Research on Rapid Detection Technology for β2-Agonists: Multi-Residue Fluorescence Immunochromatography Based on Dimeric Artificial Antigen
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus
2.3. Synthesis of Dimeric Artificial Antigen
2.4. Preparation of Polyclonal Antibodies
2.5. Preparation of EuNP Polyclonal Antibody Probes
2.6. Preparation of Colloidal Gold Polyclonal Antibody Probes
2.7. Preparation of Lateral Flow Strips
2.8. EuNP-FLFIA Detection Procedure
2.9. Parameter Optimization
2.10. Sensitivity and Specificity
2.11. Analysis of Spiked Recoveries
2.12. Analysis of Actual Samples
2.13. Data Analysis
3. Results and Discussion
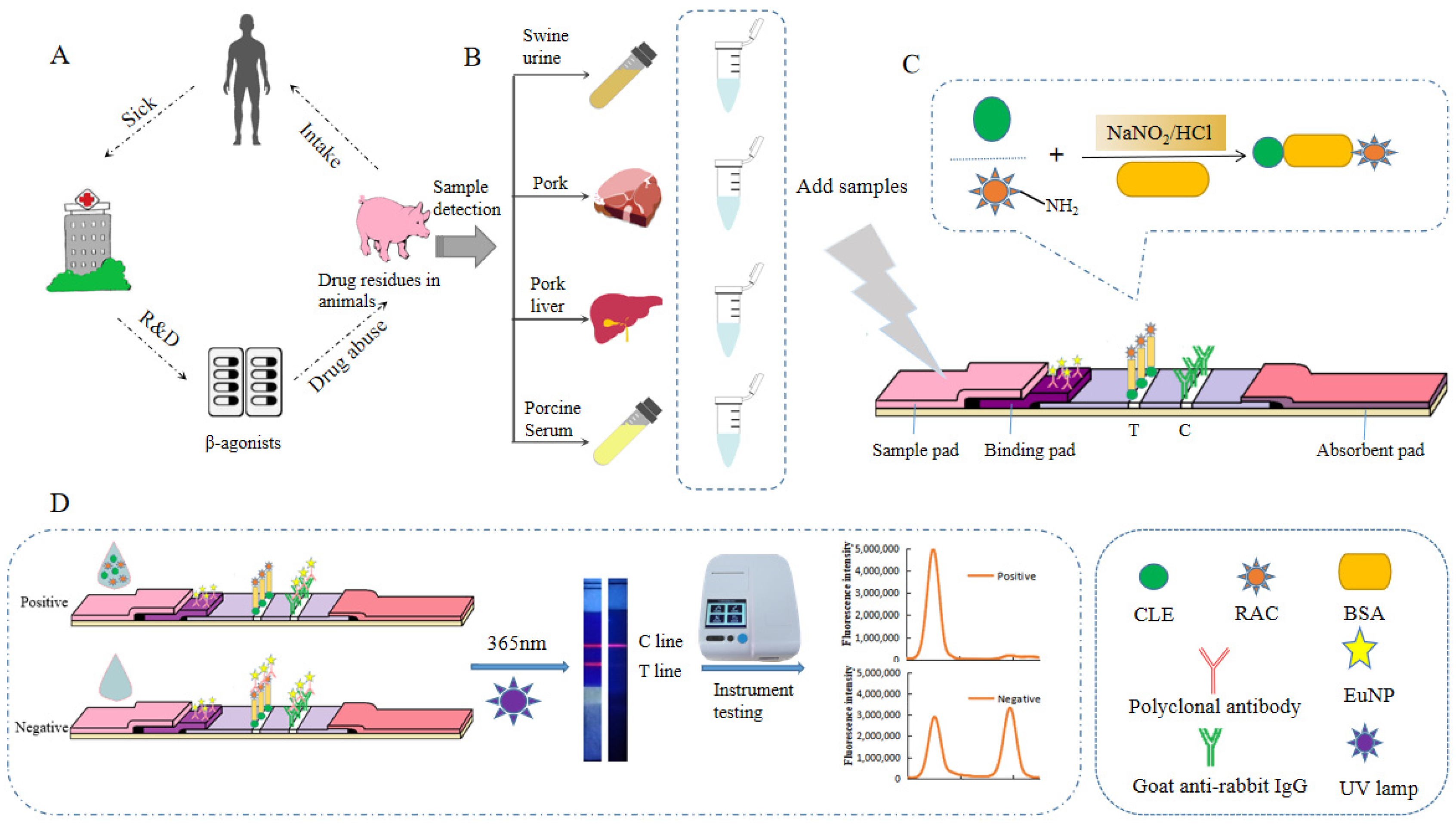
3.1. Detection Principle
3.2. Identification of the CLE-RAC Dimeric Artificial Antigen and Polyclonal Antibodies
3.3. Optimization of the EuNP-FLFIA Parameter
3.4. Sensitivity and Specificity Determination
3.5. Detection of Spiked Samples by EuNP-FLFIA
3.6. Detection of Actual Samples by EuNP-FLFIA and LC-MS/MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flavie, O.B.E.; Wang, H.V. In vitro Beta-agonist drugs modulate the proliferation and differentiation of skeletal muscle cells. Biochem. Biophys. Rep. 2021, 26, 101019. [Google Scholar] [CrossRef] [PubMed]
- Mastrianni, K.R.; Metavarayuth, K.; Brewer, W.E.; Wang, Q. Analysis of 10 β-agonists in pork meat using automated dispersive pipette extraction and LC-MS/MS. J. Chromatogr. B 2018, 1084, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Widmer, M.; Maden, K.; Butcher, P.; Walker, S. High resolution mass spectrometry-based detection and quantification of β-agonists at relevant trace levels in a variety of animal-based food matrices. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2021, 38, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, H.A.; Noordam, M.Y.; van Dooren-Flipsen, M.M.H.; Schilt, R.; Roos, A.H. Illegal use of beta-adrenergic agonists: European Community. J. Anim. Sci. 1998, 76, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.A.; Dunnavan, G. Illegal use of beta-adrenergic agonists in the United States. J. Anim. Sci. 1998, 76, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Granja, R.H.M.M.; Montes, N.A.M.; Rabone, F.; Montes, N.R.E.; Cannavan, A.; Salerno, A.G. Validation of radioimmunoassay screening methods for beta-agonists in bovine liver according to Commission Decision 2002/657/EC. Food Addit. Contam. Part A Chem. Anal. Control. Exp. Risk Assess. 2008, 25, 1475–1481. [Google Scholar] [CrossRef]
