Heat-Killed Enterococcus faecalis EF-2001 Attenuate Lipid Accumulation in Diet-Induced Obese (DIO) Mice by Activating AMPK Signaling in Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Heat-Killed Enterococcus faecalis (EF-2001)
2.2. Experimental Animals, Diet, and Treatments
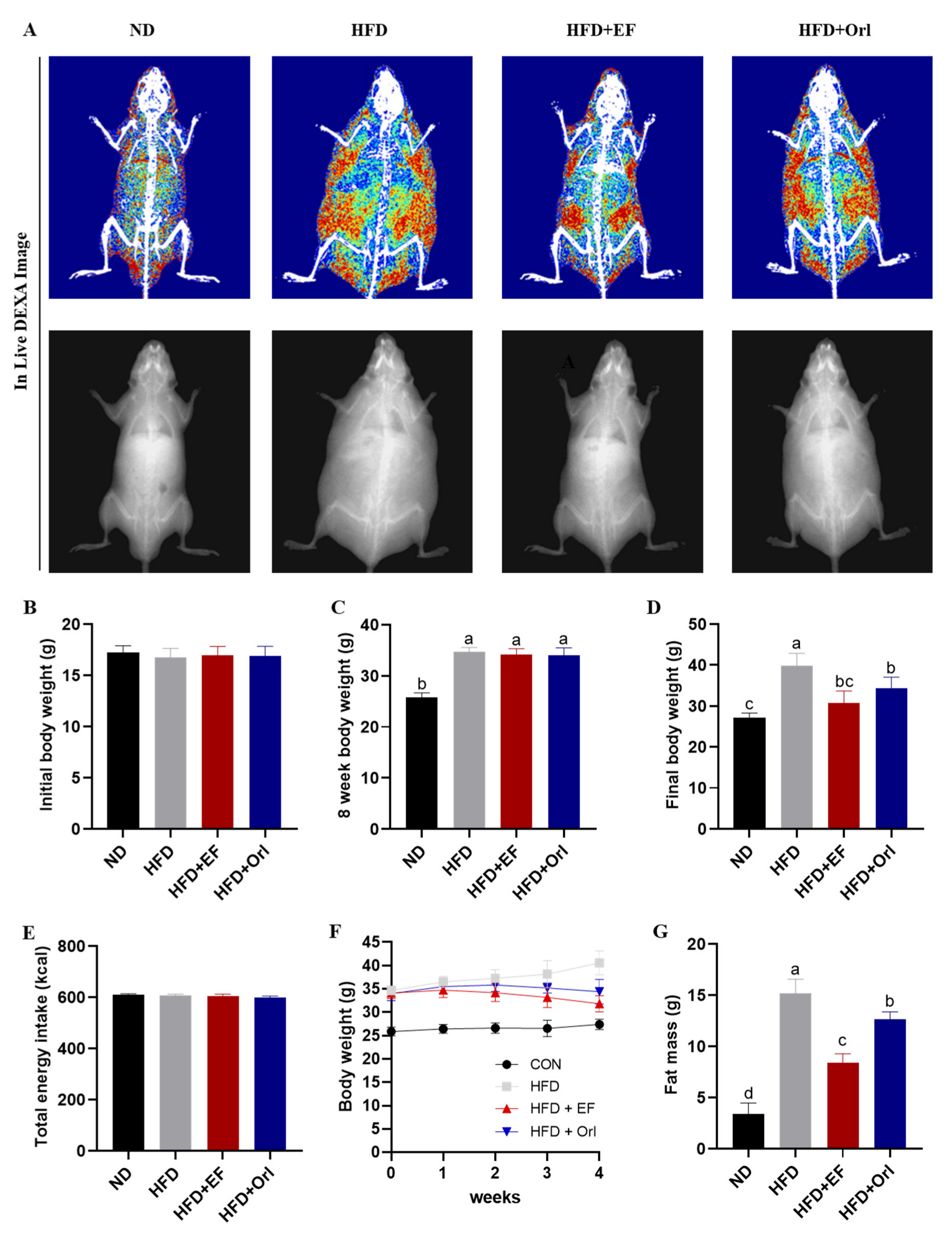
2.3. Body Composition Analysis
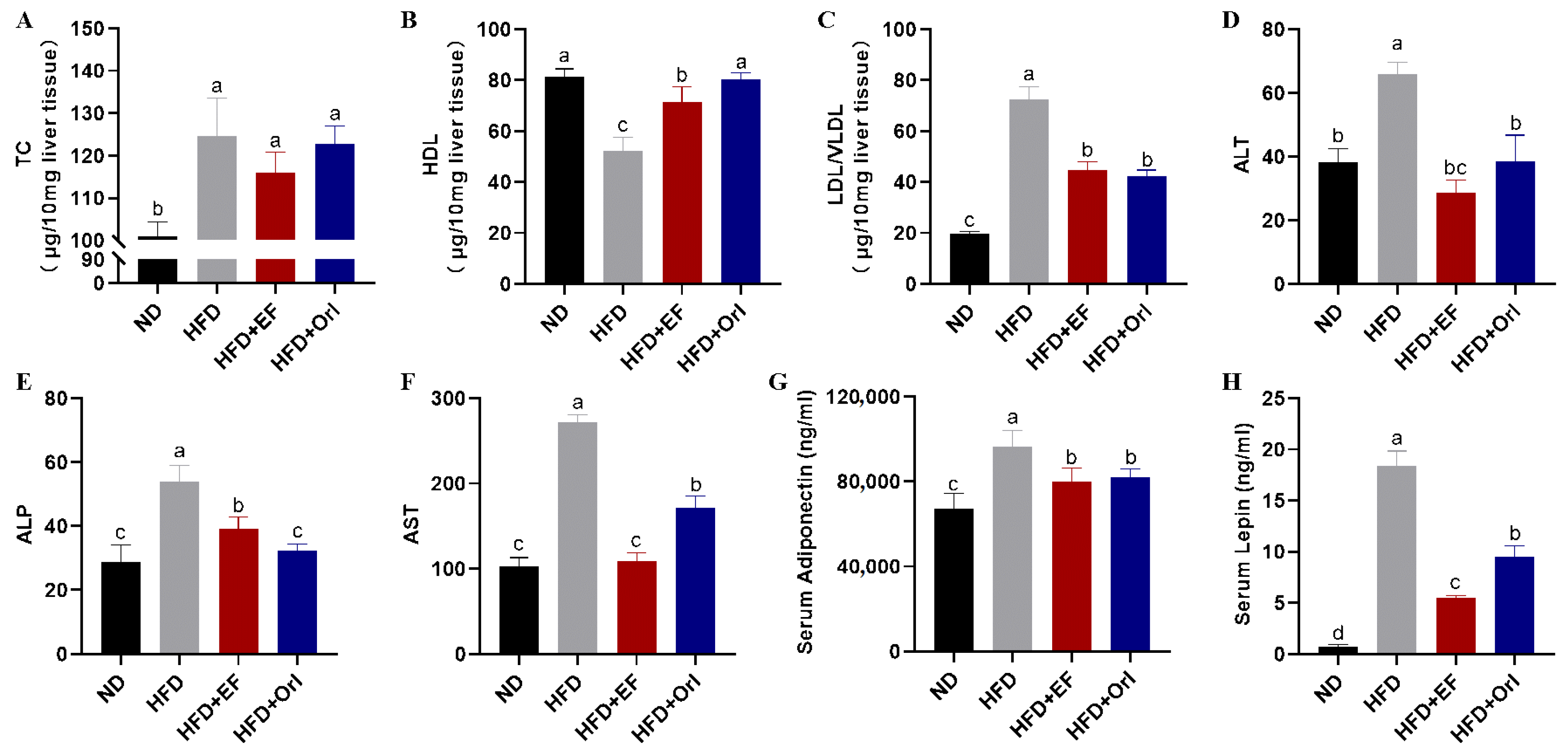
2.4. Biochemical Analysis
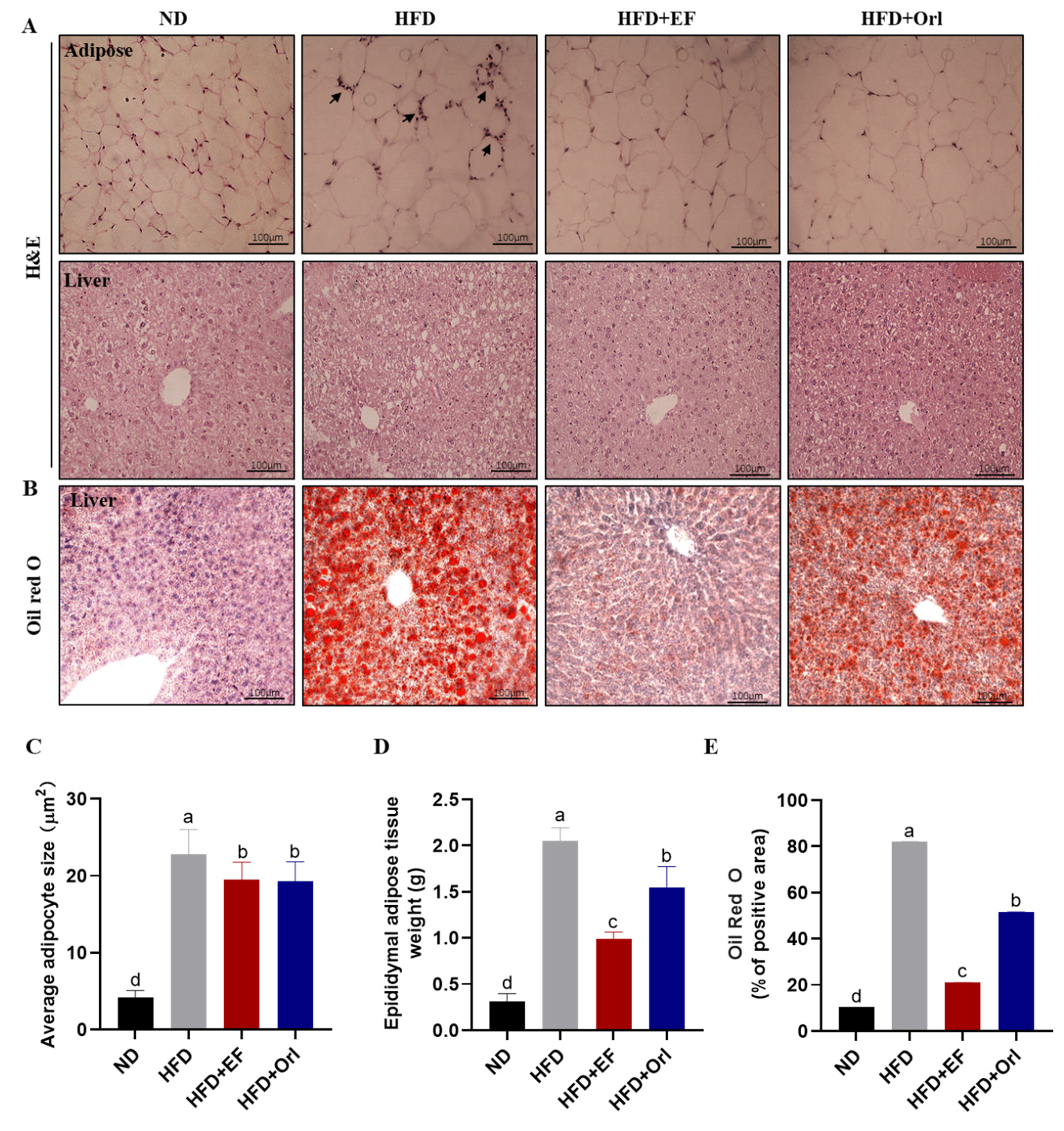
2.5. Histological Analysis
2.6. mRNA Expression Analysis
2.7. Protein Expression Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of EF-2001 on Growth Performance of DIO Mice
3.2. Effect of EF-2001 on Liver and Adipose Histopathology
3.3. Effect of EF-2001 on Liver and Serum Biochemical Parameters
3.4. Effect of EF-2001 on Hepatic Lipid-Related Gene Expression
3.5. Effect of EF-2001 on the Expression of Hepatic Lipid-Related Proteins
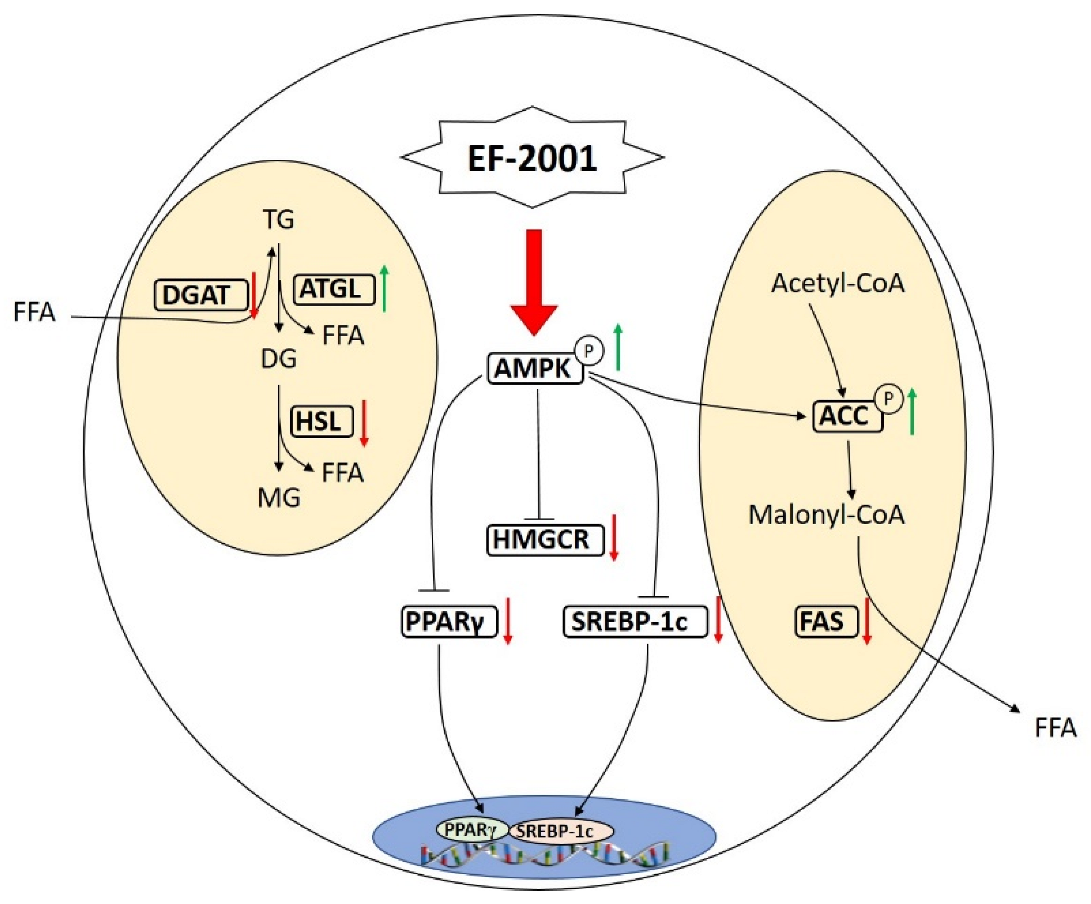
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Li, Y. An overview of lipid metabolism and nonalcoholic fatty liver disease. BioMed Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Katsiki, N.; Perez-Martinez, P.; Anagnostis, P.; Mikhailidis, D.P.; Karagiannis, A. Is nonalcoholic fatty liver disease indeed the hepatic manifestation of metabolic syndrome? Curr. Vasc. Pharmacol. 2018, 16, 219–227. [Google Scholar] [CrossRef]
- Cardoso, A.C.; de Figueiredo-Mendes, C.; Villela-Nogueira, C.A. Current management of NAFLD/NASH. Liver Int. 2021, 41, 89–94. [Google Scholar] [CrossRef]
- Gupta, N.K.; Lewis, J.H. The use of potentially hepatotoxic drugs in patients with liver disease. Aliment. Pharm. Ther. 2008, 28, 1021–1041. [Google Scholar] [CrossRef]
- Lewis, J.H.; Mortensen, M.E.; Zweig, S.; Fusco, M.J.; Medoff, J.R.; Belder, R. Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007, 46, 1453–1463. [Google Scholar]
