Arabinoxylans as Functional Food Ingredients: A Review
Abstract
:1. Introduction
2. Structure of Arabinoxylan
2.1. Structure of Wheat Arabinoxylan
2.2. Structure of Barley Arabinoxylan
2.3. Structure of Corn Arabinoxylan
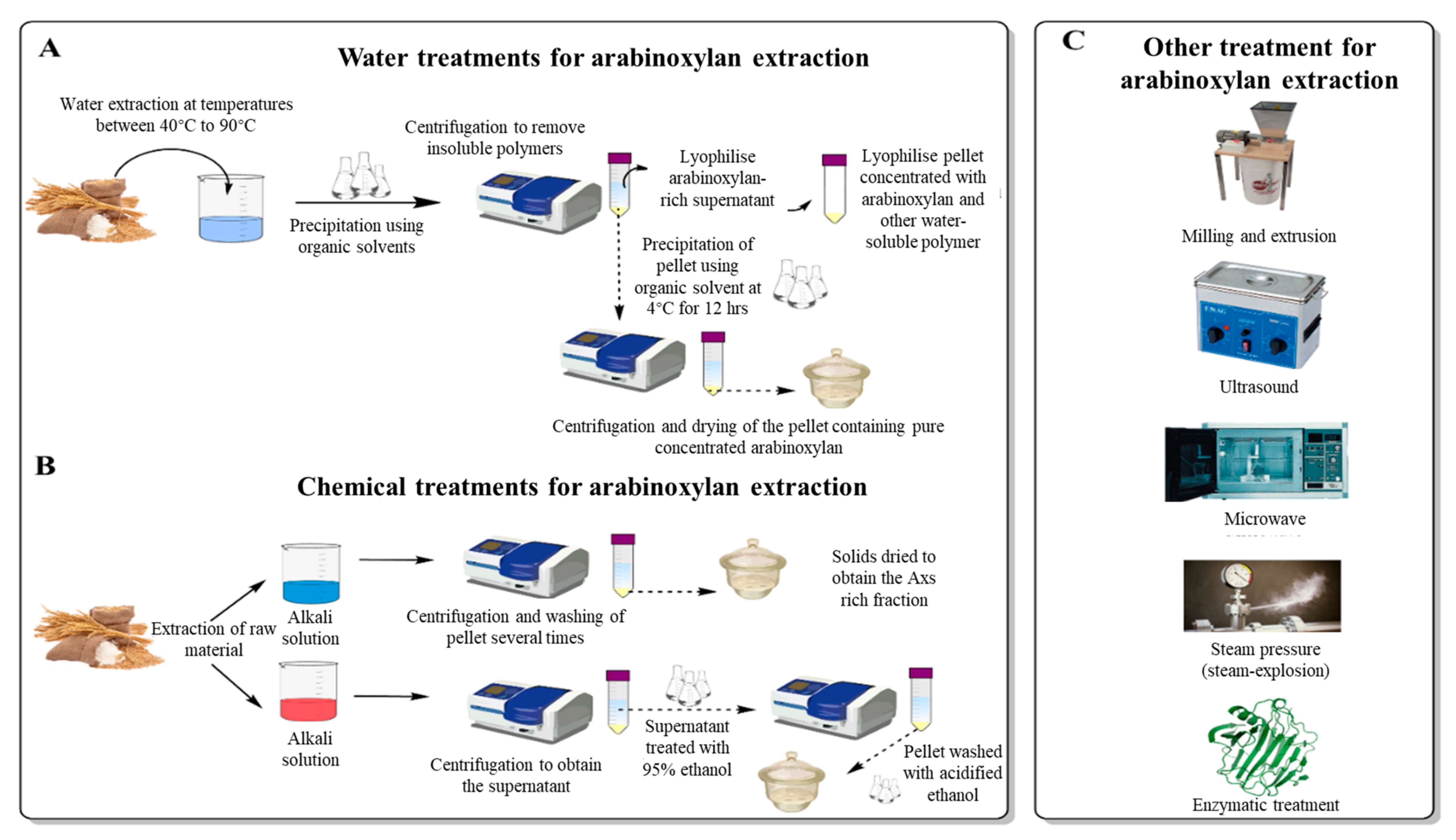
3. Extraction and Production of AXs as a Food Ingredient
3.1. Water Extraction of Arabinoxylans
3.2. Mechanical Extraction of Arabinoxylans
3.3. Chemical Extraction of Arabinoxylans
3.4. Enzymatic Extraction of Arabinoxylans
4. Health Benefits
- modification of short-chain fatty acids (SCFAs) production in the colon via regulation of gut microbiota
- antioxidant capacity
- hypoglycaemic effect/postprandial blood glucose response control
5. Physicochemical Properties of AXs
5.1. Solubility
5.2. Viscosity
5.3. Emulsifying Capacity
6. AXs Inclusion in Food Matrixes
6.1. Pasta
6.2. Cookies and Biscuits
6.3. Cakes
6.4. Bread
6.5. Beer
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Paesani, C.; Degano, A.L.; Salvucci, E.; Zalosnik, M.I.; Fabi, J.P.; Sciarini, L.S.; Perez, G.T. Soluble arabinoxylans extracted from soft and hard wheat show a differential prebiotic effect in vitro and in vivo. J. Cereal Sci. 2020, 93, 102956. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 2020, 8, 118. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Vargas-Albores, F.; Fierro-Islas, J.M.; Gollas-Galván, T.; Magdaleno-Moncayo, D.; Rascon-Chu, A.; Martínez-Porchas, M.; Lago-Lestón, A. Arabinoxylans and gelled arabinoxylans used as anti-obesogenic agents could protect the stability of intestinal microbiota of rats consuming high-fat diets. Int. J. Food Sci. Nutr. 2019, 71, 74–83. [Google Scholar] [CrossRef]
- Lin, S.; Agger, J.W.; Wilkens, C.; Meyer, A.S. Feruloylated Arabinoxylan and Oligosaccharides: Chemistry, Nutritional Functions, and Options for Enzymatic Modification. Annu. Rev. Food Sci. Technol. 2021, 12, 331–354. [Google Scholar] [CrossRef]
- Lazaridou, A.; Chornick, T.; Biliaderis, C.G.; Izydorczyk, M.S. Sequential solvent extraction and structural characterization of polysaccharides from the endosperm cell walls of barley grown in different environments. Carbohydr. Polym. 2008, 73, 621–639. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Ebringerová, A.; Heinze, T. Xylan and Xylan Derivatives—Biopolymers with Valuable Properties, 1. Naturally Occurring Xylans Structures, Isolation Procedures and Properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- He, H.-J.; Qiao, J.; Liu, Y.; Guo, Q.; Ou, X.; Wang, X. Isolation, Structural, Functional, and Bioactive Properties of Cereal Arabinoxylan—A Critical Review. J. Agric. Food Chem. 2021, 69, 15437–15457. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, M.S.; Biliaderis, C. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Fu, Y.; Liu, J.; Lin, S.; Zhang, Q.; Liu, Y.; Wu, D.; Lin, D.; Han, G.; et al. Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale. Antioxidants 2019, 8, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcotuli, I.; Hsieh, Y.S.-Y.; Lahnstein, J.; Yap, K.; Burton, R.A.; Blanco, A.; Fincher, G.B.; Gadaleta, A. Structural Variation and Content of Arabinoxylans in Endosperm and Bran of Durum Wheat (Triticum turgidum L.). J. Agric. Food Chem. 2016, 64, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.; Bar-L’Helgouac’h, C.; Champ, M.; Saulnier, L. Arabinoxylan content and grain tissue distribution are good predictors of the dietary fibre content and their nutritional properties in wheat products. Food Chem. 2020, 328, 127111. [Google Scholar] [CrossRef]
- Saulnier, L.; Sado, P.-E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281. [Google Scholar] [CrossRef]
- Saulnier, L.; Guillon, F.; Chateigner-Boutin, A.-L. Cell wall deposition and metabolism in wheat grain. J. Cereal Sci. 2012, 56, 91–108. [Google Scholar] [CrossRef]
- Comino, P.; Shelat, K.; Collins, H.; Lahnstein, J.; Gidley, M.J. Separation and Purification of Soluble Polymers and Cell Wall Fractions from Wheat, Rye and Hull less Barley Endosperm Flours for Structure-Nutrition Studies. J. Agric. Food Chem. 2013, 61, 12111–12122. [Google Scholar] [CrossRef]
- Li, L.-Y.; Wang, Y.-X.; Zhang, T.; Zhang, J.-F.; Pan, M.; Huang, X.-J.; Yin, J.-Y.; Nie, S.-P. Structural characteristics and rheological properties of alkali-extracted arabinoxylan from dehulled barley kernel. Carbohydr. Polym. 2020, 249, 116813. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Jacobs, M.; Dexter, J.E. Distribution and Structural Variation of Nonstarch Polysaccharides in Milling Fractions of Hull-less Barley with Variable Amylose Content. Cereal Chem. 2003, 80, 645–653. [Google Scholar] [CrossRef]
- Lazaridou, A.; Chornick, T.; Biliaderis, C.G.; Izydorczyk, M.S. Composition and molecular structure of polysaccharides released from barley endosperm cell walls by sequential extraction with water, malt enzymes, and alkali. J. Cereal Sci. 2008, 48, 304–318. [Google Scholar] [CrossRef]