- Medellín-Martínez, M.F.; Luna-Zavala, I.; Martínez-Delgado, M.; Pérez-Urizar, J.T.; Ramírez-Telles, J.A.; Patiño-Rodríguez, O. Sensitive Assay of Clenbuterol Residues in Beef by Ultra-High Performance Liquid Chromatography Coupled with Mass Spectrometry (UPLC-MS/MS) and Solid-Phase Extraction. Food Anal. Methods 2018, 11, 2561–2568. [Google Scholar] [CrossRef]
- Ye, D.R.; Wu, S.S.; Xu, J.Q.; Jiang, R.F.; Zhu, F.; Ouyang, G.F. Rapid Determination of Clenbuterol in Pork by Direct Immersion Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 2016, 54, 112–118. [Google Scholar] [CrossRef][Green Version]
- Chang, K.C.; Chang, Y.T.; Tsai, C.E. Determination of ractopamine and salbutamol in pig hair by liquid chromatography tandem mass spectrometry. J. Food Drug Anal. 2018, 26, 725–730. [Google Scholar] [CrossRef]
- Casey, C.R.; Andersen, W.C.; Williams, N.T.; Nickel, T.J.; Ayres, P.R. Multiclass, Multiresidue Method for the Quantification and Confirmation of 112 Veterinary Drugs in Game Meat (Bison, Deer, Elk, and Rabbit) by Rapid Polarity Switching Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 1175–1186. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Pham, T.N.M.; Doan, T.T.; Ta, T.T.; Sáiz, J.; Nguyen, T.Q.H.; Hauser, P.C.; Mai, T.D. Simple semi-automated portable capillary electrophoresis instrument with contactless conductivity detection for the determination of β-agonists in pharmacEutical and pig-feed samples. J. Chromatogr. A 2014, 1360, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhou, T.; Yin, B.; He, P. Gold nanoparticle-based colorimetric ELISA for quantification of ractopamine. Mikrochim. Acta 2018, 185, 210. [Google Scholar] [CrossRef] [PubMed]
- Gressler, V.; Feddern, V.; Peixoto, J.O.; Ledur, M.C.; Costa, O.A.D.; Lima, G.J.M.M. Application of Enzyme Digestion and Deconjugation Followed by Quick, Easy, Cheap, Effective, Rugged, Safe Extraction and Liquid Chromatography-Tandem Mass Spectrometry Methodology To Determine Ractopamine Residue in Pork. J. Food Prot. 2018, 81, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.J.; Xu, Y.; Ma, B.; Cui, H.F.; Sun, C.X.; Zhang, M.Z. MonascusCarboxyl-Functionalized, Europium Nanoparticle-Based Fluorescent Immunochromatographic Assay for Sensitive Detection of Citrinin in Fermented Food. Toxins 2019, 11, 605. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhou, Q.Q.; Guo, Y.F.; Hu, H.Z.; Zheng, Z.; Li, S.L.; Wang, Y.H.; Ma, Y.F. Rapid Detection of Ractopamine and Salbutamol in Swine Urine by Immunochromatography Based on Selenium Nanoparticles. Int. J. Nanomed. 2021, 16, 2059–2070. [Google Scholar] [CrossRef]