- Iacono, A.; Raso, G.M.; Canani, R.B.; Calignano, A.; Meli, R. Probiotics as an emerging therapeutic strategy to treat NAFLD: Focus on molecular and biochemical mechanisms. J. Nutr. Biochem. 2011, 22, 699–711. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Ahmed, A. The therapeutic implications of the gut microbiome and probiotics in patients with NAFLD. Diseases 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Cao, G.; Zhang, H.; Li, Q.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Ghosh, A.R. Probiotic Enterococcus faecalis AG5 mitigated high fat diet induced obesity and produced propionic acid stimulated apoptosis in 3T3-L1 pre-adipocyte. Life Sci. 2020, 261, 118292. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Kondoh, M.; Shimada, T.; Fukada, K.; Morita, M.; Katada, K.; Higashimura, Y.; Yoshikawa, T. Beneficial effects of heat-treated Enterococcus faecalis FK-23 on high-fat diet-induced hepatic steatosis in mice. Brit. J. Nutr. 2014, 112, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Lee, H.J.; Kim, W.J.; Han, K.I.; Iwasa, M.; Kobayashi, K.; Kim, E.K. Enterococcus faecalis EF-2001 protects DNBS-induced inflammatory bowel disease in mice model. PLoS ONE 2019, 14, e0210854. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Iwasa, M.; Han, K.I.; Kim, W.J.; Tang, Y.; Hwang, Y.J.; Kim, E.K. Heat-killed Enterococcus faecalis EF-2001 ameliorates atopic dermatitis in a murine model. Nutrients 2016, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Jeon, D.B.; Shin, H.G.; Lee, B.W.; Jeong, S.H.; Kim, J.H.; Ha, J.H.; Lee, I.C. Effect of heat-killed Enterococcus faecalis EF-2001 on ethanol-induced acute gastric injury in mice: Protective effect of EF-2001 on acute gastric ulcer. Hum. Exp. Toxicol. 2020, 39, 721–733. [Google Scholar] [CrossRef]
