Quality of Pepper Seed By-Products: A Review
Abstract
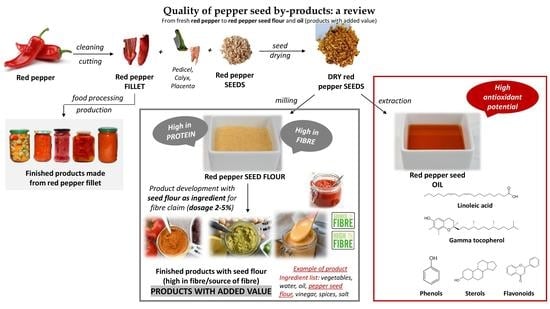
:1. Introduction
2. Chemical Composition of Pepper Seed Flour and Pepper Seed Oil
2.1. Pepper Seeds (Flour)
2.1.1. Protein Content
2.1.2. Carbohydrate and Total Dietary Fiber Content
| Sample Type | Total Dietary Fiber | Carbohydrate | Protein | Oil | References |
|---|---|---|---|---|---|
| Capsicum annuum L. (Podravka variety) | 41.2 | 3.2 | 16.7 | 27.2 | [9] |
| Capsicum annuum L. (Slavonka variety) | 42.1 | 3.4 | 16.5 | 26.7 | [9] |
| Capsicum annuum L. | 34.9 | / | 24.4 | 25.6 | [16] |
| Capsicum annuum L. (10 varieties from Turkey and Italy) | / | / | / | 8.5–32.6 | [31] |
| Capsicum frutescens L. | / | 55.1 | 26.0 | / | [11] |
| Capsicum annuum L. | / | 56.3 | 19.3 | 19.6 | [12] |
| Capsicum annuum var. annuum, var. glabriusculum Capsicum (boccatum, chinense, frutescens, pubescens) | / | / | / | 21.1–28.1 | [4] |
| / | / | / | 18.3–24.4 | ||
| Capsicum annuum (capia variety) | / | / | 21.5 | 13.6 | [1] |
| Capsicum annuum (cv. “Jinta”; hot pepper cultivar) | 38.8 | / | 21.3 | 23.7 | [19] |
| Capsicum annuum L. | 26.0 | / | 17.9 | 16.0 | [20] |
| Capsicum annuum L. | 61.0 | / | 18.3 | 11.0 | [10] |
| Capsicum annuum (capia variety) | / | / | 13.8 | 21.6 | [13] |
| Capsicum annuum L. (varieties—SZ–20, meteorite, sun dried; growing seasons 2013 and 2014) | / | / | / | 9.0–12.0 | [32] |
| Capsicum annuum L. (red pepper) | / | 43.6 | 28.3 | 18.4 | [15] |
| Capsicum annuum L. | / | / | / | 16.7 | [33] |
2.2. Pepper Seed Oil
2.2.1. Oil Production
2.2.2. Fatty Acid Profile of Pepper Seed Oil
| Sample Type | Oil Extraction Method | Palmitic C16:0 (%) | Stearic C18:0 (%) | Oleic C18:1 (%) | Linoleic C18:2 (%) | References |
|---|---|---|---|---|---|---|
| Capsicum annuum L. (Slavonka variety oil) | SC-CO2 extraction | 11.0 | 3.0 | 8.4 | 74.0 | [9] |
| Capsicum annuum L. (Slavonka variety oil) | Cold pressing | 10.9 | 3.0 | 8.4 | 77.7 | [9] |
| Capsicum annuum L. (Podravka variety oil) | SC-CO2 extraction | 11.9 | 3.3 | 8.8 | 76.0 | [9] |
| Capsicum annuum L. (Podravka variety oil) | Cold pressing | 10.8 | 3.4 | 10.4 | 75.4 | [9] |
| Capsicum annuum L. | Petroleum ether extraction | 13.8 | 3.7 | 14.6 | 67.8 | [16] |
| Capsicum annuum L. (10 varieties from Turkey and Italy) | Petroleum ether extraction | 10.7–14.2 | 2.5–4.1 | 8.9–12.5 | 69.5–74.7 | [31] |
| Capsicum annuum L. | Petroleum ether extraction | 12.3 | 3.2 | 13.0 | 71.6 | [12] |
| Capsicum (7 cultivated species) | Heptane extraction oil | 10.6–14.4 | 2.7–4.0 | 5.4–7.6 | 73.9–77.9 | [4] |
| Capsicum annuum (capia variety) | Cold pressing | 11.6 | 4.0 | 11.10 | 71.1 | [1] |
| Capsicum annuum L. | Cold pressing subcritical butane extraction | 11.5–11.6 11.4–11.7 | 2.7–2.9 2.5–3.0 | 9.8–9.9 10.0–9.6 | 72.7–72.4 73.0–72.1 | [20] |
| Capsicum annuum L. | Hexane extraction | 11.9 | 3.5 | 12.2 | 70.9 | [10] |
| Capsicum annuum L. (varieties—SZ–20, Meteorit, growing seasons 2013 and 2014) | Cold pressing | 11.1–12.2 | 3.1–3.8 | 7.9–9.6 | 70.8–74.3 | [32] |
| Capsicum annuum L. ssp. macrocarpum (Strumica valley) | Cold pressing | 10.8 | / | 4.6 | 69.6 | [39] |
| Capsicum annuum L. (red pepper) | Cold pressing, hexane extraction, supercritical CO2, microwave-assisted extraction | 13.4 12.3 11.2 11.4 | 2.5 2.5 2.4 2.4 | 9.2 9.8 8.8 8.2 | 73.7 73.9 76.3 76.5 | [15] |
| Capsicum annuum L. | Supercritical propane extraction | 11.6 | 2.4 | 11.6 | 72.6 | [41] |