- He, P.L.; Shen, L.; Liu, R.Y.; Luo, Z.P.; Li, Z. Direct detection of β-agonists by use of gold nanoparticle-based colorimetric assays. Anal. Chem. 2011, 83, 6988–6995. [Google Scholar] [CrossRef]
- Simon, T.; Shellaiah, M.; Steffi, P.; Sun, K.W.; Ko, F.H. Development of extremely stable dual functionalized gold nanoparticles for effective colorimetric detection of clenbuterol and ractopamine in human urine samples. Anal. Chim. Acta 2018, 1023, 96–104. [Google Scholar] [CrossRef]
- Song, C.M.; Zhi, A.M.; Liu, Q.T.; Yang, J.F.; Jia, G.C.; Shervin, J.; Tang, L.; Hu, X.F.; Deng, R.G.; Xu, C.L.; et al. Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip. Biosens. Bioelectron. 2013, 50, 62–65. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.L.; Jin, Z.Y.; Huang, L.P.; Dan, H.B.; Xiao, W.; Liang, J.J.; Zou, S.Y.; Tang, Y. Differential diagnosis of PRV-infected versus vaccinated pigs using a novel EuNPs-virus antigen probe-based blocking fluorescent lateral flow immunoassay. Biosens. Bioelectron. 2020, 155, 112101. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, B.; Chen, E.J.; Yu, X.P.; Ye, Z.H.; Sun, C.X.; Zhang, M.Z. Dual fluorescent immunochromatographic assay for simultaneous quantitative detection of citrinin and zearalenone in corn samples. Food Chem. 2021, 336, 127713. [Google Scholar] [CrossRef]
- Majdinasab, M.; Zareian, M.; Zhang, Q.; Li, P.W. Development of a new format of competitive immunochromatographic assay using secondary antibody-Europium nanoparticle conjugates for ultrasensitive and quantitative determination of ochratoxin A. Food Chem. 2019, 275, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Zhao, Q.; Zhou, R.; Liang, Y.C.; Zhao, W.B.; Shan, C.X. Ratiometric fluorescence sensor based on europium-grafted ZnO quantum dots for visual and colorimetric detection of tetracycline. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 259, 119901. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Luo, J.J.; Jiang, X.X.; Yang, M.H.; Rasooly, A. Gold nanocluster-europium(III) ratiometric fluorescence assay for dipicolinic acid. Mikrochim. Acta 2021, 188, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, M.; Niu, W.; Cheng, W.; Guo, Y.; Wang, Y.D.; Luo, M.; Xie, C.X.; Leng, T.T.; Zhang, X.X.; et al. In Vitro Multifunctional Protein-Decorated Bioactive Glass Nanoparticles for Tumor-Specific Therapy and Bioimaging. ACS Appl. Mater. Interfaces 2021, 13, 14985–14994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Sun, Y.Z.; Liang, D.M.; Zeng, Y.Y.; He, S.; Mari, G.M.; Peng, T.; Jiang, H.Y. Highly sensitive chromatographic time-resolved fluoroimmunoassay for rapid onsite detection of streptomycin in milk. J. Dairy Sci. 2020, 103, 8750–8760. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Shi, F.; Jiang, X.C.; Wang, L.M.; Chen, W.Q.; Zhu, C.G. Sensitive detection of small molecules by competitive immunomagnetic-proximity ligation assay. Anal. Chem. 2012, 84, 2129–2132. [Google Scholar] [CrossRef]
- Liu, S.J.; Dou, L.N.; Yao, X.L.; Zhang, W.T.; Zhao, M.; Yin, X.C.; Sun, J.; Zhang, D.H.; Wang, J.L. Nanozyme amplification mediated on-demand multiplex lateral flow immunoassay with dual-readout and broadened detection range. Biosens. Bioelectron. 2020, 169, 112610. [Google Scholar] [CrossRef]
- Ge, E.L.; Steven, S.; Wu, X.L.; Zheng, Q.K.; Kuang, H. Rapid immunochromatographic test strip detection of mabuterol and its cross-reactivity with mapenterol. Food Agric. Immunol. 2018, 29, 1028–1040. [Google Scholar] [CrossRef]
- Wang, R.G.; Zhang, W.; Wang, P.L.; Su, X.O. A paper-based competitive lateral flow immunoassay for multi β-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Mikrochim. Acta 2018, 185, 191. [Google Scholar] [CrossRef]
- Carolina, N.; Feddern, V.; Gressler, V.; Molognoni, L.; Daguer, H.; Dalla, C.O.A.; Lima, G.J.M.M.; Contreras-Castillo, C. Determination of ractopamine residue in tissues and urine from pig fed meat and bone meal. Food Addit. Contam. Part A Chem. Anal. Control. Exp. Risk Assess. 2019, 36, 424–433. [Google Scholar] [CrossRef]
- Wang, P.L.; Wang, Z.; Su, X.O. A sensitive and quantitative fluorescent multi-component immuno-chromatographic sensor for β-agonist residues. Biosens. Bioelectron. 2015, 64, 511–516. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, Z.J.; Chen, Y.; Hu, S.; Lai, W.H. Sensitive and Matrix-Tolerant Lateral Flow Immunoassay Based on Fluorescent Magnetic Nanobeads for the Detection of Clenbuterol in Swine Urine. J. Agric. Food Chem. 2019, 67, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, A.M.; Yen, C.N.; Richert, B.T.; Schinckel, A.P.; Grant, A.L.; Gerrard, D.E. Ractopamine-induced fiber type-specific gene expression in porcine skeletal muscles is independent of growth. J. Anim. Sci. 2020, 98, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Z.; Bai, A.; Sha, Y.; Ma, L.; Qin, S.; Chen, F.; Qin, S.; Wu, J. Prophylactic Efficacy of Equine Immunoglobulin F(ab’)2 Fragments Against Feline Parvovirus. Appl. Biochem. Biotechnol. 2021, 193, 3151–3162. [Google Scholar] [CrossRef]
