Effect of the Partial Substitution of Sodium Chloride on the Gel Properties and Flavor Quality of Unwashed Fish Mince Gels from Grass Carp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel Sample Preparation
2.3. Gel Properties
2.3.1. Textural Properties
2.3.2. Gel Strength
2.4. Whiteness
2.5. Water-Holding Capacity and Moisture Content
2.6. Raman Spectrometric Measurements
2.7. Gel Microstructure
2.8. Electronic Tongue Analysis
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
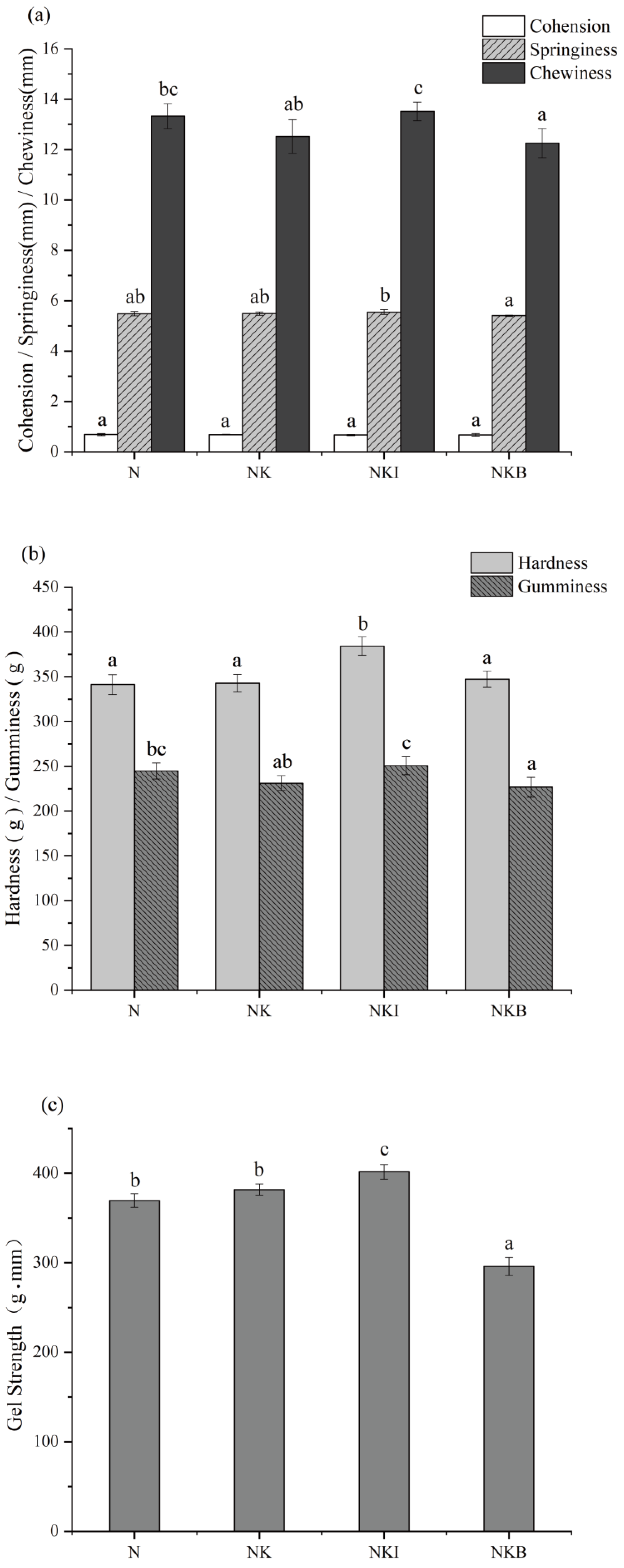
3.1. Gel Properties Analysis
3.1.1. Gel Textural Properties
3.1.2. Gel Strength
3.2. Whiteness, WHC and Moisture Content
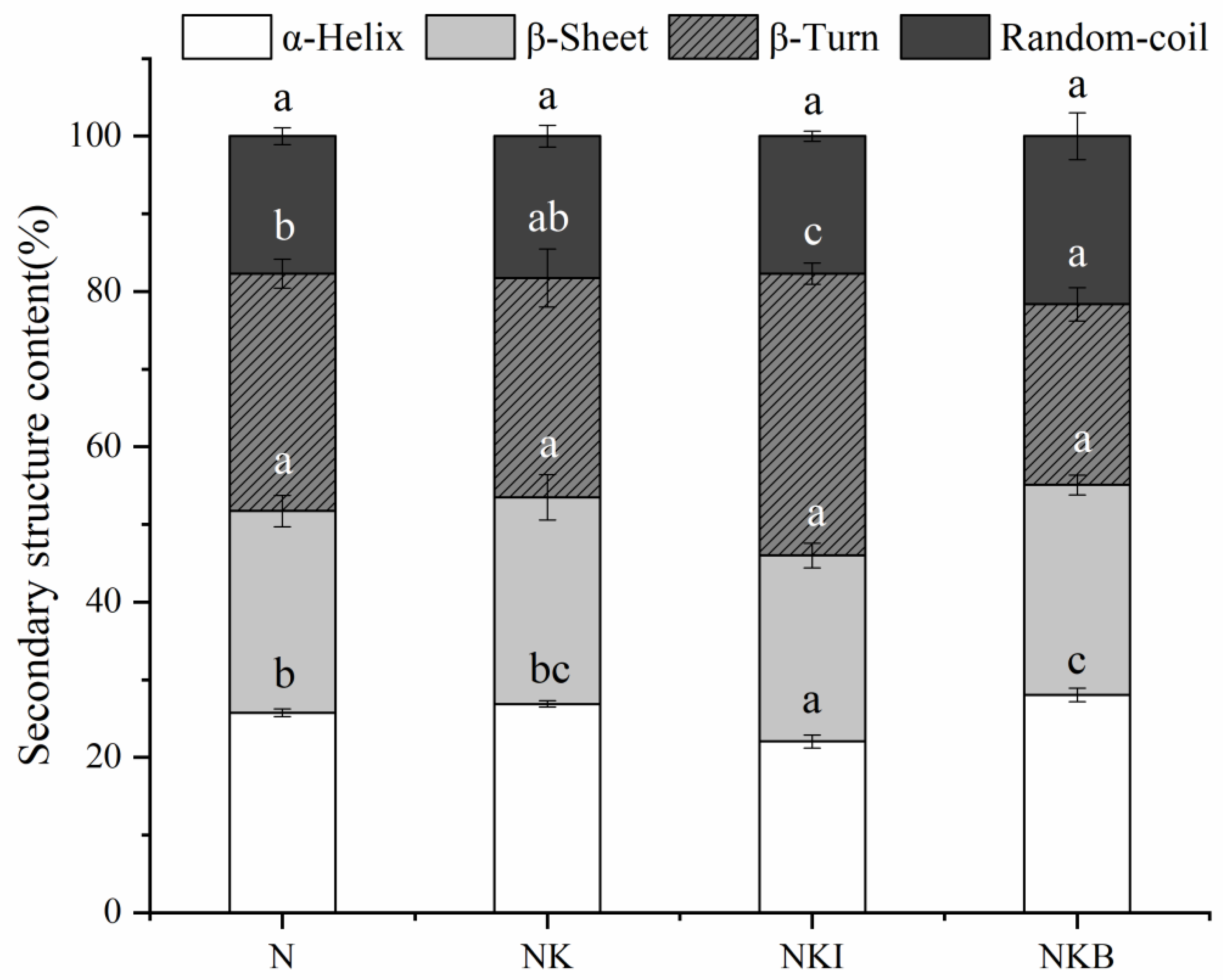
3.3. Raman Spectra Analysis
3.4. Tryptophan Microenvironment
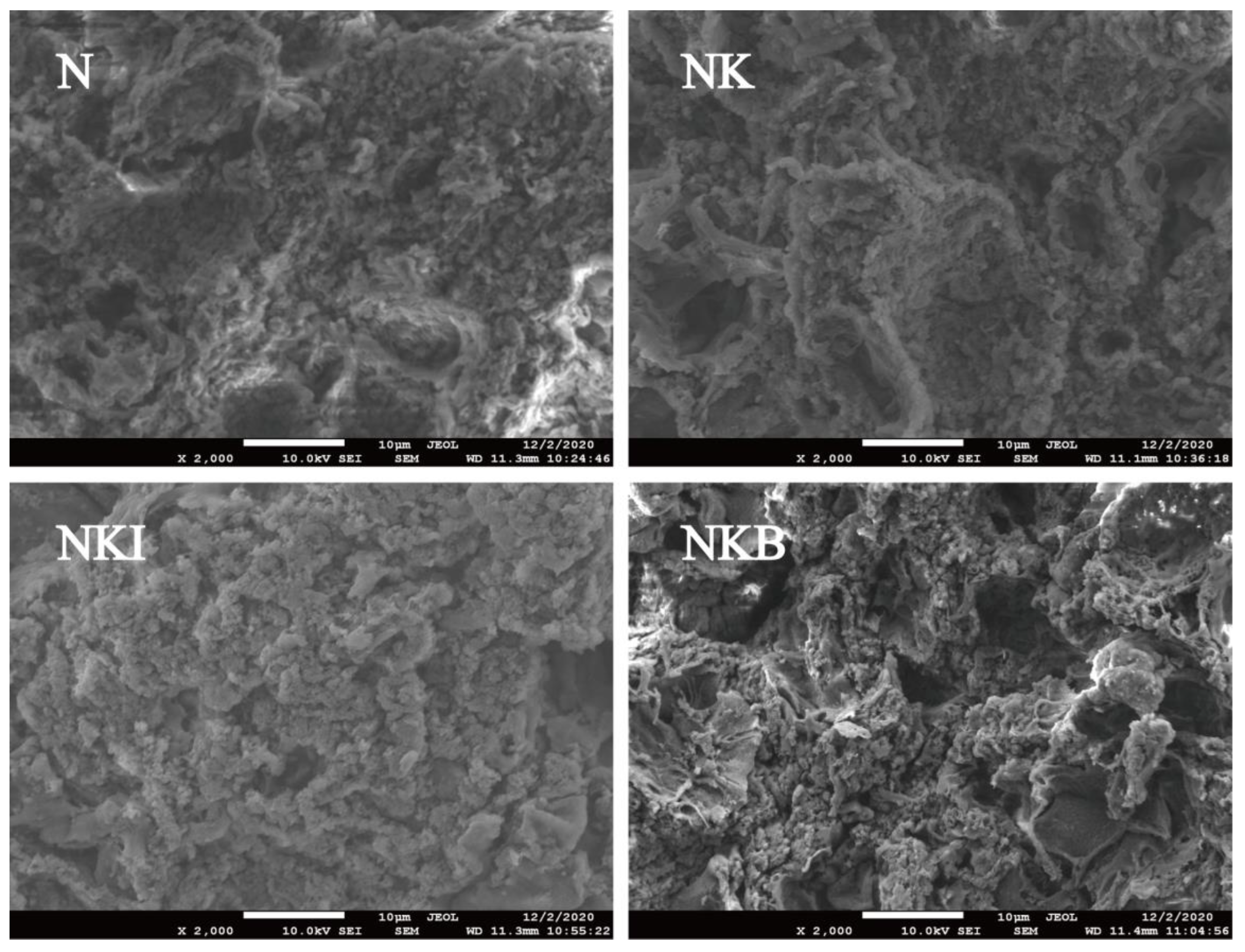
3.5. Gel Microstructure
3.6. Gel Flavor
3.6.1. Electronic Tongue Analysis
3.6.2. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.; Nunes, M.L. Reducing salt levels in seafood products. In Reducing Salt in Food, 2nd ed.; Beeren, C., Groves, K., Titoria, P.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 185–211. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, J.W. Mince from seafood processing by-product and surimi as food ingredients. In Maximising the Value of Marine By-Products, 1st ed.; Shahidi, F., Ed.; Woodhead Publishing: Sawston, UK, 2006; pp. 196–228. [Google Scholar] [CrossRef]
- Taladrid, D.; Laguna, L.; Bartolomé, B.; Moreno-arribas, M.V. Plant-derived seasonings as sodium salt replacers in food. Trends Food Sci. Technol. 2020, 99, 194–202. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Jaczynski, J. Physicochemical changes in surimi with salt substitute. Food Chem. 2012, 132, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Aliño, M.; Grau, R.; Toldrá, F.; Barat, J.M. Physicochemical changes in dry-cured hams salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Meat Sci. 2010, 86, 331–336. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Zheng, J.; Han, Y.; Ge, G.; Zhao, M.; Sun, W. Partial substitution of NaCl with chloride salt mixtures: Impact on oxidative characteristics of meat myofibrillar protein and their rheological properties. Food Hydrocoll. 2019, 96, 36–42. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Liu, Y. Characterization and evaluation of umami taste: A review. TrAC Trends Anal. Chem. 2020, 127, 115876. [Google Scholar] [CrossRef]
