Transcriptome Analysis of Halotolerant Staphylococcus saprophyticus Isolated from Korean Fermented Shrimp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. RNA Extraction, Library Construction, and Sequencing
2.3. Transcriptomic Data Analysis
2.4. Differential Expression and Functional Enrichment Analyses
3. Results
3.1. Growth of S. saprophyticus in Different NaCl Concentrations
3.2. Overview of the Transcriptome Analysis
3.3. Functional Enrichment Analysis
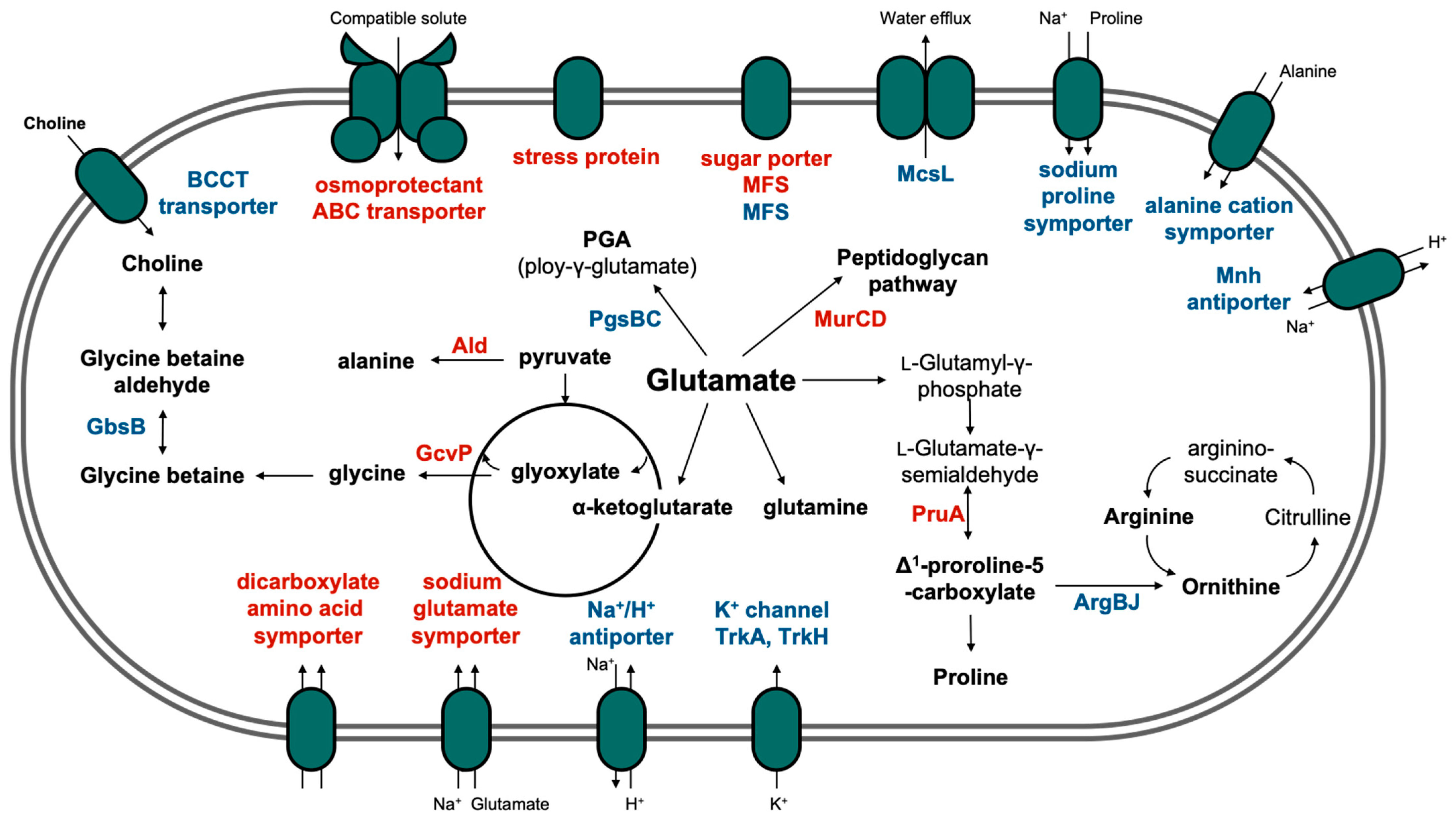
3.4. Analysis of Membrane Transporter Proteins
3.5. Pathway Analysis of Compatible Solutes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koo, O.K.; Lee, S.J.; Chung, K.R.; Jang, D.J.; Yang, H.J.; Kwon, D.Y. Korean traditional fermented fish products: Jeotgal. J. Ethn. Food. 2016, 3, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.-T.; Hwang, J.-K.; Baik, I.-H. Effects of jeotkal addition on quality of Kimchi. Korean J. Food Sci. Technol. 2004, 36, 123–128. [Google Scholar]
- Guan, L.; Cho, K.H.; Lee, J.H. Analysis of the cultivable bacterial community in jeotgal, a Korean salted and fermented seafood, and identification of its dominant bacteria. Food Microbiol. 2011, 28, 101–113. [Google Scholar] [CrossRef]
- Song, E.J.; Lee, E.S.; Park, S.L.; Choi, H.J.; Roh, S.W.; Nam, Y.D. Bacterial community analysis in three types of the fermented seafood, jeotgal, produced in South Korea. Biosci. Biotechnol. Biochem. 2018, 82, 1444–1454. [Google Scholar] [CrossRef]
- Yaakop, A.S.; Chan, K.G.; Ee, R.; Lim, Y.L.; Lee, S.K.; Manan, F.A.; Goh, K.M. Characterization of the mechanism of prolonged adaptation to osmotic stress of Jeotgalibacillus malaysiensis via genome and transcriptome sequencing analyses. Sci. Rep. 2016, 6, 33660. [Google Scholar] [CrossRef]
- Chun, B.H.; Han, D.M.; Kim, K.H.; Jeong, S.E.; Park, D.; Jeon, C.O. Genomic and metabolic features of Tetragenococcus halophilus as revealed by pan-genome and transcriptome analyses. Food Microbiol. 2019, 83, 36–47. [Google Scholar] [CrossRef]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef] [Green Version]