- Gong, Y.F.; Zhang, M.Z.; Wang, M.Z.; Chen, Z.L.; Xi, X. Development of Immuno-Based Methods for Detection of Melamine. Arab. J. Sci. Eng. 2014, 39, 5315–5324. [Google Scholar] [CrossRef]
- Marie-Claire, H.; Damià, B. Strengths and limitations of immunoassays for effective and efficient use for pesticide analysis in water samples: A review. Anal. Chim. Acta 1998, 362, 3–34. [Google Scholar] [CrossRef]
- Li, T.T.; Cao, J.J.; Li, Z.; Wang, X.; He, P.L. Broad screening and identification of β-agonists in feed and animal body fluid and tissues using ultra-high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry combined with spectra library search. Food Chem. 2016, 192, 188–196. [Google Scholar] [CrossRef]
- Wu, Q.; Song, Q.; Wang, X.X.; Yao, L.; Xu, J.G.; Lu, J.F.; Liu, G.D.; Chen, W. Simultaneous Detection of Multiple β-Adrenergic Agonists with 2-Directional Lateral Flow Strip Platform. Anal. Sci. 2020, 36, 653–658. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.Q.; Zhao, G.Y.; Dou, W.C. Ultramarine blue nanoparticles as a label for immunochromatographic on-site determination of ractopamine. Mikrochim. Acta 2020, 187, 285. [Google Scholar] [CrossRef]
- Xu, F.; Jin, Z.; Zou, S.; Chen, C.; Song, Q.; Deng, S.; Xiao, W.; Zhang, X.; Jia, A.; Tang, Y. EuNPs-mAb fluorescent probe based immunochromatographic strip for rapid and sensitive detection of porcine epidemic diarrhea virus. Talanta 2020, 214, 120865. [Google Scholar] [CrossRef]
- Wang, X.; Tang, B.; Zhao, Y.; Ding, J.; Wang, N.; Liu, Y.; Dong, Z.; Sun, X.; Xu, Q.; Liu, M.; et al. Development of a rapid and sensitive immunochromatographic strip based on EuNPs-ES fluorescent probe for the detection of early Trichinella spiralis-specific IgG antibody in pigs. Vet. Res. 2021, 52, 85. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Bu, T.; Zhang, W.T.; Yan, L.Z.; Zhang, M.Y.; Yang, Q.F.; Huang, L.J.; Yang, B.W.; Hu, N.; Suo, Y.R.; et al. An improved clenbuterol detection by immunochromatographic assay with bacteria@Au composite as signal amplifier. Food Chem. 2018, 262, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, L.Q.; Song, S.S.; Cui, G.; Zheng, Q.K.; Kuang, H.; Xu, C.L. Development of an immunochromatographic strip for the rapid detection of 10 β-agonists based on an ultrasensitive monoclonal antibody. Food Agric. Immunol. 2017, 28, 43625–43638. [Google Scholar] [CrossRef]




| CG-LFIA | ||||
| β2-Agonists | Standard Curve | R2 | IC50 (ng/mL) | LOD (ng/mL) |
| CLE | y = 42.123x + 7.6785 | 0.96 | 10.11 | 1.14 |
| RAC | y = 41.364x + 7.8854 | 0.95 | 10.42 | 1.12 |
| MAB | y = 43.167x + 6.9773 | 0.95 | 9.92 | 1.17 |
| BAM | y = 45.243x − 4.1525 | 0.95 | 15.74 | 2.04 |
