić, R. Comparison of chemical, rheological and sensory properties of kefir produced by kefir grains and commercial kefir starter. Mljekarstvo 2017, 67, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.E. Flow and thixotropic parameters for rheological characterization of hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Hoshizawa, H.; Minemura, Y.; Yoshikawa, K.; Suzuki, M.; Hanabusa, K. Thixotropic hydrogelators based on a cyclo(dipeptide) derivative. Langmuir 2013, 29, 14666–14673. [Google Scholar] [CrossRef]
- Beshkova, D.M.; Simova, E.D.; Simov, Z.I.; Frengova, G.I.; Spasov, Z.N. Pure cultures for making kefir. Food Microbiol. 2002, 19, 537–544. [Google Scholar] [CrossRef]
- Amatayakul, T.; Halmos, A.L.; Sherkat, F.; Shah, N.P. Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int. Dairy J. 2006, 16, 40–51. [Google Scholar] [CrossRef]
- Degeest, B.; Mozzi, F.; De Vuyst, L. Effect of medium composition and temperature and pH changes on exopolysaccharide yields and stability during Streptococcus thermophilus LY03 fermentations. Int. J. Food Microbiol. 2002, 79, 161–174. [Google Scholar] [CrossRef]
- Purwandari, U.; Shah, N.P.; Vasiljevic, T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. Int. Dairy J. 2007, 17, 1344–1352. [Google Scholar] [CrossRef]
- Pham, P.L.; Dupont, I.; Roy, D.; Lapointe, G.; Cerning, J. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl. Environ. Microbiol. 2000, 66, 2302–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensmira, M.; Jiang, B. Effect of some operating variables on the microstructure and physical properties of a novel kefir formulation. J. Food Eng. 2012, 108, 579–584. [Google Scholar] [CrossRef]
- Vlahopoulou, I.; Bell, A.E. Effect of various starter cultures on the viscoelastic properties of bovine and caprine yogurt gels. J. Soc. Dairy Technol. 1993, 46, 61–63. [Google Scholar] [CrossRef]
- Vlahopoulou, I.; Bell, A.E.; Wilbey, R. Effects of starter culture and its exopolysaccharides on the gelation of glucono-δ-lactone-acidified bovine and caprine milk. Int. J. Dairy Technol. 2001, 54, 135–140. [Google Scholar] [CrossRef]

| Kefir Formulations 1 | Sensory Attributes | |||
|---|---|---|---|---|
| Flavor | Body and Texture | Color and Appearance | Overall Acceptability | |
| KS | 4.46 ± 0.63 a | 4.20 ± 0.84 a | 4.00 ± 0.84 a | 4.26 ± 0.45 a |
| KS-LGG | 3.20 ± 0.67 b | 3.93 ± 1.09 a | 4.33 ± 0.61 a | 3.80 ± 0.86 a |
| KG | 3.60 ± 0.82 b | 4.26 ± 0.70 a | 4.40 ± 0.84 a | 3.86 ± 0.51 a |
| KG-LGG | 3.20 ± 0.73 b | 4.06 ± 0.79 a | 4.00 ± 0.77 a | 4.06 ± 0.70 a |
| Kefir Formulations 1 | Storage Period (d) | |||
|---|---|---|---|---|
| 1 | 7 | 14 | 21 | |
| KS | 1269.6 ± 173.7 a,A | 1300.0 ± 0.0 a,A | 1340.0 ± 28.3 a,A | 1345.0 ± 7.1 a,A |