- Vidal, V.A.S.; Santana, J.B.; Paglarini, C.S.; Maria, A.A.P.; Freitas, M.Q.; Esmerino, E.A.; Cruz, A.G.; Pollonio, M.A.R. Adding lysine and yeast extract improves sensory properties of low sodium salted meat. Meat Sci. 2020, 159, 107911. [Google Scholar] [CrossRef]
- Tan, H.; Tan, T.; Easa, A.M. The use of selected hydrocolloids and salt substitutes on structural integrity, texture, sensory properties, and shelf life of fresh no salt wheat noodles. Food Hydrocoll. 2020, 108, 105996. [Google Scholar] [CrossRef]
- Ramalingam, V.; Song, Z.; Hwang, I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Food Res. Int. 2019, 122, 174–182. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Kerry, J.P.; Burgess, C.M.; Tiwari, B.K. Salts and salt replacers. In Encyclopedia of Food Chemistry, 1st ed.; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–239. [Google Scholar] [CrossRef]
- Cezar, P.; Campagnol, B.; Alves, B.; Nascimento, N. Lysine, disodium guanylate and disodium inosinate as flavor enhancers in low-sodium fermented sausages. Meat Sci. 2012, 91, 334–338. [Google Scholar] [CrossRef]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (Aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chem. 2017, 223, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: Effects of different washing solutions. Food Hydrocoll. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Wang, R.; Gao, R.; Xiao, F.; Zhou, X.; Wang, H.; Xu, H.; Gong, C.; Huang, P.; Zhao, Y. Effect of chicken breast on the physicochemical properties of unwashed sturgeon surimi gels. LWT Food Sci. Technol. 2019, 113, 1–8. [Google Scholar] [CrossRef]
- Chéret, R.; Chapleau, N.; Delbarre-Ladrat, C.; Verrez-Bagnis, V.; De Lamballerie, M. Effects of high pressure on texture and microstructure of sea bass (Dicentrarchus labrax L.) fillets. J. Food Sci. 2005, 70, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar protein conformation enhance gel properties of mixed surimi gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, T.; Lin, H.; Chen, H.; Liu, J.; Lyu, F.; Ding, Y. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2019, 90, 82–89. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.; Joo, S. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chem. 2020, 320, 126656. [Google Scholar] [CrossRef]
- Ramadhan, K.; Huda, N.; Ahmad, R. Physicochemical and sensory characteristics of burger made from duck surimi-like material. Poult. Sci. 2012, 91, 2316–2323. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Wu, J.; Chang, R.; Lan, X.; Wei, K.; Jia, X. Effects of cations on the “salt in” of myofibrillar proteins. Food Hydrocoll. 2016, 58, 179–183. [Google Scholar] [CrossRef]
- Horita, C.N.; Morgano, M.A.; Celeghini, R.M.S.; Pollonio, M.A.R. Physico-chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci. 2011, 89, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Cezar, P.; Campagnol, B.; Alves, B.; Antonio, M.; Nascimento, N.; Aparecida, M.; Pollonio, R. Application of lysine, taurine, disodium inosinate and disodium guanylate in fermented cooked sausages with 50% replacement of NaCl by KCl. Meat Sci. 2011, 87, 239–243. [Google Scholar] [CrossRef]
- Jin, H.; Chen, J.; Zhang, J.; Sheng, L. Impact of phosphates on heat-induced egg white gel properties: Texture, water state, micro-rheology and microstructure. Food Hydrocoll. 2021, 110, 106200. [Google Scholar] [CrossRef]
- Trout, G.R.; Schmidt, G.R. Effect of phosphates on the functional properties of restructured beef rolls: The role of pH, ionic strength, and phosphate type. J. Food Sci. 1986, 51, 1416–1423. [Google Scholar] [CrossRef]
- Núñez-Flores, R.; Cando, D.; Borderías, A.J.; Moreno, H.M. Importance of salt and temperature in myosin polymerization during surimi gelation. Food Chem. 2018, 239, 1226–1234. [Google Scholar] [CrossRef]
- Yang, H.; Zhong, C.; Sun, L.; Li, Y.; Chen, H.; Wu, G. Effects of partial substitution of NaCl on myofibrillar protein properties from pearl mussel Hyriopsis cumingii muscle: Structural characteristics and aggregation behaviors. Food Chem. 2021, 356, 129734. [Google Scholar] [CrossRef]