| Capsicum annuum L. | Hexane extraction, ultrasound-assisted, pressure-assisted extraction | 13.4 13.4 13.3 | 2.8 2.8 2.7 | 9.2 9.0 9.1 | 72.4 72.5 72.6 | [33] |
2.2.3. Total Phenolic, Flavonoid and γ-Tocopherol Content
3. Product Development: Valorization of Pepper Seeds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, E.; Arsunar, E.S.; Aydeniz, B.; Güneşer, O. Cold pressed capia pepper seed (Capsicum annuum L.) oils: Composition, aroma and sensory properties. Eur. J. Lipid Sci. Technol. 2015, 117, 1016–1026. [Google Scholar] [CrossRef]
- Gurnani, N.; Gupta, M.; Mehta, D.; Kumar Mehta, B. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). J. Taibah Univ. Sci. 2016, 10, 462–470. [Google Scholar] [CrossRef] [Green Version]
- New Mexico State University. Available online: https://web.archive.org/web/20100802205934/http:/www.chilepepperinstitute.org/files/tiny_mce/file_manager/educ_info/ChileCultof%20NMSU.pdf (accessed on 15 November 2021).
- Jarret, R.L.; Levy, I.J.; Potter, T.L.; Sermak, S.C. Seed oil and fatty acid composition in Capsicum spp. J. Food Compos. Anal. 2013, 30, 102–108. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The Annual Production of Dry Chillies and Peppers Reports from United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 February 2022).
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 9, 1750–1756. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Z.; Wu, C.T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, S98–S102. [Google Scholar] [CrossRef]
- Sim, K.H.; Sil, H.Y. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int. J. Food Sci. Technol. 2008, 43, 1813–1823. [Google Scholar] [CrossRef]
- Cvetković, T.; Ranilović, J.; Gajari, D.; Tomić-Obrdalj, H.; Šubarić, D.; Moslavac, T.; Cikoš, A.M.; Jokić, S. Podravka and Slavonka Varieties of Pepper Seeds (Capsicum annuum L.) as a New Source of Highly Nutritional Edible Oil. Foods 2020, 9, 1262. [Google Scholar] [CrossRef]
- Azabou, S.; Taheur, F.B.; Jridi, M.; Bouaziz, M.; Nasri, M. Discarded seeds from red pepper (Capsicum annuum) processing industry as a sustainable source of high added-value compounds and edible oil. Environ. Sci. Pollut. Res. 2017, 24, 22196–22203. [Google Scholar] [CrossRef]
- Firatligil-Durmus, E.; Evranuz, O. Response surface methodology for protein extraction optimization of red pepper seed (Capsicum frutescens). LWT-Food Sci. Technol. 2010, 43, 226–231. [Google Scholar] [CrossRef]
- Embaby, H.S.; Mokhtar, S.M. Chemical composition and nutritive value of lantana and sweet pepper seeds and Nabak seed kernels. J. Food Sci. 2011, 76, 736–741. [Google Scholar] [CrossRef]
- Bostanci, H.; Ok, S.; Yilmaz, E. Valorization of capia pepperseed flour I: Spreadable new products development. Waste Biomass Valor. 2019, 10, 681–690. [Google Scholar] [CrossRef]
- Yilmaz, E. Valorization of Capia Pepperseed Flour in Breakfast Sauce Production. Waste Biomass Valor. 2020, 11, 6803–6813. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind. Crops Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- El-Adaway, T.A.; Taha, K.M. Characteristics and composition of watermelon, pumpkin, and paprika seed oils and flours. J. Agric. Food Chem. 2001, 49, 253–1259. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Energy and Protein Requirements; Reports of FAO Nutritional Meeting Series 52; FAO: Rome, Italy, 1973; Available online: https://apps.who.int/iris/bitstream/handle/10665/41042/WHO_TRS_522_eng.pdf?sequence=1&isAllowed=y (accessed on 15 December 2021).