- Zheng, X.; Li, L.; Wang, X. Molecular Characterization of Arabinoxylans from Hull-Less Barley Milling Fractions. Molecules 2011, 16, 2743–2753. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Kumar, S.; Ahluwalia, V.; Kansal, S.K.; Elumalai, S. Extraction of arabinoxylan from corncob through modified alkaline method to improve xylooligosaccharides synthesis. Bioresour. Technol. Rep. 2018, 3, 51–58. [Google Scholar] [CrossRef]
- Saulnier, L.; Marot, C.; Chanliaud, E.; Thibault, J.-F. Cell wall polysaccharide interactions in maize bran. Carbohydr. Polym. 1995, 26, 279–287. [Google Scholar] [CrossRef]
- Montgomery, R.; Smith, F. Structure of Corn Hull Hemicellulose. Part III. Identification of the Methylated Aldobiouronic Acid Obtained from Methyl Corn Hull Hemicellulose1,2. J. Am. Chem. Soc. 1957, 79, 695–697. [Google Scholar] [CrossRef]
- Whistler, R.L.; Corbett, W.M. Oligosaccharides from Partial Acid Hydrolysis of Corn Fiber Hemicellulose1,2. J. Am. Chem. Soc. 1955, 77, 6328–6330. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, C.; Li, W.; Ashworth, J. Characterization of Nitric Oxide Modulatory Activities of Alkaline-Extracted and Enzymatic-Modified Arabinoxylans from Corn Bran in Cultured Human Monocytes. J. Agric. Food Chem. 2016, 64, 8128–8137. [Google Scholar] [CrossRef]
- Hashimoto, S.; Shogren, M.D.; Bolte, L.C.; Pomeranz, Y. Cereal Pentosans: Their Estimation and Significance III Pentosans in Abraded Grains and Milling Products. Cereal Chem. 1987, 64, 39–41. [Google Scholar]
- Fadel, A.; Plunkett, A.; Li, W.; Ranneh, Y.; Gyamfi, V.E.T.; Salmon, Y.; Nyaranga, R.R.; Ashworth, J. Arabinoxylans from rice bran and wheat immunomodulatory potentials: A review article. Nutr. Food Sci. 2018, 48, 97–110. [Google Scholar] [CrossRef]
- Shibuya, N.; Iwasaki, T. Structural features of rice bran hemicellulose. Phytochemistry 1985, 24, 285–289. [Google Scholar] [CrossRef]
- Vinkx, J.A.; Delcour, J.A. Rye (Secale cereale L.) Arabinoxylans: A Critical Review. J. Cereal Sci. 1996, 24, 1–14. [Google Scholar] [CrossRef]
- Aspinall, G.O.; Sturgeon, R.J. 900. Cereal gums. Part II. The constitution of an araboxylan from rye flour. J. Chem. Soc. 1957, 4469–4471. [Google Scholar] [CrossRef]
- Åman, P.; Bengtsson, S. Periodate oxidation and degradation studies on the major water-soluble arabinoxylan in rye grain. Carbohydr. Polym. 1991, 15, 405–414. [Google Scholar] [CrossRef]
- Nilsson, M.; Saulnier, L.; Andersson, R.; Åman, P. Water unextractable polysaccharides from three milling fractions of rye grain. Carbohydr. Polym. 1996, 30, 229–237. [Google Scholar] [CrossRef]
- Westerlund, E.; Andersson, R.; Åman, P. Isolation and chemical characterization of water-soluble mixed-linked β-glucans and arabinoxylans in oat milling fractions. Carbohydr. Polym. 1993, 20, 115–123. [Google Scholar] [CrossRef]
- Tian, L.; Gruppen, H.; Schols, H.A. Characterization of (Glucurono)arabinoxylans from Oats Using Enzymatic Fingerprinting. J. Agric. Food Chem. 2015, 63, 10822–10830. [Google Scholar] [CrossRef]
- Dornez, E.; Gebruers, K.; Wiame, S.; Delcour, J.A.; Courtin, C.M. Insight into the Distribution of Arabinoxylans, Endoxylanases, and Endoxylanase Inhibitors in Industrial Wheat Roller Mill Streams. J. Agric. Food Chem. 2006, 54, 8521–8529. [Google Scholar] [CrossRef]
- Gebruers, K.; Dornez, E.; Boros, D.; Fraś, A.; Dynkowska, W.; Bedő, Z.; Rakszegi, M.; Delcour, J.A.; Courtin, C.M. Variation in the Content of Dietary Fiber and Components Thereof in Wheats in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9740–9749. [Google Scholar] [CrossRef]
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Kaur, A.; Yadav, M.P.; Singh, B.; Bhinder, S.; Simon, S.; Singh, N. Isolation and characterization of arabinoxylans from wheat bran and study of their contribution to wheat flour dough rheology. Carbohydr. Polym. 2019, 221, 166–173. [Google Scholar] [CrossRef]
- Ordaz-Ortiz, J.J.; Saulnier, L. Structural variability of arabinoxylans from wheat flour. Comparison of water-extractable and xylanase-extractable arabinoxylans. J. Cereal Sci. 2005, 42, 119–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Gao, X.; Ying, R.; Huang, M. Effects of lamellar organization and arabinoxylan substitution rate on the properties of films simulating wheat grain aleurone cell wall. Carbohydr. Polym. 2021, 270, 117819. [Google Scholar] [CrossRef] [PubMed]
- Philippe, S.; Barron, C.; Robert, P.; Devaux, M.-F.; Saulnier, L.; Guillon, F. Characterization Using Raman Microspectroscopy of Arabinoxylans in the Walls of Different Cell Types during the Development of Wheat Endosperm. J. Agric. Food Chem. 2006, 54, 5113–5119. [Google Scholar] [CrossRef]
- Antoine, C.; Peyron, S.; Mabille, F.; Lapierre, C.; Bouchet, B.; Abecassis, A.J.; Rouau, X. Individual Contribution of Grain Outer Layers and Their Cell Wall Structure to the Mechanical Properties of Wheat Bran. J. Agric. Food Chem. 2003, 51, 2026–2033. [Google Scholar] [CrossRef]