- Detkova, E.N.; Boltyanskaya, Y.V. Osmoadaptation of haloalkaliphilic bacteria: Role of osmoregulators and their possible practical application. Microbiology 2007, 76, 511–522. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Jin, H.M.; Hahn, Y.; Madsen, E.L.; Jeon, C.O. Metatranscriptomic analysis of lactic acid bacterial gene expression during kimchi fermentation. Int. J. Food Microbiol. 2013, 163, 171–179. [Google Scholar] [CrossRef]
- Pawar, S.; Yao, X.; Lu, C. Transcriptomic data for analyzing global gene expression patterns in Methicillin-resistance Staphylococcus aureus in response to spermine and oxacillin stress. Data Br. 2018, 21, 2230–2236. [Google Scholar] [CrossRef]
- Senizza, A.; Callegari, M.L.; Senizza, B.; Minuti, A.; Rocchetti, G.; Morelli, L.; Patrone, V. Effects of linoleic acid on gut-derived Bifidobacterium breve DSM 20213: A transcriptomic approach. Microorganisms 2019, 7, 710. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.B.; Hwang, S.; Baek, S.-W.; Lee, S.; Bang, W.Y.; Moon, K.H. In vitro Edwardsiella piscicida CK108 transcriptome profiles with subinhibitory concentrations of phenol and formalin reveal new insights into bacterial pathogenesis mechanisms. Microorganisms 2020, 8, 1068. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Hou, Y.; Soteyome, T.; Zeng, B.; Su, J.; Li, L.; Li, B.; Chen, D.; Li, Y.; et al. Transcriptomics study on Staphylococcus aureus biofilm under low concentration of ampicillin. Front. Microbiol. 2018, 9, 2413. [Google Scholar] [CrossRef] [Green Version]
- Vermassen, A.; Dordet-Frisoni, E.; de La Foye, A.; Micheau, P.; Laroute, V.; Leroy, S.; Talon, R. Adaptation of Staphylococcus xylosus to nutrients and osmotic stress in a salted meat model. Front. Microbiol. 2016, 7, 87. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Jung, J.; Jeon, C.O.; Park, W. Comparative genomic and transcriptomic analyses of NaCl-tolerant Staphylococcus sp. OJ82 isolated from fermented seafood. Appl. Microbiol. Biotechnol. 2014, 98, 807–822. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2017. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 September 2021).