- Yilmaz, E.; Hüriyet, Z. Physico-Chemical and Functional Properties of Extracted Capia Pepperseed (Capsicum annuum L.) Proteins. Waste Biomass Valor. 2017, 8, 871–881. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, K.; Tian, M. Chemical composition and nutritive value of hot pepper seed (Capsicum annuum) grown in Northeast Region of China. Food Sci. Technol. 2015, 35, 659–663. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.B.; Pang, H.L.; Lu, K.K.; Liu, H.M.; Wang, X.D.; Quin, G.Y. Process optimization and characterization of fragrant oil from red pepper (Capsicum annuum L.) seed extracted by subcritical butane extraction. J. Sci. Food Agric. 2017, 97, 1894–1903. [Google Scholar] [CrossRef]
- Kadri, N.; Khettal, B.; Aid, Y.; Kherfellah, S.; Sobhi, W.; Barragan-Montero, V. Some physicochemical characteristics of pinus (Pinus halepensis Mill., Pinus pinea L., Pinus pinaster and Pinus canariensis) seeds from North Algeria, their lipid profiles and volatile contents. Food Chem. 2015, 188, 184–192. [Google Scholar] [CrossRef]
- Li, G.; Song, C.; You, J.; Sun, Z.; Xia, L.; Suo, Y. Optimisation of red pepper seed oil extraction using supercritical CO2 and analysis of the composition by reversed-phase HPLC-FLD-MS/MS. Int. J. Food Sci. Technol. 2011, 46, 44–51. [Google Scholar] [CrossRef]
- Aw, T.L.; Swanson, B.G. Influence of tannin on (Phaseolus vulgaris) protein digestibility and quality. J. Food Sci. 1985, 50, 67–70. [Google Scholar] [CrossRef]
- Rahma, E.H.; El-Bedawy, A.A.; El-Adawy, T.A.; Goma, M.A. Changes in chemical and antinutritional factors and functional properties of faba beans during germination. Lebensm. Wiss. Technol. 1987, 20, 271–276. [Google Scholar]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Jesús Periago, M. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Int. Life Sci. Inst. 2020, 67, 188–205. [Google Scholar] [CrossRef]
- Rosell, C.M.; Santos, E.; Collar, C. Physico-chemical properties of commercial fibers from different sources: A comparative approach. Food Res. Int. 2009, 42, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1924&from=en (accessed on 10 November 2021).