- Barron, C.; Surget, A.; Rouau, X. Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J. Cereal Sci. 2007, 45, 88–96. [Google Scholar] [CrossRef]
- Parker, M.L.; Ng, A.; Waldron, K.W. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L. cv. Avalon) grains. J. Sci. Food Agric. 2005, 85, 2539–2547. [Google Scholar] [CrossRef]
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative structural organisation model for wheat endosperm cell walls: Cellulose as an important constituent. Carbohydr. Polym. 2018, 196, 199–208. [Google Scholar] [CrossRef]
- Maes, C.; Delcour, J. Structural Characterisation of Water-extractable and Water-unextractable Arabinoxylans in Wheat Bran. J. Cereal Sci. 2002, 35, 315–326. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165. [Google Scholar] [CrossRef]
- Viëtor, R.; Angelino, S.; Voragen, A. Structural features of arabinoxylans from barley and malt cell wall material. J. Cereal Sci. 1992, 15, 213–222. [Google Scholar] [CrossRef]
- Trogh, I.; Courtin, C.M.; Delcour, J.A. Isolation and Characterization of Water-Extractable Arabinoxylan from Hull-less Barley Flours. Cereal Chem. 2004, 81, 576–581. [Google Scholar] [CrossRef]
- Yadav, M.P.; Moreau, R.A.; Hicks, K.B. Phenolic Acids, Lipids, and Proteins Associated with Purified Corn Fiber Arabinoxylans. J. Agric. Food Chem. 2006, 55, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Johnston, D.B.; Hotchkiss, A.; Hicks, K.B. Corn fiber gum: A potential gum arabic replacer for beverage flavor emulsification. Food Hydrocoll. 2007, 21, 1022–1030. [Google Scholar] [CrossRef]
- Doner, L.W.; Johnston, D.B.; Singh, V. Analysis and Properties of Arabinoxylans from Discrete Corn Wet-Milling Fiber Fractions. J. Agric. Food Chem. 2001, 49, 1266–1269. [Google Scholar] [CrossRef]
- Doner, L.W.; Hicks, K.B. Isolation of Hemicellulose from Corn Fiber by Alkaline Hydrogen Peroxide Extraction. Cereal Chem. 1997, 74, 176–181. [Google Scholar] [CrossRef]
- Yadav, M.P.; Kale, M.S.; Hicks, K.B.; Hanah, K. Isolation, characterization and the functional properties of cellulosic arabinoxylan fiber isolated from agricultural processing by-products, agricultural residues and energy crops. Food Hydrocoll. 2017, 63, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Fadel, A.; Mahmoud, A.M.; Ashworth, J.J.; Li, W.; Ng, Y.L.; Plunkett, A. Health-related effects and improving extractability of cereal arabinoxylans. Int. J. Biol. Macromol. 2018, 109, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, M.; Macri, L.; MacGregor, A. Structure and physicochemical properties of barley non-starch polysaccharides—I. Water-extractable β-glucans and arabinoxylans. Carbohydr. Polym. 1998, 35, 249–258. [Google Scholar] [CrossRef]
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Effect of water-extractable arabinoxylans from wheat aleurone and bran on lipid peroxidation and factors influencing their antioxidant capacity. Bioact. Carbohydrates Diet. Fibre 2017, 10, 20–26. [Google Scholar] [CrossRef]
- Jacquemin, L.; Zeitoun, R.; Sablayrolles, C.; Pontalier, P.-Y.; Rigal, L. Evaluation of the technical and environmental performances of extraction and purification processes of arabinoxylans from wheat straw and bran. Process Biochem. 2012, 47, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Jacquemin, L.; Mogni, A.; Zeitoun, R.; Guinot, C.; Sablayrolles, C.; Saulnier, L.; Pontalier, P.-Y. Comparison of different twin-screw extraction conditions for the production of arabinoxylans. Carbohydr. Polym. 2015, 116, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demuth, T.; Betschart, J.; Nyström, L. Structural modifications to water-soluble wheat bran arabinoxylan through milling and extrusion. Carbohydr. Polym. 2020, 240, 116328. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.A.; Andersson, R.; Jonsäll, A.; Andersson, J.; Fredriksson, H. Effect of Different Extrusion Parameters on Dietary Fiber in Wheat Bran and Rye Bran. J. Food Sci. 2017, 82, 1344–1350. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Liu, Y.; Zhang, H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014, 150, 482–488. [Google Scholar] [CrossRef]
- Reis, S.F.; Coelho, E.; Coimbra, M.A.; Abu-Ghannam, N. Improved efficiency of brewer’s spent grain arabinoxylans by ultrasound-assisted extraction. Ultrason. Sonochem. 2015, 24, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Roos, A.A.; Persson, T.; Krawczyk, H.; Zacchi, G.; Stålbrand, H. Extraction of water-soluble hemicelluloses from barley husks. Bioresour. Technol. 2009, 100, 763–769. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.; Candelas-Cadillo, M.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of hemicelluloses from grape pomace using response surface methodology. Carbohydr. Polym. 2016, 138, 180–191. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Saraiva, J.A.; Coimbra, M.A. Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydr. Polym. 2014, 99, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Andreani, E.S.; Karboune, S. Production of Extracts Composed of Pectic Oligo/Polysaccharides and Polyphenolic Compounds from Cranberry Pomace by Microwave-Assisted Extraction Process. Food Bioprocess Technol. 2021, 14, 634–649. [Google Scholar] [CrossRef]