- Zhou, Y.; Watkins, P.; Oiseth, S.; Cochet-Broch, M.; Sikes, A.L.; Chen, C.; Buckow, R. High pressure processing improves the sensory quality of sodium-reduced chicken sausage formulated with three anion types of potassium salt. Food Control. 2021, 126, 108008. [Google Scholar] [CrossRef]
- Greiff, K.; Mathiassen, J.R.; Misimi, E.; Hersleth, M. Gradual reduction in sodium content in cooked ham, with corresponding change in sensorial properties measured by sensory evaluation and a multimodal machine vision system. PLoS ONE 2015, 10, e0137805. [Google Scholar] [CrossRef]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Physicochemical properties, gel-forming ability and myoglobin content of sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) surimi produced by conventional method and alkaline solubilisation process. Eur. Food Res. Technol. 2006, 222, 58–63. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Emara, A.M.; Wang, Z.; Chen, X.; He, Z. Study on the mechanism of KCl replacement of NaCl on the water retention of salted pork. Food Chem. 2020, 332, 127414. [Google Scholar] [CrossRef]
- Greiff, K.; Aursand, I.G.; Erikson, U.; Josefsen, K.D.; Rustad, T. Effects of type and concentration of salts on physicochemical properties in fish mince. LWT Food Sci. Technol. 2015, 64, 220–226. [Google Scholar] [CrossRef]
- Mi, H.; Su, Q.; Chen, J.; Yi, S.; Li, X.; Li, J. Starch-fatty acid complexes improve the gel properties and enhance the fatty acid content of Nemipterus virgatus surimi under high-temperature treatment. Food Chem. 2021, 362, 130253. [Google Scholar] [CrossRef] [PubMed]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Gluten, Gliadins and Glutenins as Studied with Spectroscopic Methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Ptaszyńska, A.A.; Kowalski, R.; Waśko, P.; Gruszecki, W.I. Influence of dietary fibre on gluten proteins structure—A study on model flour with application of FT-Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 309–316. [Google Scholar] [CrossRef]
- Guo, M.; Liu, S.; Ismail, M.; Farid, M.M.; Ji, H.; Mao, W.; Gao, J.; Li, C. Changes in the myosin secondary structure and shrimp surimi gel strength induced by dense phase carbon dioxide. Food Chem. 2017, 227, 219–226. [Google Scholar] [CrossRef]