| BRO | y = 49.039x − 11.854 | 0.97 | 16.94 | 2.75 |
| MAP | y = 46.332x − 18.323 | 0.96 | 29.83 | 4.08 |
| CIM | y = 46.332x − 15.323 | 0.96 | 25.70 | 2.14 |
| CLEN | y = 44.707x + 3.6362 | 0.95 | 10.89 | 1.38 |
| CIMB | y = 48.816x − 17.775 | 0.97 | 24.43 | 3.72 |
| CLE+RAC | y = 43.072x + 6.5865 | 0.96 | 10.19 | 1.20 |
| Mixed solution | y = 41.921x + 3.8361 | 0.96 | 12.59 | 1.40 |
| EuNP-FLFIA | ||||
| β2-Agonists | Standard Curve | R2 | IC50 (ng/mL) | LOD (ng/mL) |
| CLE | y = 43.349x + 50.829 | 0.98 | 0.96 | 0.11 |
| RAC | y = 44.468x + 50.396 | 0.98 | 0.98 | 0.12 |
| MAB | y = 43.966x + 52.329 | 0.97 | 0.89 | 0.11 |
| BAM | y = 39.138x + 44.763 | 0.97 | 1.36 | 0.13 |
| BRO | y = 36.708x + 42.77 | 0.97 | 1.58 | 0.13 |
| MAP | y = 34.149x + 34.426 | 0.98 | 2.86 | 0.19 |
| CIM | y = 33.415x + 35.994 | 0.97 | 2.62 | 0.17 |
| CLEN | y = 42.33x + 49.775 | 0.97 | 1.01 | 0.11 |
| CIMB | y = 34.463x + 41.135 | 0.97 | 1.80 | 0.13 |
| CLE+RAC | y = 44.033x + 50.504 | 0.98 | 0.97 | 0.12 |
| Mixed solution | y = 37.379x + 46.526 | 0.97 | 1.24 | 0.11 |
| Target | Spiked Level (ng/mL) | Intra-Assay a | Target | Spiked Level (ng/mL) | Intra-Assay a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Detected Amount (ng/mL) | Recovery Rate | RSD (n = 3) | Detected Amount (ng/mL) | Recovery Rate | RSD (n = 3) | ||||
| Clenbuterol | 0.5 | 0.44 | 88.00% | 6.53% | Ractopamine | 0.5 | 0.57 | 114.00% | 6.05% |
| 1.0 | 1.09 | 109.00% | 4.56% | 1.0 | 1.06 | 106.00% | 2.28% | ||
| 2.0 | 2.18 | 109.00% | 8.01% | 2.0 | 2.21 | 110.50% | 4.49% | ||
| Mabuterol | 0.5 | 0.42 | 84.00% | 5.56% | Bambuterol | 0.5 | 0.49 | 98.00% | 5.86% |
| 1.0 | 0.85 | 85.00% | 3.59% | 1.0 | 0.87 | 87.00% | 4.92% | ||
| 2.0 | 1.96 | 98.00% | 3.37% | 2.0 | 1.73 | 86.50% | 2.37% | ||
| Brombuterol | 0.5 | 0.52 | 104.00% | 5.83% | Mapenterol | 0.5 | 0.47 | 94.00% | 4.60% |
| 1.0 | 1.14 | 114.00% | 6.43% | 1.0 | 0.94 | 94.00% | 4.52% | ||
| 2.0 | 2.16 | 108.00% | 5.81% | 2.0 | 1.77 | 88.50% | 4.20% | ||
| Cimaterol | 0.5 | 0.55 | 110.00% | 6.83% | Clenproperol | 0.5 | 0.47 | 94.00% | 6.77% |
| 1.0 | 1.12 | 112.00% | 5.82% | 1.0 | 0.91 | 91.00% | 4.60% | ||
| 2.0 | 1.83 | 91.50% | 2.80% | 2.0 | 2.09 | 104.50% | 1.98% | ||
| Cimbuterol | 0.5 | 0.48 | 96.00% | 5.82% | CLE + RAC | 0.5 | 0.55 | 110.00% | 5.85% |
| 1.0 | 0.88 | 88.00% | 1.12% | (1:1) | 1.0 | 1.02 | 102.00% | 4.78% | |
| 2.0 | 1.99 | 99.50% | 1.45% | 2.0 | 2.12 | 106.00% | 2.64% | ||
| Sample | EuNP-FLFIA a (ng/mL) | LC-MS/MS a (ng/mL) | Sample | EuNP-FLFIA a (ng/mL) | LC-MS/MS a (ng/mL) |