- Kong, F.; Wang, L.; Chen, H.; Zhao, X. Improving storage property of wheat bran by steam explosion. Int. J. Food Sci. Technol. 2021, 56, 287–292. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Mattila, O.; Sozer, N.; Poutanen, K.; Koksel, H.; Nordlund, E. Effect of steam explosion on enzymatic hydrolysis and baking quality of wheat bran. J. Cereal Sci. 2017, 78, 25–32. [Google Scholar] [CrossRef]
- Sui, W.; Xie, X.; Liu, R.; Wu, T.; Zhang, M. Effect of wheat bran modification by steam explosion on structural characteristics and rheological properties of wheat flour dough. Food Hydrocoll. 2018, 84, 571–580. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Labidi, J. Simultaneous microwave-ultrasound assisted extraction of bioactive compounds from bark. Chem. Eng. Process. Process Intensif. 2020, 156, 108100. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.-F.; Geng, Z.; Sun, J.; Sun, R.; Hei, B.; Lin, L.; Wu, S.; Je, J. Characterisation of degraded organosolv hemicelluloses from wheat straw. Polym. Degrad. Stab. 2006, 91, 1880–1886. [Google Scholar] [CrossRef]
- Fincher, G.B.; Stone, B.A. Cell walls and their components in cereal grain technology. Adv. Cereal Sci. Technol. 1986, 8, 207–295. [Google Scholar]
- Cyran, M.; Courtin, C.M.; Delcour, J.A. Heterogeneity in the Fine Structure of Alkali-Extractable Arabinoxylans Isolated from Two Rye Flours with High and Low Breadmaking Quality and Their Coexistence with Other Cell Wall Components. J. Agric. Food Chem. 2004, 52, 2671–2680. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, H.; Southgate, D.A.T. Review of Methods of Analysis. In Food Composition Data: Production, Management, and Use; FAO: Rome, Italy, 2003. [Google Scholar]
- Aguedo, M.; Fougnies, C.; Dermience, M.; Richel, A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohydr. Polym. 2014, 105, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Effect of processing time, temperature and alkali concentration on yield extraction, structure and gelling properties of corn fiber arabinoxylans. Food Hydrocoll. 2016, 60, 21–28. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, S.W.; Gu, X.; Zhang, J. Isolation and structural characterization of water unextractable arabinoxylans from Chinese black-grained wheat bran. Carbohydr. Polym. 2011, 85, 615–621. [Google Scholar] [CrossRef]
- Schooneveld-Bergmans, M.; Beldman, G.; Voragen, A. Structural Features of (Glucurono)Arabinoxylans Extracted from Wheat Bran by Barium Hydroxide. J. Cereal Sci. 1999, 29, 63–75. [Google Scholar] [CrossRef]
- Ogawa, K.; Takeuchi, M.; Nakamura, N. Immunological Effects of Partially Hydrolyzed Arabinoxylan from Corn Husk in Mice. Biosci. Biotechnol. Biochem. 2005, 69, 19–25. [Google Scholar] [CrossRef]
- Bender, D.; Nemeth, R.; Wimmer, M.; Götschhofer, S.; Biolchi, M.; Török, K.; Tömösközi, S.; D’Amico, S.; Schoenlechner, R. Optimization of Arabinoxylan Isolation from Rye Bran by Adapting Extraction Solvent and Use of Enzymes. J. Food Sci. 2017, 82, 2562–2568. [Google Scholar] [CrossRef] [Green Version]
- Persson, T.; Ren, J.L.; Joelsson, E.; Jönsson, A.-S. Fractionation of wheat and barley straw to access high-molecular-mass hemicelluloses prior to ethanol production. Bioresour. Technol. 2009, 100, 3906–3913. [Google Scholar] [CrossRef]
- Mathew, S.; Karlsson, E.N.; Adlercreutz, P. Extraction of soluble arabinoxylan from enzymatically pretreated wheat bran and production of short xylo-oligosaccharides and arabinoxylo-oligosaccharides from arabinoxylan by glycoside hydrolase family 10 and 11 endoxylanases. J. Biotechnol. 2017, 260, 53–61. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.; Yin, J.; Ma, L.; Li, D. Optimisation of double-enzymatic extraction of arabinoxylan from fresh corn fibre. J. Food Sci. Technol. 2020, 57, 4649–4659. [Google Scholar] [CrossRef]
- Escarnot, E.; Aguedo, M.; Paquot, M. Enzymatic hydrolysis of arabinoxylans from spelt bran and hull. J. Cereal Sci. 2012, 55, 243–253. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Li, S.; Song, X.; Li, Z.; Zhang, D. Exploration of the association between dietary fiber intake and depressive symptoms in adults. Nutrition 2018, 54, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Byeon, S.; Shin, D.-M. Sources of Dietary Fiber Are Differently Associated with Prevalence of Depression. Nutrition 2020, 12, 2813. [Google Scholar] [CrossRef]
- Berding, K.; Carbia, C.; Cryan, J.F. Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis. Exp. Biol. Med. 2021, 246, 796–811. [Google Scholar] [CrossRef]