- Dordevic, D.; Janickova, S.; Capikova, J.; Tremlova, B.; Kushkevych, I. Chemical and sensory properties of fruit jams affected by bamboo fiber fortification. Biointerface Res. Appl. Chem. 2020, 10, 5247–5251. [Google Scholar]
- Matthäus, B.; Őzcan, M.M. Chemical evaluation of some paprika (Capsicum annuum L.) seed oils. Eur. J. Lipid Sci. Technol. 2009, 111, 1249–1254. [Google Scholar] [CrossRef]
- Koncsek, A.; Helyes, L.; Daoog, H.G. Bioactive compounds of cold pressed spice paprika seeds oils. J. Food Process. Preserv. 2017, 42, e13403. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of the compounds and characteristics of pepper seed oil by pressure-assisted, ultrasound-assisted and convencional solvent extraction. Innov. Food Sci. Emerg. 2019, 54, 78–86. [Google Scholar] [CrossRef]
- Reddy, B.S.; Sajorini, G. Chemical and nutritional evaluation of chilli (Capsicum annuum) seed oil. J. Am. Oil Chem. Soc. 1987, 64, 1419–1422. [Google Scholar] [CrossRef]
- Jokić, S.; Aladić, K. Chapter: Hempseed Oil: Compounds and Production. In Edible Oil: Compounds, Production and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2019; pp. 173–192. [Google Scholar]
- Jokić, S.; Vidović, S.; Aladić, K. Chapter: Supercritical fluid extraction of edible Oils. In Supercritical Fluids: Fundamentals, Properties and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 205–228. [Google Scholar]
- Li, M.; Wen, X.; Peng, Y.; Wang, Y.; Wang, K.; Ni, Y. Functional properties of protein isolates from bell pepper. (Capcicum annuum L. var. annuum) seeds. LWT-Food Sci. Technol. 2018, 97, 802–810. [Google Scholar] [CrossRef]
- Yılmaz, E.; Hüriyet, Z.; Arifoğlu, N.; Dündar Emir, D. Functional properties of the capia pepper seed defatted press cakes. Waste Biomass Valori. 2017, 8, 783–791. [Google Scholar] [CrossRef]
- Kostadinović Veličkovska, S.; Mot, A.C.; Mitrev, S.; Goluboski, S.; Bruhl, L.; Mirhosseini, H.; Silaghi-Dumitrescu, R.; Matthäus, B. Bioactive compounds and “in vitro” antioxidant activity of some traditional and non-traditional cold-pressed edible oils from Macedonia. J. Food Technol. 2018, 55, 1614–1623. [Google Scholar] [CrossRef]
- Commision Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0432&from=EN (accessed on 9 November 2021).
- Zhang, R.-Y.; Liu, H.-M.; Ma, Y.-X.; Wang, X.-D. Characterization of fragrant oil extracted from pepper seed during subcritical propane extraction. LWT-Food Sci. Technol. 2019, 110, 110–116. [Google Scholar] [CrossRef]
- Gunstone, F.D. Vegetable Oils in Food Technology Composition, Properties and Uses, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 116, 137, 139. [Google Scholar]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemisty and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Saldeen, K.; Saldeen, T. Importance of tocopherols beyond α-tocopherol: Evidence from animal and human studies. Nutr. Res. 2005, 25, 877–889. [Google Scholar] [CrossRef]
- Saldeen, T.; Li, D.; Metha, J.L. Differential effects of alpha- and gama-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thromogenesis. J. Am. Coll. Cardiol. 1999, 34, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ–tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Branen, A.L. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc. 1975, 52, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Fukushima, S.; Hasegawa, A.; Shibata, M.; Ogiso, T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J. Natl. Cancer I. 1983, 70, 343–347. [Google Scholar]
- Larson, R. The antioxidants of higher plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional foods: Their role in disease prevention and health. Food Technol. 1998, 52, 63–69. [Google Scholar]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Taxonomic classification helps identify flavonoid-containing foods on a semiquantitative food frequency questionnaire. J. Am. Diet. Assoc. 1998, 98, 677–685. [Google Scholar] [CrossRef]
- Gorinstein, S.; Cvikrova, M.; Machackova, I.; Haruenkit, R.; Park, Y.S.; Jung, S.T.; Yamamoto, K.; Ayala, A.L.M.; Katrich, E.; Trakhtenberg, S. Characterization ofantioxidant compounds in Jaffa sweeties and white grapefruits. Food Chem. 2004, 84, 503–510. [Google Scholar] [CrossRef]