- Krueger, F. Trim Galore! A Wrapper Tool around Cutadapt and FastQC. 2012. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 15 September 2021).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Biorxiv 2014, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. 2019. Available online: https://bioconductor.org/packages/release/bioc/vignettes/EnhancedVol-cano/inst/doc/EnhancedVolcano.html#references (accessed on 29 December 2021).
- Yan, L. ggvenn: Draw Venn Diagram by ‘ggplot2’. 2021. Available online: https://cran.r-project.org/web/packages/ggvenn/ggvenn.pdf (accessed on 29 December 2021).
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant graphics for data analysis (2nd ed.). Meas.-Interdiscip. Res. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.7. 2021. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 29 December 2021).
- Youn, J.-W.; Jolkver, E.; Krämer, R.; Marin, K.; Wendisch, V.F. Characterization of the dicarboxylate transporter DctA in Corynebacterium glutamicum. J. Bacteriol. 2009, 191, 5480–5488. [Google Scholar] [CrossRef] [Green Version]
- Sakuraba, H.; Kawakami, R.; Takahashi, H.; Ohshima, T. Novel archaeal Alanine: Glyoxylate aminotransferase from Thermococcus litoralis. J. Bacteriol. 2004, 186, 5513–5518. [Google Scholar] [CrossRef] [Green Version]
- Mueller, E.A.; Levin, P.A. Bacterial cell wall quality control during environmental stress. Mbio 2020, 11, e02456-20. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; Guinane, C.M.; Crispie, F.; Kilcawley, K.N.; McSweeney, P.L.; Rea, M.C. Genome sequence of Staphylococcus saprophyticus DPC5671, a strain isolated from cheddar cheese. Genome Announc. 2017, 5, e00193-17. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Jeong, D.-W.; Lee, J.-H. Genetic diversity and antibiotic resistance of Staphylococcus saprophyticus isolates from fermented foods and clinical samples. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 659–668. [Google Scholar] [CrossRef]
- Khusro, A.; Aarti, C.; Salem, A.Z.M.; Barbabosa-Pilego, A. Techno-functional traits and safety aspects of coagulase-negative Staphylococcus saprophyticus isolated from traditional fermented food. Food Biotechnol. 2020, 34, 77–99. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Brinks, E.; Beshiru, A.; Igbinosa, I.H.; Stein, M.; Cho, G.S.; Franz, C.M.A.P. Draft genome sequences of five Staphylococcus saprophyticus strains isolated from african fermented nono in Nigeria. Microbiol. Resour. Announc. 2021, 10, e00315–e00321. [Google Scholar] [CrossRef]