- Lynch, K.M.; Strain, C.R.; Johnson, C.; Patangia, D.; Stanton, C.; Koc, F.; Gil-Martinez, J.; O’riordan, P.; Sahin, A.W.; Ross, R.P.; et al. Extraction and characterisation of arabinoxylan from brewers spent grain and investigation of microbiome modulation potential. Zeitschrift für Ernährungswissenschaft 2021, 60, 4393–4411. [Google Scholar] [CrossRef]
- Walker, A.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2010, 5, 220–230. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.; Louis, P. The impact of nutrition on intestinal bacterial communities. Curr. Opin. Microbiol. 2017, 38, 59–65. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Chen, T.; Ma, D.; Yao, T.; Gu, F.; Lim, J.; Tuinstra, M.R.; Hamaker, B.R. High arabinoxylan fine structure specificity to gut bacteria driven by corn genotypes but not environment. Carbohydr. Polym. 2021, 257, 117667. [Google Scholar] [CrossRef]
- Paesani, C.; Sciarini, L.S.; Moiraghi, M.; Salvucci, E.; Prado, S.B.; Pérez, G.T.; Fabi, J.P. Human colonic in vitro fermentation of water-soluble arabinoxylans from hard and soft wheat alters Bifidobacterium abundance and short-chain fatty acids concentration. LWT 2020, 134, 110253. [Google Scholar] [CrossRef]
- Pereira, G.V.; Abdel-Hamid, A.M.; Dutta, S.; D’alessandro-Gabazza, C.N.; Wefers, D.; Farris, J.A.; Bajaj, S.; Wawrzak, Z.; Atomi, H.; Mackie, R.I.; et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat. Commun. 2021, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Millet, S.; Van Oeckel, M.J.; Aluwé, M.; Delezie, E.; De Brabander, D.L. Prediction of In Vivo Short-Chain Fatty Acid Production in Hindgut Fermenting Mammals: Problems and Pitfalls. Crit. Rev. Food Sci. Nutr. 2010, 50, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Walton, G.E.; Lu, C.; Trogh, I.; Arnaut, F.; Gibson, G.R. A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan-oligosaccharides enriched bread in healthy volunteers. Nutr. J. 2012, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Yuwang, P.; Sulaeva, I.; Hell, J.; Henniges, U.; Böhmdorfer, S.; Rosenau, T.; Chitsomboon, B.; Tongta, S. Phenolic compounds and antioxidant properties of arabinoxylan hydrolysates from defatted rice bran. J. Sci. Food Agric. 2017, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Yang, T.; Chen, D.; Xiao, Y.; Qin, W.; Wu, D.; Zhang, Q.; Lin, D.; Liu, Y.; et al. Interactive effects of molecular weight and degree of substitution on biological activities of arabinoxylan and its hydrolysates from triticale bran. Int. J. Biol. Macromol. 2021, 166, 1409–1418. [Google Scholar] [CrossRef]
- Malunga, L.N.; Eck, P.; Beta, T. Inhibition of Intestinalα-Glucosidase and Glucose Absorption by Feruloylated Arabinoxylan Mono- and Oligosaccharides from Corn Bran and Wheat Aleurone. J. Nutr. Metab. 2016, 2016, 1932532. [Google Scholar] [CrossRef] [Green Version]
- Hartvigsen, M.L.; Jeppesen, P.B.; Lærke, H.N.; Njabe, E.N.; Knudsen, K.E.B.; Hermansen, K. Concentrated Arabinoxylan in Wheat Bread Has Beneficial Effects as Rye Breads on Glucose and Changes in Gene Expressions in Insulin-Sensitive Tissues of Zucker Diabetic Fatty (ZDF) Rats. J. Agric. Food Chem. 2013, 61, 5054–5063. [Google Scholar] [CrossRef]
- Boll, E.V.J.; Ekström, L.; Courtin, C.; Delcour, J.; Nilsson, A.C.; Björck, I.M.E.; Östman, E.M. Effects of wheat bran extract rich in arabinoxylan oligosaccharides and resistant starch on overnight glucose tolerance and markers of gut fermentation in healthy young adults. Eur. J. Nutr. 2016, 55, 1661–1670. [Google Scholar] [CrossRef]
- Vogel, B.; Gallaher, D.D.; Bunzel, M. Influence of Cross-Linked Arabinoxylans on the Postprandial Blood Glucose Response in Rats. J. Agric. Food Chem. 2012, 60, 3847–3852. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Kang, J.; Wang, N.; Xiao, M.; Li, Z.; Wang, C.; Guo, Q.; Hu, X. Arabinoxylan from wheat bran: Molecular degradation and functional investigation. Food Hydrocoll. 2020, 107, 105914. [Google Scholar] [CrossRef]
- Cyrañ, M.; Izydorczyk, M.S.; MacGregor, A.W. Structural Characteristics of Water-Extractable Nonstarch Polysaccharides from Barley Malt. Cereal Chem. 2002, 79, 359–366. [Google Scholar] [CrossRef]
- Shrestha, U.R.; Smith, S.; Pingali, S.V.; Yang, H.; Zahran, M.; Breunig, L.; Wilson, L.A.; Kowali, M.; Kubicki, J.D.; Cosgrove, D.J.; et al. Arabinose substitution effect on xylan rigidity and self-aggregation. Cellulose 2019, 26, 2267–2278. [Google Scholar] [CrossRef] [Green Version]
- Andrewartha, K.A.; Phillips, D.R.; Stone, B.A. Solution properties of wheat-flour arabinoxylans and enzymically modified arabinoxylans. Carbohydr. Res. 1979, 77, 191–204. [Google Scholar] [CrossRef]
- Dervilly, G.; Leclercq, C.; Zimmermann, D.; Roue, C.; Thibault, J.-F.; Saulnier, L. Isolation and characterization of high molar mass water-soluble arabinoxylans from barley and barley malt. Carbohydr. Polym. 2001, 47, 143–149. [Google Scholar] [CrossRef]