| KS-LGG | 1225.8 ± 6.2 a,A | 1315.0 ± 7.1 a,A | 1175.0 ± 120.2 a,A | 1250.0 ± 56.6 a,A |
| KG | 1197.1 ± 50.2 a,A | 1225.0 ± 77.8 a,A | 1240.0 ± 56.6 a,A | 1200.0 ± 0.0 a,A |
| KG-LGG | 1262.9 ± 25.5 a,A | 1360 ± 56.6 a,A | 1275 ± 49.5 a,A | 1260 ± 14.4 a,A |
| Kefir Formulations 1 | Storage Period (d) | |||
|---|---|---|---|---|
| 1 | 7 | 14 | 21 | |
| KS | 3094.5 ± 481.5 a,A | 3116.5 ± 30.4 a,A | 3067.5 ± 152.0 a,A | 3075.5 ± 0.7 a,A |
| KS-LGG | 3010 ± 42.4 a,A | 3160.5 ± 29.0 a,A | 2667 ± 272.9 a,A | 2834 ± 183.8 a,A |
| KG | 2646 ± 152.7 a,A | 2592.5 ± 191.6 a,A | 2657 ± 161.2 a,A | 2520 ± 19.8 a,A |
| KG-LGG | 2975.5 ± 40.3 a,A | 3186.5 ± 180.3 a,A | 2925 ± 100.4 a,A | 2837.5 ± 57.3 a,A |
| Kefir Formulations 1 | Storage Period (d) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d 1 | d 7 | d 14 | d 21 | |||||||||
| G′ | G″ | tan(δ) | G′ | G″ | tan(δ) | G′ | G″ | tan(δ) | G′ | G″ | tan(δ) | |
| KS | 200.6 ± 73.4 a,A | 53.5 ± 20.0 a,A | 0.266 ± 0.002 a,A | 177.4 ± 5.7 a,A | 44.2 ± 1.0 a,A | 0.249 ± 0.019 a,A | 181.8 ± 1.27 a,A | 46.2 ± 2.6 a,A | 0.254 ± 0.012 a,A | 190.3 ± 24.0 a,A | 46.4 ±5.6 a,A | 0.244 ± 0.001 a,A |
| KS-LGG | 156.8 ± 12.2 a,A | 42.3 ± 1.80 a,A | 0.270 ± 0.009 a,A | 176.5 ± 17.3 a,A | 46.2 ± 1.6 a,A | 0.262 ± 0.016 a,A | 171.1 ± 17.8 a,A | 43.4 ± 1.5 a,A | 0.254 ± 0.017 a,A | 160.3 ± 9.0 a,A | 42.3 ± 1.3 a,A | 0.253 ± 0.005 a,A |
| KG | 146.6 ± 0.1 a,A | 40.3 ± 3.1 a,A | 0.274 ± 0.021 a,A | 186.9 ± 31.3 a,A | 46.8 ± 8.5 a,A | 0.250 ± 0.003 a,A | 153.0 ± 28.2 a,A | 38.3 ± 7.8 a,A | 0.249 ± 0.005 a,A | 119.3 ± 7.4 a,A | 30.1 ± 0.7 a,A | 0.252 ± 0.009 a,A |
| KG-LGG | 203.8 ± 2.3 a,A | 53.7 ± 0.1 a,A | 0.263 ± 0.002 a,A | 185.5 ± 16.9 a,A | 46.0 ± 4.3 a,A | 0.248 ± 0.009 a,A | 143.1 ± 11.0 a,A | 35.2 ± 3.7 a,A | 0.246 ± 0.007 a,A | 153.9 ± 26.2 a,A | 36.9 ± 0.0 a,A | 0.239 ± 0.003 a,A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefvand, A.; Huang, X.; Zarei, M.; Saris, P.E.J. Lacticaseibacillus rhamnosus GG Survival and Quality Parameters in Kefir Produced from Kefir Grains and Natural Kefir Starter Culture. Foods 2022, 11, 523. https://doi.org/10.3390/foods11040523
Yousefvand A, Huang X, Zarei M, Saris PEJ. Lacticaseibacillus rhamnosus GG Survival and Quality Parameters in Kefir Produced from Kefir Grains and Natural Kefir Starter Culture. Foods. 2022; 11(4):523. https://doi.org/10.3390/foods11040523
Chicago/Turabian StyleYousefvand, Amin, Xin Huang, Mehdi Zarei, and Per Erik Joakim Saris. 2022. "Lacticaseibacillus rhamnosus GG Survival and Quality Parameters in Kefir Produced from Kefir Grains and Natural Kefir Starter Culture" Foods 11, no. 4: 523. https://doi.org/10.3390/foods11040523