- Bremer, E.; Kramer, R. Responses of microorganisms to osmotic stress. Annu. Rev. Microbiol. 2019, 73, 313–334. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bremer, E. Guardians in a stressful world: The Opu family of compatible solute transporters from Bacillus subtilis. Biol. Chem. 2017, 398, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Vaish, M.; Price-Whelan, A.; Reyes-Robles, T.; Liu, J.; Jereen, A.; Christie, S.; Alonzo, F.; Benson, M.A.; Torres, V.J.; Krulwich, T.A. Roles of Staphylococcus aureus Mnh1 and Mnh2 antiporters in salt tolerance, alkali tolerance, and pathogenesis. J. Bacteriol. 2018, 200, e00611–e00617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, D.-W.; Heo, S.; Ryu, S.; Blom, J.; Lee, J.-H. Genomic insights into the virulence and salt tolerance of Staphylococcus equorum. Sci. Rep. 2017, 7, 5383. [Google Scholar] [CrossRef] [Green Version]
- Teichmann, L.; Chen, C.; Hoffmann, T.; Smits, S.H.J.; Schmitt, L.; Bremer, E. From substrate specificity to promiscuity: Hybrid ABC transporters for osmoprotectants. Mol. Microbiol. 2017, 104, 761–780. [Google Scholar] [CrossRef] [Green Version]
- Asai, K.; Baik, S.-H.; Kasahara, Y.; Moriya, S.; Ogasawara, N. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology 2000, 146, 263–271. [Google Scholar] [CrossRef]
- Rahman, M.; Ismat, F.; Jiao, L.; Baldwin, J.M.; Sharples, D.J.; Baldwin, S.A.; Patching, S.G. Characterisation of the DAACS family Escherichia coli glutamate/aspartate-proton symporter GltP using computational, chemical, biochemical and biophysical methods. J. Membr. Biol. 2017, 250, 145–162. [Google Scholar] [CrossRef]
- Oren, A. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 1999, 63, 334–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves-Moreno, D.; Wos-Oxley, M.L.; Jauregui, R.; Medina, E.; Oxley, A.P.; Pieper, D.H. Exploring the transcriptome of Staphylococcus aureus in its natural niche. Sci. Rep. 2016, 6, 33174. [Google Scholar] [CrossRef] [Green Version]
- Peddie, B.A.; Wong-She, J.; Randall, K.; Lever, M.; Chambers, S.T. Osmoprotective properties and accumulation of betaine analogues by Staphylococcus aureus. FEMS Microbiol. Lett. 1998, 160, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Hoffmann, T.; Zelder, O.; Felle, M.F.; Bremer, E. Enhanced glutamate synthesis and export by the thermotolerant emerging industrial workhorse Bacillus methanolicus in response to high osmolarity. Front. Microbiol. 2021, 12, 640980. [Google Scholar] [CrossRef]
- Saum, S.H.; Müller, V. Regulation of osmoadaptation in the moderate halophile Halobacillus halophilus: Chloride, glutamate and switching osmolyte strategies. Saline Syst. 2008, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Description | Locus ID | Gene | Product | Gene Expression (log2 FC) * | ||
|---|---|---|---|---|---|---|
| 0% vs. 10% | 0% vs. 20% | 10% vs. 20% | ||||
| Stress | DV527_RS05825 | Universal stress protein | 1.88 | 5.89 | 4.01 | |
| DV527_RS01570 | Universal stress protein | 0.35 | 3.63 | 3.28 | ||
| DV527_RS05850 | Universal stress protein | 1.24 | 3.09 | 1.86 | ||
| DV527_RS03625 | Asp23/Gls24 family envelope stress response protein | 1.55 | 2.87 | 1.33 | ||
| DV527_RS10290 | GlsB/YeaQ/YmgE family stress response membrane protein | 2.21 | 2.69 | 0.49 | ||