|---|---|---|---|---|---|
| Urine 01 | / b | / | Urine 11 | / | / |
| Urine 02 | / | / | Urine 12 | 1.78 ± 0.09 | 1.93 ± 0.12 |
| Urine 03 | / | / | Urine 13 | / | / |
| Urine 04 | / | / | Urine 14 | / | / |
| Urine 05 | / | / | Urine 15 | / | / |
| Urine 06 | 0.81 ± 0.22 | 0.80 ± 0.09 | Urine 16 | / | / |
| Urine 07 | / | / | Urine 17 | / | / |
| Urine 08 | / | / | Urine 18 | / | / |
| Urine 09 | 0.67 ± 0.13 | 0.88 ± 0.15 | Urine 19 | / | / |
| Urine 10 | / | / | Urine 20 | 0.33 ± 0.04 | 0.36 ± 0.02 |
| Muscle 01 | / | / | Muscle 10 | / | / |
| Muscle 02 | / | / | Muscle 11 | / | / |
| Muscle 03 | / | / | Muscle 12 | / | / |
| Muscle 04 | / | / | Muscle 13 | 0.41 ± 0.23 | 0.45 ± 0.16 |
| Muscle 05 | / | / | Muscle 14 | / | / |
| Muscle 06 | / | / | Muscle 15 | / | / |
| Muscle 07 | / | / | Muscle 16 | / | / |
| Muscle 08 | 1.27 ± 0.14 | 1.38 ± 0.11 | Muscle 17 | / | / |
| Muscle 09 | / | / | Muscle 18 | / | / |
| Liver 01 | / | / | Liver 07 | / | / |
| Liver 02 | 10.22 ± 0.31 | 10.31 ± 0.20 | Liver 08 | / | / |
| Liver 03 | / | / | Liver 09 | / | / |
| Liver 04 | / | / | Liver 10 | 15.88 ± 0.24 | 16.02 ± 0.14 |
| Liver 05 | / | / | Liver 11 | / | / |
| Liver 06 | / | / | Liver 12 | / | / |
| Serum 01 | / | / | Serum 06 | 2.11 ± 0.11 | 2.13 ± 0.03 |
| Serum 02 | / | / | Serum 07 | / | / |
| Serum 03 | 4.21 ± 0.08 | 4.27 ± 0.05 | Serum 08 | / | / |
| Serum 04 | / | / | Serum 09 | / | / |
| Serum 05 | / | / | Serum 10 | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Ma, B.; Wang, Y.; Chen, E.; Li, J.; Zhang, M. Research on Rapid Detection Technology for β2-Agonists: Multi-Residue Fluorescence Immunochromatography Based on Dimeric Artificial Antigen. Foods 2022, 11, 863. https://doi.org/10.3390/foods11060863
Liu M, Ma B, Wang Y, Chen E, Li J, Zhang M. Research on Rapid Detection Technology for β2-Agonists: Multi-Residue Fluorescence Immunochromatography Based on Dimeric Artificial Antigen. Foods. 2022; 11(6):863. https://doi.org/10.3390/foods11060863
Chicago/Turabian StyleLiu, Miaomiao, Biao Ma, Yaping Wang, Erjing Chen, Jiali Li, and Mingzhou Zhang. 2022. "Research on Rapid Detection Technology for β2-Agonists: Multi-Residue Fluorescence Immunochromatography Based on Dimeric Artificial Antigen" Foods 11, no. 6: 863. https://doi.org/10.3390/foods11060863
APA StyleLiu, M., Ma, B., Wang, Y., Chen, E., Li, J., & Zhang, M. (2022). Research on Rapid Detection Technology for β2-Agonists: Multi-Residue Fluorescence Immunochromatography Based on Dimeric Artificial Antigen. Foods, 11(6), 863. https://doi.org/10.3390/foods11060863