- Yang, L.; Yang, M.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Structural and emulsion stabilization comparison of four gelatins from two freshwater and two marine fish skins. Food Chem. 2022, 371, 131129. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, L.; Chi, Y.; Chi, Y. Forces involved in freeze-induced egg yolk gelation: Effects of various bond dissociation reagents on gel properties and protein structure changes. Food Chem. 2022, 371, 131190. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Shi, T.; Zhang, W.; Kong, Y.; Yuan, L.; Gao, R. Improvement of gel properties of low salt surimi using low-dose L-arginine combined with oxidized caffeic acid. LWT Food Sci. Technol. 2021, 145, 111303. [Google Scholar] [CrossRef]
- Takeuchi, H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolym. Biospectroscopy Sect. 2003, 72, 305–317. [Google Scholar] [CrossRef]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Assaf, A.; Thouand, G.; Le-Bail, A. Study of structural changes of gluten proteins during bread dough mixing by Raman spectroscopy. Food Chem. 2021, 358, 129916. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Szymańska-Chargot, M.; Krawęcka, A.; Sobota, A.; Miś, A. Effect of moisturizing pre-treatment of dietary fibre preparations on formation of gluten network during model dough mixing—A study with application of FT-IR and FT-Raman spectroscopy. LWT Food Sci. Technol. 2020, 121, 108959. [Google Scholar] [CrossRef]
- Andreetta-Gorelkina, I.V.; Greiff, K.; Rustad, T.; Aursand, I.G. Reduction of salt in haddock mince: Effect of different salts on the solubility of proteins. J. Aquat. Food Prod. Technol. 2016, 25, 518–530. [Google Scholar] [CrossRef] [Green Version]
- Jayasundar, R.; Singh, A.; Kumar, D. Challenges in using electronic tongue to study rasa of plants: I. Finding the right tool for the right job. J. Ayurveda Integr. Med. 2021, 12, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Baines, D.; Brown, M. Flavor enhancers: Characteristics and uses. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Todrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 716–723. [Google Scholar] [CrossRef]