| DV527_RS11920 | General stress protein | 0.52 | 1.54 | 1.02 | ||
| DV527_RS11570 | 50S ribosomal protein L25/general stress protein Ctc | 0.31 | −0.96 | −1.27 | ||
| DV527_RS06680 | Asp23/Gls24 family envelope stress response protein | −0.84 | −2.23 | −1.39 | ||
| Osmoprotectant | DV527_RS02415 | Osmoprotectant ABC transporter substrate-binding protein | −0.87 | 1.36 | 2.23 | |
| DV527_RS05510 | Osmoprotectant ABC transporter substrate-binding protein | −1.59 | −0.79 | 0.80 | ||
| Channel | DV527_RS12460 | Mechanosensitive ion channel family protein | −1.75 | −2.42 | −0.67 | |
| DV527_RS07575 | mscL | Large conductance mechanosensitive channel protein MscL | −1.98 | −4.73 | −2.75 | |
| Symporter | DV527_RS02905 | gltS | Sodium/glutamate symporter | 0.67 | 1.68 | 1.02 |
| DV527_RS05020 | Dicarboxylate/amino acid: cation symporter | 0.47 | 2.04 | 1.57 | ||
| DV527_RS04605 | putP | Sodium/proline symporter PutP | −3.66 | −4.06 | −0.40 | |
| DV527_RS07535 | Alanine:cation symporter family protein | −0.49 | −1.72 | −1.23 | ||
| Na+/H+ antiporter | DV527_RS09405 | mnhB1 | Na+/H+ antiporter Mnh1 subunit B | −0.38 | ||
| DV527_RS09410 | mnhC1 | Na+/H+ antiporter Mnh1 subunit C | −1.15 | −1.76 | −0.61 | |
| DV527_RS09415 | mnhD1 | Na+/H+ antiporter Mnh1 subunit D | −0.25 | −0.36 | ||
| DV527_RS10700 | mnhB2 | Na+/H+ antiporter Mnh2 subunit B | −0.64 | −1.28 | −0.63 | |
| DV527_RS10695 | mnhC2 | Na+/H+ antiporter Mnh2 subunit C | −1.31 | −2.13 | −0.82 | |
| DV527_RS10690 | mnhD2 | Na+/H+ antiporter Mnh2 subunit D | −0.91 | −1.44 | −0.53 | |
| DV527_RS10685 | mnhE2 | Na+/H+ antiporter Mnh2 subunit E | −1.86 | −2.82 | −0.96 | |
| DV527_RS10680 | mnhF2 | Na+/H+ antiporter Mnh2 subunit F | −1.01 | −0.51 | ||
| DV527_RS02145 | Sodium:proton antiporter | −0.52 | −3.42 | −2.90 | ||
| DV527_RS11800 | Sodium-dependent transporter | −0.66 | −3.86 | −3.20 | ||
| Potassium | DV527_RS08810 | TrkA family potassium uptake protein | −0.32 | 0.50 | ||
| DV527_RS09095 | TrkH family potassium uptake protein | −0.27 | −0.95 | −0.69 | ||
| Glycine betaine | DV527_RS07570 | BCCT family transporter | −1.31 | −2.37 | −1.06 | |
| DV527_RS07250 | BCCT family transporter | −0.49 | −4.89 | −4.40 | ||
| DV527_RS01165 | BCCT family transporter | −2.58 | −5.73 | −3.16 | ||
| DV527_RS01180 | betA | Choline dehydrogenase | −1.36 | −6.02 | −4.66 | |
| DV527_RS01175 | betB | Betaine–aldehyde dehydrogenase | −5.74 | −4.69 | ||
| MFS transporter | DV527_RS02780 | Sugar porter family MFS transporter | −0.48 | 2.56 | 3.03 | |
| DV527_RS12535 | Sugar porter family MFS transporter | −1.01 | 2.03 | 3.03 | ||
| DV527_RS02165 | MFS transporter | 1.45 | 4.96 | 3.51 | ||
| DV527_RS11830 | MFS transporter | −0.25 | 0.70 | 0.95 | ||
| DV527_RS02855 | Multidrug efflux MFS transporter | −0.64 | −1.53 | −0.90 | ||
| DV527_RS02475 | MFS transporter | −1.61 | −2.52 | −0.91 | ||
| Description | Locus ID | Gene | Product | Gene Expression (log2 FC) * | ||