- Pitkänen, L.; Tuomainen, P.; Virkki, L.; Tenkanen, M. Molecular characterization and solution properties of enzymatically tailored arabinoxylans. Int. J. Biol. Macromol. 2011, 49, 963–969. [Google Scholar] [CrossRef]
- Qiu, S.; Yadav, M.P.; Yin, L. Characterization and functionalities study of hemicellulose and cellulose components isolated from sorghum bran, bagasse and biomass. Food Chem. 2017, 230, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Singh, B.; Yadav, M.P.; Bhinder, S.; Singh, N. Isolation of arabinoxylan and cellulose-rich arabinoxylan from wheat bran of different varieties and their functionalities. Food Hydrocoll. 2021, 112, 106287. [Google Scholar] [CrossRef]
- Kale, M.S.; Yadav, M.P.; Chau, H.K.; Hotchkiss, A. Molecular and functional properties of a xylanase hydrolysate of corn bran arabinoxylan. Carbohydr. Polym. 2018, 181, 119–123. [Google Scholar] [CrossRef]
- Đorđević, T.; Milošević, M.; Antov, M. Advance diversity of enzymatically modified arabinoxylan from wheat chaff. Food Chem. 2021, 339, 128093. [Google Scholar] [CrossRef]
- Kale, M.S.; Pai, D.A.; Hamaker, B.R.; Campanella, O.H. Structure–function relationships for corn bran arabinoxylans. J. Cereal Sci. 2010, 52, 368–372. [Google Scholar] [CrossRef]
- Gendron, R.; Daigneault, L.E. Rheology of Thermoplastic Foam Extrusion Process. In Foam Extrusion: Principles and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Li, J.; Du, J. Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer. Molecules 2019, 24, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubun, S.; Yadav, M.P.; Moreau, R.A.; Williams, P.A. Components responsible for the emulsification properties of corn fibre gum. Food Hydrocoll. 2014, 41, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Runge, T. Emulsifying properties of succinylated arabinoxylan-protein gum produced from corn ethanol residuals. Food Hydrocoll. 2016, 52, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Brijs, K.; Ingelbrecht, J.A.; Courtin, C.M.; Schlichting, L.; Marchylo, B.A.; Delcour, J.A. Combined Effects of Endoxylanases and Reduced Water Levels in Pasta Production. Cereal Chem. 2004, 81, 361–368. [Google Scholar] [CrossRef]
- Ingelbrecht, J.A.; Verwimp, T.; Grobet, P.J.; Delcour, J.A. Behavior of Triticum durum Desf. Arabinoxylans and Arabinogalactan Peptides during Industrial Pasta Processing. J. Agric. Food Chem. 2001, 49, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Ingelbrecht, J.A.; Moers, K.; Abécassis, J.; Rouau, X.; Delcour, J.A. Influence of Arabinoxylans and Endoxylanases on Pasta Processing and Quality. Production of High-Quality Pasta with Increased Levels of Soluble Fiber. Cereal Chem. 2001, 78, 721–729. [Google Scholar] [CrossRef]
- Turner, M.A.; Soh, C.H.; Ganguli, N.K.; Sissons, M.J. A survey of water-extractable arabinopolymers in bread and durum wheat and the effect of water-extractable arabinoxylan on durum dough rheology and spaghetti cooking quality. J. Sci. Food Agric. 2008, 88, 2551–2555. [Google Scholar] [CrossRef]
- Bettge, A.D.; Morris, C.F. Relationships among Grain Hardness, Pentosan Fractions, and End-Use Quality of Wheat. Cereal Chem. 2000, 77, 241–247. [Google Scholar] [CrossRef]
- Guttieri, M.J.; Souza, E.J.; Sneller, C. Nonstarch Polysaccharides in Wheat Flour Wire-Cut Cookie Making. J. Agric. Food Chem. 2008, 56, 10927–10932. [Google Scholar] [CrossRef]
- Heredia-Sandoval, N.G.; Granados-Nevárez, M.D.C.; De La Barca, A.M.C.; Lara, F.V.; Malunga, L.N.; Apea-Bah, F.B.; Beta, T.; Islas-Rubio, A.R. Phenolic Acids, Antioxidant Capacity, and Estimated Glycemic Index of Cookies Added with Brewer’s Spent Grain. Mater. Veg. 2019, 75, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Igrejas, G.; Martinant, J.-P.; Bouguennec, A.; Villain, A.C.; Saulnier, L.; Popineau, Y.; Branlard, G. Genetical, Biochemical and Technological Parameters Associated with Biscuit Quality. I. Prediction Using Grain Hardness and Water Extractable Arabinoxylans. J. Cereal Sci. 2002, 36, 115–124. [Google Scholar] [CrossRef]
- Kiszonas, A.M.; Fuerst, E.P.; Morris, C.F. Wheat Arabinoxylan Structure Provides Insight into Function. Cereal Chem. 2013, 90, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Pareyt, B.; Goovaerts, M.; Broekaert, W.F.; Delcour, J.A. Arabinoxylan oligosaccharides (AXOS) as a potential sucrose replacer in sugar-snap cookies. LWT 2011, 44, 725–728. [Google Scholar] [CrossRef]
- Souza, E.J.; Guttieri, M.J.; Sneller, C. Selecting Soft Wheat Genotypes for Whole Grain Cookies. Crop Sci. 2011, 51, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Hartikainen, K.; Poutanen, K.; Katina, K. Influence of Bioprocessed Wheat Bran on the Physical and Chemical Properties of Dough and on Wheat Bread Texture. Cereal Chem. 2014, 91, 115–123. [Google Scholar] [CrossRef]