- Gu, Y.H.; Yamasita, T.; Kang, K.M. Subchronic oral dose toxicity study of Enterococcus faecalis 2001 (EF 2001) in mice. Toxicol. Res. 2018, 34, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Develop. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Ye, J.; Wu, Y.; Li, F.; Wu, T.; Shao, C.; Lin, Y.; Zhong, B. Effect of orlistat on liver fat content in patients with nonalcoholic fatty liver disease with obesity: Assessment using magnetic resonance imaging-derived proton density fat fraction. Ther. Adv. Gastroenter. 2019, 12, 1756284819879047. [Google Scholar] [CrossRef]
- Fan, M.; Lee, J.I.; Ryu, Y.B.; Choi, Y.J.; Tang, Y.; Oh, M.; Kim, E.K. Comparative Analysis of Metabolite Profiling of Momordica charantia Leaf and the Anti-Obesity Effect through Regulating Lipid Metabolism. Int. J. Env. Res. Pub. Health 2021, 18, 5584. [Google Scholar] [CrossRef]
- Choi, Y.J.; Fan, M.; Tang, Y.; Yang, H.P.; Hwang, J.Y.; Kim, E.K. In vivo effects of polymerized anthocyanin from grape skin on benign prostatic hyperplasia. Nutrients 2019, 11, 2444. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Choi, Y.J.; Tang, Y.; Bae, S.M.; Yang, H.P.; Kim, E.K. Efficacy and mechanism of polymerized anthocyanin from grape-skin extract on high-fat-diet-induced nonalcoholic fatty liver disease. Nutrients 2019, 11, 2586. [Google Scholar] [CrossRef] [Green Version]
- Trepiana, J.; Krisa, S.; Renouf, E.; Portillo, M.P. Resveratrol metabolites are able to reduce steatosis in cultured hepatocytes. Pharmaceuticals 2020, 13, 285. [Google Scholar] [CrossRef]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817. [Google Scholar]
- Liu, Y.; Zheng, D.; Su, L.; Wang, Q.; Li, Y. Protective effect of polysaccharide from Agaricus bisporus in Tibet area of China against tetrachloride-induced acute liver injury in mice. Int. J. Biol. Macromol. 2018, 118, 1488–1493. [Google Scholar] [CrossRef]
- Mohankumar, N.; Ranjan, P.; Kumari, A. Drug-induced liver injury: Diagnosing (and treating) it early. J. Fam. Pract. 2015, 64, 634–644. [Google Scholar]
- Mesilati-Stahy, R.; Argov-Argaman, N. Changes in lipid droplets morphometric features in mammary epithelial cells upon exposure to non-esterified free fatty acids compared with VLDL. PLoS ONE 2018, 13, e0209565. [Google Scholar] [CrossRef]
- Liu, K.D.; Acharjee, A.; Hinz, C.; Liggi, S.; Murgia, A.; Denes, J.; Griffin, J.L. Consequences of Lipid Remodeling of Adipocyte Membranes Being Functionally Distinct from Lipid Storage in Obesity. J. Proteome Res. 2020, 19, 3919–3935. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The subtle balance between lipolysis and lipogenesis: A critical point in metabolic homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Cappel, D.A.; Deja, S.; Duarte, J.A.; Kucejova, B.; Iñigo, M.; Fletcher, J.A.; Burgess, S.C. Pyruvate-carboxylase-mediated anaplerosis promotes antioxidant capacity by sustaining TCA cycle and redox metabolism in liver. Cell Metab. 2019, 29, 1291–1305. [Google Scholar] [CrossRef]
- Hu, S.; Gao, S.; Zhu, J.; Gan, X.; Chen, X.; He, H.; Wang, J. Differential actions of diacylglycerol acyltransferase (DGAT) 1 and 2 in regulating lipid metabolism and progesterone secretion of goose granulosa cells. J. Steroid Biochem. 2020, 202, 105721. [Google Scholar] [CrossRef]
- Leamy, A.K.; Hasenour, C.M.; Egnatchik, R.A.; Trenary, I.A.; Yao, C.H.; Patti, G.J.; Young, J.D. Knockdown of triglyceride synthesis does not enhance palmitate lipotoxicity or prevent oleate-mediated rescue in rat hepatocytes. Biochim. Biophys Acta Mol. Cell Biol. Lipids 2016, 1861, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Cao, H.; Yan, J.; Huang, R.; Ying, H. ‘Micro-managers’ of hepatic lipid metabolism and NAFLD. WIRES RNA 2015, 6, 581–593. [Google Scholar] [CrossRef]
- Tardelli, M.; Bruschi, F.V.; Trauner, M. The role of metabolic lipases in the pathogenesis and management of liver disease. Hepatology 2020, 72, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; Van De Weijer, T.; Zechner, R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Haemmerle, G.; Zimmermann, R.; Hayn, M.; Theussl, C.; Waeg, G.; Wagner, E.; Zechner, R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002, 277, 4806–4815. [Google Scholar] [CrossRef] [Green Version]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 101092. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Samali, A. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, R.; Ma, Z. Autophagy and energy metabolism. In Autophagy: Biology and Diseases; Advances in Experimental Medicine and Biology 1206; Springer: Singapore, 2019; pp. 329–357. [Google Scholar]
- Bairwa, S.C.; Parajuli, N.; Dyck, J.R. The role of AMPK in cardiomyocyte health and survival. BBA Mol. Basis Dis. 2016, 1862, 2199–2210. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Vinayagam, R.; Jayachandran, M.; Chung, S.S.M.; Xu, B. Guava leaf inhibits hepatic gluconeogenesis and increases glycogen synthesis via AMPK/ACC signaling pathways in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 1012–1017. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Steinberg, G.R. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Liou, C.J.; Wei, C.H.; Chen, Y.L.; Cheng, C.Y.; Wang, C.L.; Huang, W.C. Fisetin protects against hepatic steatosis through regulation of the Sirt1/AMPK and fatty acid β-oxidation signaling pathway in high-fat diet-induced obese mice. Cell. Physiol. Biochem. 2018, 49, 1870–1884. [Google Scholar] [CrossRef]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Sim, D.Y.; Lee, H.M.; Lee, H.J.; Kim, S.H. Hypolipogenic effect of shikimic acid via inhibition of MID1IP1 and phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019, 20, 582. [Google Scholar] [CrossRef] [Green Version]
- Angin, Y.; Beauloye, C.; Horman, S.; Bertrand, L. Regulation of carbohydrate metabolism, lipid metabolism, and protein metabolism by AMPK. Exp. Suppl. 2016, 107, 23–43. [Google Scholar] [PubMed]
- Gwon, S.Y.; Ahn, J.; Jung, C.H.; Moon, B.; Ha, T.Y. Shikonin attenuates hepatic steatosis by enhancing Beta oxidation and energy expenditure via AMPK activation. Nutrients 2020, 12, 1133. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chen, Y.L.; Huang, W.C.; Liou, C.J. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Bioph. Res. Commun. 2018, 495, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Jang, J.; Chung, S.I.; Yoon, Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin. Int. J. Mol. Med. 2016, 37, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J.; Zhao, Y.; Lu, F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complem. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera suppresses SREBP-1c and ACC expression to lower serum triglycerides in high-fat diet-induced hyperlipidaemic rats. J. Funct. Foods 2018, 40, 722–728. [Google Scholar] [CrossRef]
- Castro, L.F.C.; Tocher, D.R.; Monroig, O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Cui, K.; Shafique, L.; Luo, C.; Wang, Z.; Liu, Q. Fatty acid biosynthesis and transcriptional regulation of Stearoyl-CoA Desaturase 1 (SCD1) in buffalo milk. BMC Genet. 2020, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Li, L.; Liang, N.; Zhang, L.; Xu, X.; Chen, S.; Yin, H. Acetaldehyde Dehydrogenase 2 regulates HMG-CoA reductase stability and cholesterol synthesis in the liver. Redox Biol. 2021, 41, 101919. [Google Scholar] [CrossRef]
- Hall, J.M.; Powell, H.R.; Rajic, L.; Korach, K.S. The Role of Dietary Phytoestrogens and the Nuclear Receptor PPAR γ in Adipogenesis: An in vitro study. Environ. Health Persp. 2019, 127, 037007. [Google Scholar] [CrossRef]


| Target Genes | GenBank Accession | Primer Sequence | |
|---|---|---|---|
| PPAR-γ | NM_001308354.1 | Forward | 5-GAA AGA CAA CGG ACA AAT CAC-3 |
| Reverse | 5-GAA ACT GGC ACC CTT GAA-3 | ||
| HMGCR | NM_001360165.1 | Forward | 5-AGA ATA ATG TGC TAA GTA GTG CTA A-3 |
| Reverse | 5-GCC TCT CTG AAC AAA GAC TC-3 | ||
| SREBP-1C | NM_001358315.1 | Forward | 5-CTT CTG GAG ACA TCG CAA AC-3 |
| Reverse | 5-GGT AGA CAA CAG CCG CAT C-3 | ||
| FAS | NM_007988.3 | Forward | 5-CTT GGG TGC TGA CTA CAA CC-3 |
| Reverse | 5-GCC CTC CCG TAC ACT CAC TC-3 | ||
| HSL | NM_010719.5 | Forward | 5-AAG GAC TCA CCG CTG ACT TCC-3 |
| Reverse | 5-GCC TGT CTC GTT GCG TTT GTA-3 | ||
| ATGL | NM_025802.3 | Forward | 5-GAC CTG ATG ACC ACC CTT TCC-3 |
| Reverse | 5-TGC TAC CCG TCT GCT CTT TCA-3 | ||
| DGAT | NM_010046.3 | Forward | 5-CCT CAG CCT TCT TCC ATG AG-3 |
| Reverse | 5-ACT GGG GCA TCG TAG TTG AG-3 | ||
| GAPDH | NM_001289726.1 | Forward | 5-GCA CAG TCA AGG CCG AGA AT-3 |
| Reverse | 5-GCC TTC TCC ATG GTG GTG AA-3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, M.; Choi, Y.-J.; Wedamulla, N.E.; Tang, Y.; Han, K.I.; Hwang, J.-Y.; Kim, E.-K. Heat-Killed Enterococcus faecalis EF-2001 Attenuate Lipid Accumulation in Diet-Induced Obese (DIO) Mice by Activating AMPK Signaling in Liver. Foods 2022, 11, 575. https://doi.org/10.3390/foods11040575
Fan M, Choi Y-J, Wedamulla NE, Tang Y, Han KI, Hwang J-Y, Kim E-K. Heat-Killed Enterococcus faecalis EF-2001 Attenuate Lipid Accumulation in Diet-Induced Obese (DIO) Mice by Activating AMPK Signaling in Liver. Foods. 2022; 11(4):575. https://doi.org/10.3390/foods11040575
Chicago/Turabian StyleFan, Meiqi, Young-Jin Choi, Nishala Erandi Wedamulla, Yujiao Tang, Kwon Il Han, Ji-Young Hwang, and Eun-Kyung Kim. 2022. "Heat-Killed Enterococcus faecalis EF-2001 Attenuate Lipid Accumulation in Diet-Induced Obese (DIO) Mice by Activating AMPK Signaling in Liver" Foods 11, no. 4: 575. https://doi.org/10.3390/foods11040575
APA StyleFan, M., Choi, Y.-J., Wedamulla, N. E., Tang, Y., Han, K. I., Hwang, J.-Y., & Kim, E.-K. (2022). Heat-Killed Enterococcus faecalis EF-2001 Attenuate Lipid Accumulation in Diet-Induced Obese (DIO) Mice by Activating AMPK Signaling in Liver. Foods, 11(4), 575. https://doi.org/10.3390/foods11040575