- Maruji, Y.; Shimizu, M.; Murata, M.; Ando, M.; Sakaguchi, M.; Hirata, T. Multiple taste functions of the umami substances in muscle extracts of yellowtail and bastard halibut. Fish. Sci. 2010, 76, 521–528. [Google Scholar] [CrossRef]
- Dörr, O.S.; Brezina, S.; Rauhut, D.; Mibus, H. Plant architecture and phytochemical composition of basil (Ocimum basilicum L.) under the influence of light from microwave plasma and high-pressure sodium lamps. J. Photochem. Photobiol. B Biol. 2020, 202, 111678. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]




| Group | NaCl | KCl | DIMP | Basil |
|---|---|---|---|---|
| N | 1.80% | - | - | - |
| NK | 1.26% | 0.54% | - | - |
| NKI | 1.26% | 0.45% | 0.09% | - |
| NKB | 1.26% | 0.45% | - | 0.09% |
| Group | L* | a* | b* | Whiteness | WHC (%) | Moisture Content (%) |
|---|---|---|---|---|---|---|
| N | 82.62 ± 0.22b | −2.77 ± 0.02a | 4.33 ± 0.07a | 81.88 ± 0.22b | 83.12 ± 0.54a | 81.16 ± 0.42a |
| NK | 83.64 ± 0.13c | −2.53 ± 0.02b | 4.77 ± 0.04b | 82.77 ± 0.12c | 83.17 ± 0.69a | 81.09 ± 1.65a |
| NKI | 82.44 ± 0.17b | −2.81 ± 0.02a | 4.54 ± 0.15a | 81.65 ± 0.13b | 83.58 ± 1.33a | 81.41 ± 0.42a |
| NKB | 81.18 ± 0.10a | −2.18 ± 0.03c | 5.59 ± 0.09c | 80.25 ± 0.12a | 81.27 ± 1.52a | 81.27 ± 1.64a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, R.; Li, G.; Zhuang, S.; Yu, Q.; Luo, Y.; Tan, Y.; Dai, R.; Hong, H. Effect of the Partial Substitution of Sodium Chloride on the Gel Properties and Flavor Quality of Unwashed Fish Mince Gels from Grass Carp. Foods 2022, 11, 576. https://doi.org/10.3390/foods11040576
Pi R, Li G, Zhuang S, Yu Q, Luo Y, Tan Y, Dai R, Hong H. Effect of the Partial Substitution of Sodium Chloride on the Gel Properties and Flavor Quality of Unwashed Fish Mince Gels from Grass Carp. Foods. 2022; 11(4):576. https://doi.org/10.3390/foods11040576
Chicago/Turabian StylePi, Ruobing, Gaojing Li, Shuai Zhuang, Qinye Yu, Yongkang Luo, Yuqing Tan, Ruitong Dai, and Hui Hong. 2022. "Effect of the Partial Substitution of Sodium Chloride on the Gel Properties and Flavor Quality of Unwashed Fish Mince Gels from Grass Carp" Foods 11, no. 4: 576. https://doi.org/10.3390/foods11040576