- Adams, V.; Ragaee, S.; Goff, H.D.; Abdelaal, E.M. Properties of Arabinoxylans in Frozen Dough Enriched with Wheat Fiber. Cereal Chem. 2017, 94, 242–250. [Google Scholar] [CrossRef]
- Arif, S.; Ahmed, M.; Chaudhry, Q.; Hasnain, A. Effects of water extractable and unextractable pentosans on dough and bread properties of hard wheat cultivars. LWT 2018, 97, 736–742. [Google Scholar] [CrossRef]
- Cardone, G.; D’Incecco, P.; Pagani, M.A.; Marti, A. Sprouting improves the bread-making performance of whole wheat flour (Triticum aestivum L.). J. Sci. Food Agric. 2020, 100, 2453–2459. [Google Scholar] [CrossRef]
- Coda, R.; Katina, K.; Rizzello, C.G. Bran bioprocessing for enhanced functional properties. Curr. Opin. Food Sci. 2015, 1, 50–55. [Google Scholar] [CrossRef]
- Courtin, C.M.; Gelders, G.G.; Delcour, J.A. Use of Two Endoxylanases with Different Substrate Selectivity for Understanding Arabinoxylan Functionality in Wheat Flour Breadmaking. Cereal Chem. 2001, 78, 564–571. [Google Scholar] [CrossRef]
- Damen, B.; Pollet, A.; Dornez, E.; Broekaert, W.; Van Haesendonck, I.; Trogh, I.; Arnaut, F.; Delcour, J.A.; Courtin, C.M. Xylanase-mediated in situ production of arabinoxylan oligosaccharides with prebiotic potential in whole meal breads and breads enriched with arabinoxylan rich materials. Food Chem. 2012, 131, 111–118. [Google Scholar] [CrossRef]
- De Schryver, P.; Seseña, S.; Decaigny, B.; Van De Wiele, T.; Verstraete, W.; Boon, N. Xylanases from microbial origin induce syrup formation in dough. J. Cereal Sci. 2008, 47, 18–28. [Google Scholar] [CrossRef]
- Döring, C.; Hussein, M.A.; Jekle, M.; Becker, T. On the assessments of arabinoxylan localization and enzymatic modifications for enhanced protein networking and its structural impact on rye dough and bread. Food Chem. 2017, 229, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Dornez, E.; Verjans, P.; Broekaert, W.F.; Cappuyns, A.M.; Van Impe, J.F.; Arnaut, F.; Delcour, J.A.; Courtin, C.M. In Situ Production of Prebiotic AXOS by Hyperthermophilic Xylanase B fromThermotoga maritimain High-Quality Bread. Cereal Chem. 2011, 88, 124–129. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, X.; Tian, M.; Zhou, Y.; Yang, R.; Gu, Z.; Wang, P. Impact of water extractable arabinoxylan with different molecular weight on the gelatinization and retrogradation behavior of wheat starch. Food Chem. 2020, 318, 126477. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, L.; Yang, W.; Ding, L.; Awais, M.; Wang, L.; Zhou, S. Improving the physicochemical properties of whole wheat model dough by modifying the water-unextractable solids. Food Chem. 2018, 259, 18–24. [Google Scholar] [CrossRef]
- Katina, K.; Juvonen, R.; Laitila, A.; Flander, L.; Nordlund, E.; Kariluoto, S.; Piironen, V.; Poutanen, K. Fermented Wheat Bran as a Functional Ingredient in Baking. Cereal Chem. 2012, 89, 126–134. [Google Scholar] [CrossRef]
- Li, Q.; Liu, R.; Wu, T.; Zhang, M. Interactions between soluble dietary fibers and wheat gluten in dough studied by confocal laser scanning microscopy. Food Res. Int. 2017, 95, 19–27. [Google Scholar] [CrossRef]
- Messia, M.; Reale, A.; Maiuro, L.; Candigliota, T.; Sorrentino, E.; Marconi, E. Effects of pre-fermented wheat bran on dough and bread characteristics. J. Cereal Sci. 2016, 69, 138–144. [Google Scholar] [CrossRef]
- Primo-Martin, C.; Martinez-Anaya, M. Influence of Pentosanase and Oxidases on Water-extractable Pentosans during a Straight Breadmaking Process. J. Food Sci. 2003, 68, 31–41. [Google Scholar] [CrossRef]
- Shah, A.R.; Shah, R.; Madamwar, D. Improvement of the quality of whole wheat bread by supplementation of xylanase from Aspergillus foetidus. Bioresour. Technol. 2006, 97, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Zhang, Y.; Campanella, O.H. Physicochemical properties of arabinoxlans in refrigerated dough. Food Res. Int. 2010, 43, 2119–2125. [Google Scholar] [CrossRef]
- Simsek, S.; Whitney, K.; Ohm, J.-B.; Mergoum, M. Refrigerated Dough Quality of Hard Red Spring Wheat: Effect of Genotype and Environment on Dough Syruping and Arabinoxylan Production. Cereal Chem. 2011, 88, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Steinmacher, N.C.; Honna, F.A.; Gasparetto, A.V.; Anibal, D.; Grossmann, M.V.E. Bioconversion of brewer’s spent grains by reactive extrusion and their application in bread-making. LWT 2012, 46, 542–547. [Google Scholar] [CrossRef]
- Wang, M.; van Vliet, T.; Hamer, R.J. Evidence that pentosans and xylanase affect the re-agglomeration of the gluten network. J. Cereal Sci. 2004, 39, 341–349. [Google Scholar] [CrossRef]
- Wang, P.; Tao, H.; Jin, Z.; Xu, X. Impact of water extractable arabinoxylan from rye bran on the frozen steamed bread dough quality. Food Chem. 2016, 200, 117–124. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, X.; Zhang, Z.; Zhou, T.; Gao, R.; Li, Y.; Ding, X. Effect of β-endoxylanase and α-arabinofuranosidase enzymatic hydrolysis on nutritional and technological properties of wheat brans. Food Chem. 2020, 302, 125332. [Google Scholar] [CrossRef]