|---|---|---|---|---|---|---|
| 0% vs. 10% | 0% vs. 20% | 10% vs. 20% | ||||
| Alanine | DV527_RS05830 | ald | Alanine dehydrogenase | 1.01 | 4.05 | 3.04 |
| DV527_RS05660 | murC | UDP-N-acetylmuramate-l-alanine ligase | 1.04 | 1.74 | 0.69 | |
| DV527_RS08415 | murD | UDP-N-acetylmuramoyl-l-alanine-d-glutamate ligase | 1.75 | 1.00 | −0.74 | |
| DV527_RS05740 | Alanine–glyoxylate aminotransferase family protein | −0.82 | 0.80 | 1.62 | ||
| Proline | DV527_RS05540 | Proline dehydrogenase family protein | −0.59 | 4.14 | 4.73 | |
| DV527_RS02405 | Betaine/proline/choline family ABC transporter ATP-binding protein | −0.70 | 1.24 | 1.94 | ||
| Glycine | DV527_RS06635 | gcvPB | Aminomethyl-transferring glycine dehydrogenase subunit GcvPB | 1.83 | 4.33 | 2.51 |
| DV527_RS06630 | gcvPA | Aminomethyl-transferring glycine dehydrogenase subunit GcvPA | 1.70 | 4.13 | 2.43 | |
| DV527_RS06625 | gcvT | Glycine cleavage system aminomethyltransferase GcvT | 1.31 | 3.74 | 2.44 | |
| DV527_RS11235 | Glycine C-acetyltransferase | 0.77 | 2.48 | 1.71 | ||
| DV527_RS00920 | Choloylglycine hydrolase family protein | 1.51 | 2.18 | 0.67 | ||
| DV527_RS03365 | Lipid II:glycine glycyltransferase FemX | −1.04 | −0.76 | 0.27 | ||
| Glutamate | DV527_RS01735 | pruA | l-glutamate γ-semialdehyde dehydrogenase | 0.91 | 6.02 | 5.12 |
| DV527_RS02350 | FMN-binding glutamate synthase family protein | 1.55 | 3.60 | 2.04 | ||
| DV527_RS06030 | hemL | Glutamate-1-semialdehyde 2,1-aminomutase | 1.90 | 2.65 | 0.75 | |
| DV527_RS12045 | Poly-γ-glutamate hydrolase family protein | 1.16 | 2.42 | 1.26 | ||
| DV527_RS07840 | Poly-γ-glutamate hydrolase family protein | 1.14 | 1.83 | 0.69 | ||
| DV527_RS08520 | racE | glutamate racemase | 0.27 | 1.52 | 1.25 | |
| DV527_RS11825 | Poly-γ-glutamate hydrolase family protein | 2.60 | 0.53 | −2.07 | ||
| DV527_RS11740 | gltB | glutamate synthase large subunit | −3.21 | −0.42 | 2.79 | |
| DV527_RS01265 | argJ | Bifunctional glutamate N-acetyltransferase acetyltransferase ArgJ | −1.38 | −1.12 | ||
| DV527_RS01910 | pgsC | Poly-γ-glutamate biosynthesis protein PgsC | −1.75 | −1.77 | ||
| DV527_RS01270 | argB | acetylglutamate kinase | −2.14 | −1.78 | ||
| DV527_RS01905 | pgsB | Poly-γ-glutamate synthase PgsB | −1.94 | −1.52 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, E.; Hwang, S.; Cha, J. Transcriptome Analysis of Halotolerant Staphylococcus saprophyticus Isolated from Korean Fermented Shrimp. Foods 2022, 11, 524. https://doi.org/10.3390/foods11040524
Jo E, Hwang S, Cha J. Transcriptome Analysis of Halotolerant Staphylococcus saprophyticus Isolated from Korean Fermented Shrimp. Foods. 2022; 11(4):524. https://doi.org/10.3390/foods11040524
Chicago/Turabian StyleJo, Eunhye, Sungmin Hwang, and Jaeho Cha. 2022. "Transcriptome Analysis of Halotolerant Staphylococcus saprophyticus Isolated from Korean Fermented Shrimp" Foods 11, no. 4: 524. https://doi.org/10.3390/foods11040524