- Amarowicz, R.; Naczk, M.; Shahidi, F. Antioxidant activity of crude tannins of canola and rapeseed hulls. J. Am. Oil Chem. Soc. 2000, 77, 957–961. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Richter, J.; Kabrodt, K.; Lücke, I.M.; Schellenberg, I.; Herrling, T. The antioxidative power AP—A new quantitative dependent (2D) parameter for the determination of the antioxidant capacity and reactivity of different plants. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 846–850. [Google Scholar] [CrossRef]
- Silva, L.R.; Azevedo, J.; Pereira, M.J.; Valentao, P.; Andrade, P.B. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem. Toxicol. 2013, 53, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Bostanci, H.; Ok, S. Valorization of Capia Pepperseed Flour-II: Sensory Properties and Storage Stability of the New Spreadable Pastes. Waste Biomass Valor. 2019, 10, 3163–3171. [Google Scholar] [CrossRef]



| Sample Type | Oil Extraction Method | γ-Tocopherol | Total Phenolic | Flavonoids | References |
|---|---|---|---|---|---|
| Capsicum annuum L. oil (Slavonka variety) | SC-CO2 extraction | 16.0 mg/100 g | / | / | [9] |
| Capsicum annuum L. oil (Slavonka variety) | Cold pressed | 65.3 mg/100 g | / | / | [9] |
| Capsicum annuum L. oil (Podravka variety) | SC-CO2 extraction | 44.7 mg/100 g | / | / | [9] |
| Capsicum annuum L. oil (Podravka variety) | Cold pressed | 80.1 mg/100 g | / | / | [9] |
| Capsicum annuum L. (Slavonka variety) pepper seeds | / | / | 149.9 mg/100 g | / | [9] |
| Capsicum annuum L. (Podravka variety) pepper seeds | / | / | 158.2 mg/100 g | / | [9] |
| Capsicum annuum L. red pepper seed | / | / | 29.1 mg GAE/g | 21.3 mg CAE/g | [8] |
| Capsicum annuum L. red pepper pericarp | / | / | 47.5 mg GAE/g | 27.5 mg CAE/g | [8] |
| Capsicum annuum L. (10 varieties from Turkey and Italy) oil | Petroleum ether | 306.6–602.6 mg/kg | / | / | [31] |
| Capsicum annuum L. oil (capia variety) | Cold pressed from roasted and enzyme-treated seeds | 152.9–169.1 mg/kg | 18.3–24.0 µg GA/100 g | / | [1] |
| Capsicum annuum L. seeds | / | / | 21.5 mg GAE/g | 43.4 µg QE/g | [10] |
| Capsicum annuum L. oil (varieties—SZ-20, meteorite, sun dried; growing seasons 2013 and 2014) | Cold pressed | 56.9–83.6 mg/100 g | / | / | [32] |
| Capsicum annuum L. ssp. macrocarpum oil (Strumica valley) | Cold pressed | 25.7 mg/100 g | 117.4 mg GAE/L oil | / | [39] |
| Capsicum annuum L. oil | Subcritical propane extraction | 21.9 mg/100 g | / | / | [41] |
| Capsicum annuum L. oil | Cold pressed | 113.2 mg/kg | 8.3 mg/100 g | 1.6 mg/100 g | [15] |
| Hexane extraction | 94.4 mg/kg | 10.5 mg/100 g | 1.8 mg/100 g | ||
| Subcritical CO2 | 130.6 mg/kg | 12.6 mg/100 g | 2.1 mg/100 g | ||
| Microwave-assisted extraction | 136.5 mg/kg | 11.2 mg/100 g | 1.9 mg/100 g | ||
| Capsicum annuum L. oil | Cold pressed | 80.4 mg/kg | / | / | [33] |
| Hexane extraction | 77.1 mg/kg | / | / | ||
| Ultrasonic assisted | 74.5 mg/kg | / | / |
| Ingredient | Dosage (%) | New Product Development | Conclusion | References |
|---|---|---|---|---|
| Pepper seed flour | 20.0 | Breakfast sauce (vegetable and spice type) | Pepper seed flour could be valorized in various types of breakfast sauce, but bitterness should be moderate during such applications | [14] |
| Pepper seed flour | 23.7–30.1 | Spreadable pastes (chocolate and molasses type) | Valorization of pepper seeds flour in spreadable product formulations is possible, as well as creation nutritive valuable products | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvetković, T.; Ranilović, J.; Jokić, S. Quality of Pepper Seed By-Products: A Review. Foods 2022, 11, 748. https://doi.org/10.3390/foods11050748
Cvetković T, Ranilović J, Jokić S. Quality of Pepper Seed By-Products: A Review. Foods. 2022; 11(5):748. https://doi.org/10.3390/foods11050748
Chicago/Turabian StyleCvetković, Tanja, Jasmina Ranilović, and Stela Jokić. 2022. "Quality of Pepper Seed By-Products: A Review" Foods 11, no. 5: 748. https://doi.org/10.3390/foods11050748