- Yegin, S.; Altinel, B.; Tuluk, K. A novel extremophilic xylanase produced on wheat bran from Aureobasidium pullulans NRRL Y-2311-1: Effects on dough rheology and bread quality. Food Hydrocoll. 2018, 81, 389–397. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, P.; Yang, J.; Ren, D.; Lu, Z.; Zhao, H.; Lu, F. Oxidative crosslinking of water-extractable wheat arabinoxylans by recombinant lipoxygenase and its effect on bread properties. LWT 2019, 103, 1–7. [Google Scholar] [CrossRef]
- Solomou, K.; Alyassin, M.; Angelis-Dimakis, A.; Campbell, G.M. Arabinoxylans: A new class of food ingredients arising from synergies with biorefining, and illustrating the nature of biorefinery engineering. Food Bioprod. Process. 2021, 132, 83–98. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schutter, D.P.; Courtin, C.M. Arabinoxylan from non-malted cereals can act as mouthfeel contributor in beer. Carbohydr. Polym. 2020, 239, 116257. [Google Scholar] [CrossRef] [PubMed]
- Lebesi, D.M.; Tzia, C. Effect of the Addition of Different Dietary Fiber and Edible Cereal Bran Sources on the Baking and Sensory Characteristics of Cupcakes. Food Bioprocess Technol. 2011, 4, 710–722. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Influence of barley non-starchy polysaccharides on selected quality attributes of sponge cakes. LWT 2017, 85, 252–261. [Google Scholar] [CrossRef]
- Moiraghi, M.; de la Hera, E.; Pérez, G.T.; Gómez, M. Effect of wheat flour characteristics on sponge cake quality. J. Sci. Food Agric. 2013, 93, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Ciccoritti, R.; Nocente, F.; Sgrulletta, D.; Gazza, L. Cooking quality, biochemical and technological characteristics of bran-enriched pasta obtained by a novel pasta-making process. LWT 2018, 101, 10–16. [Google Scholar] [CrossRef]
- Oliete, B.; Pérez, G.T.; Gómez, M.; Ribotta, P.D.; Moiraghi, M.; León, A.E. Use of wheat, triticale and rye flours in layer cake production. Int. J. Food Sci. Technol. 2010, 45, 697–706. [Google Scholar] [CrossRef]
- Haghighi-Manesh, S.; Azizi, M.H. Integrated extrusion-enzymatic treatment of corn bran for production of functional cake. Food Sci. Nutr. 2018, 6, 1870–1878. [Google Scholar] [CrossRef]
- Paesani, C.; Bravo-Núñez, Á.; Gómez, M. Effect of stabilized wholegrain maize flours on the quality characteristics of gluten-free layer cakes. LWT 2020, 135, 109959. [Google Scholar] [CrossRef]
- Saeed, F.; Pasha, I.; Anjum, F.M.; Sultan, M.T. Arabinoxylans and Arabinogalactans: A Comprehensive Treatise. Crit. Rev. Food Sci. Nutr. 2011, 51, 467–476. [Google Scholar] [CrossRef]
- Pietiäinen, S.; Moldin, A.; Ström, A.; Malmberg, C.; Langton, M. Effect of physicochemical properties, pre-processing, and extraction on the functionality of wheat bran arabinoxylans in breadmaking—A review. Food Chem. 2022, 383, 132584. [Google Scholar] [CrossRef]
- Koegelenberg, D.; Chimphango, A.F. Effects of wheat-bran arabinoxylan as partial flour replacer on bread properties. Food Chem. 2017, 221, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Michniewicz, J.; Biliaderis, G.; Bushuk, W. Effect of added pentosans on some properties of wheat bread. Food Chem. 1992, 43, 251–257. [Google Scholar] [CrossRef]
- Hromádková, Z.; Stavová, A.; Ebringerová, A.; Hirsch, J. Effect of Buckwheat Hull Hemicelluloses Addition on the Bread-Making Quality of Wheat Flour. J. Food Nutr. Res. 2007, 46, 158–166. [Google Scholar]
- Nishitsuji, Y.; Whitney, K.; Nakamura, K.; Hayakawa, K.; Simsek, S. Changes in structure and solubility of wheat arabinoxylan during the breadmaking process. Food Hydrocoll. 2020, 109, 106129. [Google Scholar] [CrossRef]
- Labat, E.; Morel, M.H.; Rouau, X. Effects of Laccase and Ferulic Acid on Wheat Flour Doughs. Cereal Chem. 2000, 77, 823–828. [Google Scholar] [CrossRef]
- Courtin, C.M.; Delcour, J.A. Arabinoxylans and Endoxylanases in Wheat Flour Bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
- Pavlovich-Abril, A.; Rouzaud-Sández, O.; Carvajal-Millan, E.; Navarro, R.E.; Robles-Sánchez, R.M.; Barrón-Hoyos, J.M. Molecular characterization of water extractable arabinoxylans isolated from wheat fine bran and their effect on dough viscosity. LWT 2016, 74, 484–492. [Google Scholar] [CrossRef]
- Guo, X.-N.; Yang, S.; Zhu, K.-X. Impact of arabinoxylan with different molecular weight on the thermo-mechanical, rheological, water mobility and microstructural characteristics of wheat dough. Int. J. Food Sci. Technol. 2018, 53, 2150–2158. [Google Scholar] [CrossRef]
- Zhang, L.; van Boven, A.; Mulder, J.; Grandia, J.; Chen, X.D.; Boom, R.M.; Schutyser, M.A. Arabinoxylans-enriched fractions: From dry fractionation of wheat bran to the investigation on bread baking performance. J. Cereal Sci. 2019, 87, 1–8. [Google Scholar] [CrossRef]
- