Characterization and Effect of Refining on the Oil Extracted from Durum Wheat By-Products
Abstract
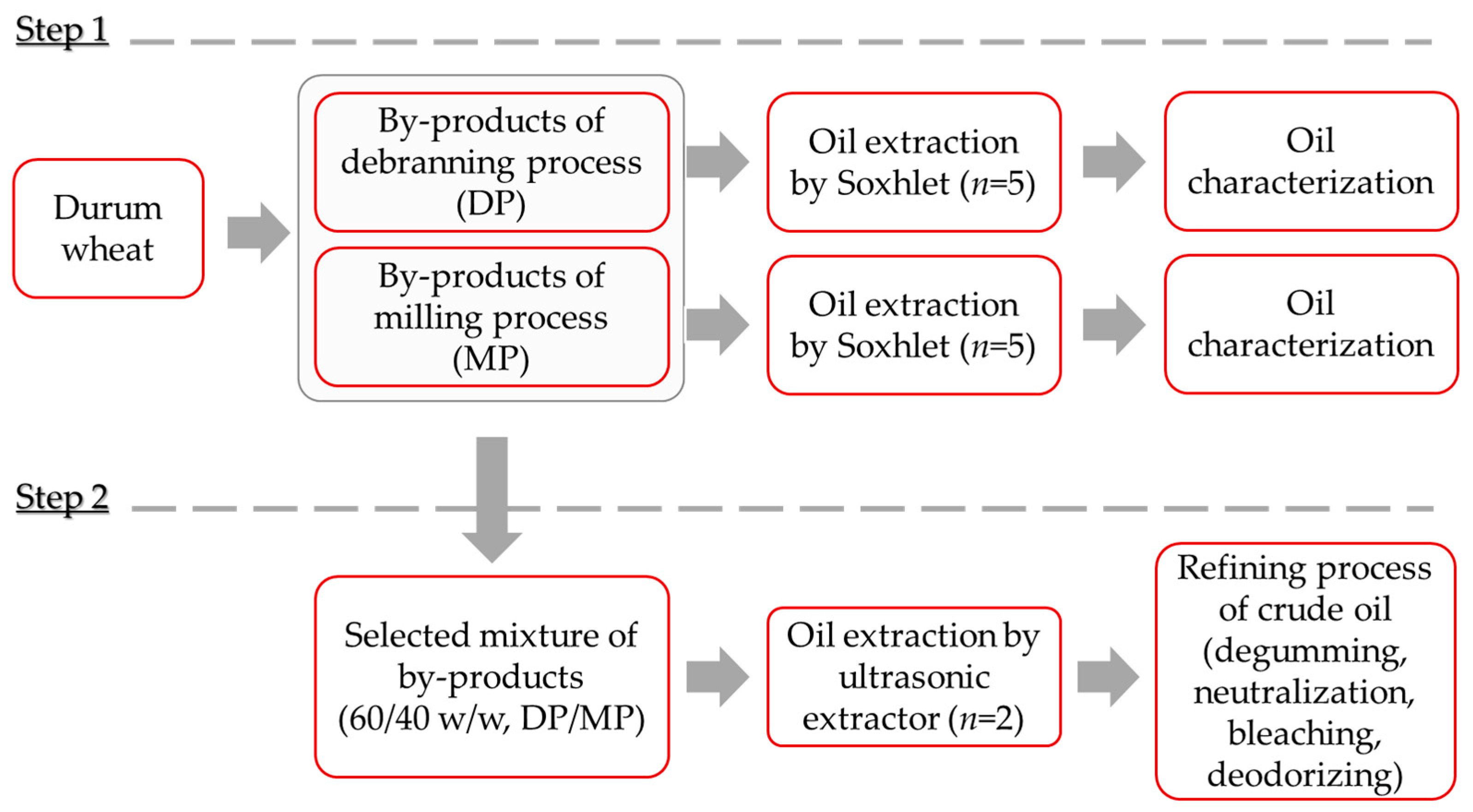
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sampling
2.3. Oil Extraction
2.4. Refining Process
2.4.1. Degumming
2.4.2. Neutralization
2.4.3. Bleaching
2.4.4. Deodorization
2.5. Analytical Determinations
2.5.1. Routine Analyses and Fatty Acids Composition
2.5.2. Carotenoids Determination
2.5.3. Tocotrienols and Tocopherols Determination
2.5.4. Polar compounds Separation and High-Performance Size-Exclusion Chromatography (HPSEC) Determination
2.5.5. Phytosterols and Policosanols Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Oils from Debranning and Milling Fraction
3.2. Effect of the Refining Process on Durum Wheat Oil Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narducci, V.; Finotti, E.; Galli, V.; Carcea, M. Lipids and fatty acids in Italian durum wheat (Triticum durum Desf.) cultivars. Foods 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarroug, Y.; Mejri, J.; Dhawefi, N.; Ali, S.B.S.; Felah, M.E.; Hassouna, M. Comparison of chemical composition of two durum wheat (Triticum durum L.) and bread wheat (Triticum aestivum L.) germ oils. Ekin J. Crop Breed. Genet. 2015, 1, 69–73. [Google Scholar]
- Güven, M.; Kara, H.H. Some chemical and physical properties, fatty acid composition and bioactive compounds of wheat germ oils extracted from different wheat cultivars. J. Agric. Sci. 2016, 22, 433–443. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Omega 3 fatty acids and cardiovascular disease. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β+ γ-and δ-tocopherol) levels in plant oils. Flavour Frag. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Lafiandra, D.; Masci, S.; Sissons, M.; Dornez, E.; Delcour, J.A.; Courtin, C.M.; Caboni, M.F. Kernel components of technological value. In Durum Wheat Chemistry and Technology, 2nd ed.; Sissons, M., Abecassis, J., Marchylo, B., Carcea, M., Eds.; AACC International Inc.: St. Paul, MN, USA, 2012; pp. 85–124. [Google Scholar]
- Prueckler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Hoeltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT-Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Cardenia, V.; Sgarzi, F.; Mandrioli, M.; Tribuzio, G.; Rodriguez-Estrada, M.T.; Toschi, T.G. Durum wheat bran by-products: Oil and phenolic acids to be valorized by industrial symbiosis. Eur. J. Lipid Sci. Technol. 2018, 120, 1700209. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Rescio, L.; Mita, G.; Caretto, S. Durum wheat by-products as natural sources of valuable nutrients. Phytochem. Rev. 2012, 11, 255–262. [Google Scholar] [CrossRef]
- Digesù, A.M.; Platani, C.; Cattivelli, L.; Mangini, G.; Blanco, A. Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J. Cereal Sci. 2009, 50, 210–218. [Google Scholar] [CrossRef]
- Durmaz, G.; Gökmen, V. Effect of refining on bioactive composition and oxidative stability of hazelnut oil. Food Res. Int. 2019, 116, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; Bertoz, V.; Pacetti, D.; Moret, S.; Conte, L. Effect of the refining process on total hydroxytyrosol, tyrosol, and tocopherol contents of olive oil. Foods 2020, 9, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EEC Regulation no. 2568/91. Off. J. Eur. Communities 1991, 248, 1–83.
- Makhlouf, F.Z.; Squeo, G.; Barkat, M.; Trani, A.; Caponio, F. Antioxidant activity, tocopherols and polyphenols of acornoil obtained from Quercus species grown in Algeria. Food Res. Int. 2018, 114, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Fortunato, S.; Tamborrino, A.; Squeo, G.; Bianchi, B.; Caponio, F. Development of a modified malaxer reel: Influence on mechanical characteristic and virgin olive oil quality and composition. LWT-Food Sci. Technol. 2021, 135, 110290. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Summo, C.; Pasqualone, A. Influence of the type of olive-crusher used on the quality of extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2003, 105, 201–206. [Google Scholar] [CrossRef]
- Miazzi, M.M.; di Rienzo, V.; Mascio, I.; Montemurro, C.; Sion, S.; Sabetta, W.; Vivaldi, G.A.; Camposeo, S.; Caponio, F.; Squeo, G.; et al. REGEROP: An integrated project for the recovery of ancient and rare olive germplasm. Front. Plant Sci. 2020, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Harrabi, S.; Ferchichi, A.; Bacheli, A.; Fellah, H. Policosanol composition, antioxidant and anti-arthritic activities of milk thistle (Silybium marianum L.) oil at different seed maturity stages. Lipids Health Dis. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.G.; Krishna, G.A.G. Studies on the nutraceuticals composition of wheat derived oils wheat bran oil and wheat germ oil. J. Food Sci. Technol. 2015, 2, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- Özcan, M.M.; Rosa, A.; Dessi, M.A.; Marongiu, B.; Piras, A.; Al-Juhaimi, F.Y.I. Quality of wheat germ oil obtained by cold pressing and supercritical carbon dioxide extraction. Czech J. Food Sci. 2013, 31, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Cunnane, S.C.; Anderson, M.J. Pure linoleate deficiency in the rat: Influence on growth, accumulation of n-6 polyunsaturated, and [1-14C]linoleate oxidation. J. Lipid Res. 1997, 38, 805–812. [Google Scholar] [CrossRef]
- Ghafoor, K.; Özcan, M.M.; Al-Juhaimi, F.; Babiker, E.E.; Sarker, Z.I.; Ahmed, I.A.M.; Ahmed, M.A. Nutritional composition, extraction, and utilization of wheat germ oil: A review. Eur. J. Lipid Sci. Technol. 2017, 119, 1600160. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, T.; Shi, L.; Gong, M.; Jin, J.; Zhang, Y.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. The relationship between lipid phytochemicals, obesity and its related chronic diseases. Food Funct. 2018, 9, 6048–6062. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Hidalgo, A. Wheat germ: Not only a by-product. Int. J. Food Sci. Nutr. 2012, 63, 71–74. [Google Scholar] [CrossRef]
- Yang, C.S.; Lu, G.; Ju, J.; Li, G.X. Inhibition of inflammation and carcinogenesis in the lung and colon by tocopherols. Ann. N. Y. Acad. Sci. 2010, 1203, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, B.; Swick, J.; Ronnenberg, A.G. Vitamin E and adiponectin: Proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr. Res. 2010, 69, 155–161. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Vaisali, C.; Charanyaa, S.; Belur, P.D.; Regupathi, I. Refining of edible oils: A critical appraisal of current and potential technologies. Int. J. Food Sci. Technol. 2015, 50, 13–23. [Google Scholar] [CrossRef]
- Gomes, T.; Caponio, F. Possibility to improve the quality characteristics of olive-pomace oils and to enhance differentiation with refined olive-pomace oil. J. Sci. Food Agric. 2001, 81, 62–67. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Pérez-Camino, M.C.; Márquez-Ruiz, G. High performance size exclusion chromatography of polar compounds in heated and non-heated fats. LWT-Food Sci. Technol. 1988, 90, 308–311. [Google Scholar] [CrossRef]
- Gomes, T.; Caponio, F. Investigation on the degree of oxidation and hydrolysis of refined olive oils. An approach for better product characterization. Ital. J. Food Sci. 1997, 9, 277–285. [Google Scholar]
- Gomes, T.; Caponio, F.; Delcuratolo, D. Fate of oxidized triglycerides during refining of seed oils. J. Agric. Food Chem. 2003, 51, 4647–4651. [Google Scholar] [CrossRef] [PubMed]
- Billek, G. Health aspects of thermoxidized oils and fats. Eur. J. Lipid Sci. Technol. 2000, 102, 587–593. [Google Scholar] [CrossRef]
- Saguy, I.S.; Dana, D. Integrated approach to deep fat frying: Engineering, nutrition, health and consumer aspects. J. Food Eng. 2003, 56, 143–152. [Google Scholar] [CrossRef]
- Frankel, E.; Neff, W.E.; Selke, E.; Brooks, D.D. Analysis of autoxidized fats by gas chromatography-mass spectrometry: X. Volatile thermal decomposition products of methyl linoleate dimers. Lipids 1988, 23, 295–298. [Google Scholar] [CrossRef]
- Gomes, T.; Delcuratolo, D.; Paradiso, V.M. Pro-oxidant action of polar triglyceride oligopolymers in edible vegetable oils. Eur. Food Res. Technol. 2008, 226, 1409–1414. [Google Scholar] [CrossRef]
- Gomes, T.; Delcuratolo, D.; Paradiso, V.M.; Summo, C.; Caponio, F. Pro-oxidant activity of oxidized triacylglycerols in olive oil and comparison with pro-oxidant action of polar triacylglycerol oligopolymers. LWT-Food Sci. Technol. 2011, 44, 1236–1239. [Google Scholar] [CrossRef]
- Gomes, T.; Caponio, F. Evaluation of the state of oxidation of olive-pomace oils. The influence of the refining process. J. Agric. Food Chem. 1998, 46, 1137–1142. [Google Scholar] [CrossRef]
- Gomes, T.; Caponio, F. A study of oxidation and polymerization compounds during vegetable oil refining. Riv. Ital. Sostanze Grasse 1996, 73, 97–100. [Google Scholar]
- Wang, T.; Johnson, L.A. Refining high-free fatty acid wheat germ oil. J. Am. Oil Chem. Soc. 2001, 78, 71–76. [Google Scholar] [CrossRef]
- Zhu, M.; Wen, X.; Zhao, J.; Liu, F.; Ni, Y.; Ma, L.; Li, J. Effect of industrial chemical refining on the physicochemical properties and the bioactive minor components of peanut oil. J. Am. Oil Chem. Soc. 2016, 93, 285–294. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, R.; Wang, Z.; Wang, B.; Yang, Y.; Ju, X.; He, R. The effect of refining process on the physicochemical properties and micronutrients of rapeseed oils. PLoS ONE 2019, 14, e0212879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codex Alimentarius. Standard for Edible Fats and Oils Not Covered by Individual Standards, CXS 19-1981. 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 15 January 2022).
- Hopia, A. Analysis of high molecular weight autoxidation products using high performance size exclusion chromatography: II. Changes during processing. LWT-Food Sci. Technol. 1993, 26, 568–571. [Google Scholar] [CrossRef]
- Arroyo, R.; Cuesta, C.; Garrido-Polonio, C.; López-Varela, S.; Sánchez-Muñiz, F.J. High-performance size-exclusion chromatography studies on polar components formed in sunflower oils used for frying. J. Am. Oil Chem. Soc. 1992, 69, 557–563. [Google Scholar] [CrossRef]
- Garrido-Polonio, C.; Sánchez-Muñiz, F.J.; Arroyo, R.; Cuesta, C. Small scale frying of potatoes in sunflower oil: Thermoxidative alteration of the fat content in the fried product. Eur. J. Nutr. 1994, 33, 267–276. [Google Scholar] [CrossRef]
- Summo, C.; Caponio, F.; Paradiso, V.M.; Bilancia, M.T.; Pasqualone, A.; Cosmai, L.; Gomes, T. Oxidation compounds in extra virgin olive oils, fresh or stored, after frying. Ital. J. Food Sci. 2014, 26, 176–182. [Google Scholar]
- Rodríguez, G.; Squeo, G.; Estivi, L.; Quezada Berru, S.; Buleje, D.; Caponio, F.; Brandolini, A.; Hidalgo, A. Changes in stability, tocopherols, fatty acids and antioxidant capacity of sacha inchi (Plukenetia volubilis) oil during French fries deep-frying. Food Chem. 2021, 340, 127942. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T. Examination of lipid fraction quality of margarine. J. Food Sci. 2004, 69, 63–66. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Summo, C. Assessment of the oxidative and hydrolytic degradation of oils used as liquid medium of in-oil preserved vegetables. J. Food Sci. 2003, 68, 147–151. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Pasqualone, A.; Summo, C. Use of the high performance size exclusion chromatography analysis for the measurement of the degree of hydrolytic and oxidative degradation of the lipid fraction of biscuits. Food Chem. 2007, 102, 232–236. [Google Scholar] [CrossRef]
- Summo, C.; Bilancia, M.T.; Caponio, F. Assessment of the oxidative and hydrolytic degradation of the lipid fraction of mortadella by means of HPSEC analyses of polar compounds. Meat Sci. 2008, 79, 722–726. [Google Scholar] [CrossRef]
- Caponio, F.; Summo, C.; Bilancia, M.T.; Paradiso, V.M.; Sikorska, E.; Gomes, T. High performance size-exclusion chromatography analysis of polar compounds applied to refined, mild deodorized, extra virgin olive oils and their blends: An approach to their differentiation. LWT-Food Sci. Technol. 2011, 44, 1726–1730. [Google Scholar] [CrossRef]
- Eder, S.R. Über die bildung von artefakten bei der dämpfung von speiseölen und-fetten. Fett Lipid 1982, 84, 136–141. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Demonty, I. Phytosterols: Natural compounds with established and emerging health benefits. OCL 2007, 14, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Piironen, V.; Lampi, A. Occurrence and Levels of Phytosterols in Foods. In Phytosterols as Functional Food Components and Nutraceuticals; Dutta, P.C., Ed.; Marcel Dekker: New York, NY, USA, 2004; pp. 1–32. [Google Scholar]
- Weerawatanakorn, M.; Meerod, K.; Wongwaiwech, D.; Ho, C.T. Policosanols: Chemistry, occurrence, and health effects. Curr. Pharmacol. Rep. 2019, 5, 131–149. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, T. Phytosterols in cereal by-products. J. Am. Oil Chem. Soc. 2005, 82, 439–444. [Google Scholar] [CrossRef]
- Codex Alimentarius. Standard for Named Vegetable Oils, CXS 210-1999. 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 15 January 2022).

| Fatty Acids (%) | DP | MP |
|---|---|---|
| SFA | 17.28 ± 0.51 b | 18.67 ± 0.37 a |
| MUFA | 21.01 ± 0.52 a | 18.86 ± 1.81 b |
| PUFA | 61.71 ± 0.12 | 62.46 ± 1.51 |
| Compounds (mg/kg) | DP | MP |
|---|---|---|
| Total carotenoids | 42.44 ± 1.22 a | 37.88 ± 5.07 a |
| Total tocotrienols | 1336.33 ± 66.56 a | 1085.76 ± 121.66 b |
| Total tocopherols | 461.77 ± 31.49 b | 1050.63 ± 117.54 a |
| Tocotrienols + tocopherols | 1798.10 ± 31.49 b | 2136.39 ± 101.28 a |
| Parameters | Crude Oil | Degummed Oil | Neutralized Oil | Bleached Oil | Deodorized Oil |
|---|---|---|---|---|---|
| FFA (%) | 11.58 ± 0.31 a | 10.16 ± 0.38 b | 0.64 ± 0.01 c | 0.40 ± 0.04 c | 0.32 ± 0.04 c |
| PV (meq O2/kg) | 4.45 ± 0.65 b | 3.89 ± 0.16 b | 7.65 ± 0.35 a | 4.78 ± 0.07 b | 2.05 ± 0.07 c |
| TAGP (%) | 0.14 ± 0.02 c | 0.15 ± 0.01 c | 0.13 ± 0.01 c | 0.26 ± 0.01 b | 0.41 ± 0.01 a |
| ox-TAG (%) | 2.28 ± 0.22 a | 2.21 ± 0.08 a | 2.63 ± 0.12 a | 2.15 ± 0.11 ab | 1.58 ± 0.06 c |
| DAG (%) | 5.34 ± 0.25 a | 5.33 ± 0.21 a | 5.29 ± 0.04 a | 5.11 ± 0.12 a | 5.09 ± 0.09 a |
| PCs (%) | 19.34 ± 0.15 a | 17.86 ± 0.68 a | 8.69 ± 0.15 b | 7.92 ± 0.06 b | 7.39 ± 0.13 b |
| Compounds | Mean Values |
|---|---|
| Cholesterol | 0.2% |
| 24-Methylenecholesterol | 0.7% |
| Campesterol | 13.1% |
| Campestanol | 15.1% |
| Stigmasterol | 1.8% |
| ∆-7-Campesterol | 1.4% |
| Clerosterol | 0.5% |
| β-Sitosterol | 34.8% |
| Sitostanol | 16.6% |
| ∆-5-Avenasterol | 7.9% |
| ∆-7(9,11)-Stigmastadienol | 1.9% |
| ∆-5,24-Stigmastadienol | 1.1% |
| ∆-7-Stigmastenol | 1.5% |
| ∆-7-Avenasterol | 3.4% |
| Total sterols (mg/kg) | 20,975 |
| Total policosanols (mg/kg) | 754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squeo, G.; Silletti, R.; Napoletano, G.; Greco Miani, M.; Difonzo, G.; Pasqualone, A.; Caponio, F. Characterization and Effect of Refining on the Oil Extracted from Durum Wheat By-Products. Foods 2022, 11, 683. https://doi.org/10.3390/foods11050683
Squeo G, Silletti R, Napoletano G, Greco Miani M, Difonzo G, Pasqualone A, Caponio F. Characterization and Effect of Refining on the Oil Extracted from Durum Wheat By-Products. Foods. 2022; 11(5):683. https://doi.org/10.3390/foods11050683
Chicago/Turabian StyleSqueo, Giacomo, Roccangelo Silletti, Giulia Napoletano, Marcello Greco Miani, Graziana Difonzo, Antonella Pasqualone, and Francesco Caponio. 2022. "Characterization and Effect of Refining on the Oil Extracted from Durum Wheat By-Products" Foods 11, no. 5: 683. https://doi.org/10.3390/foods11050683








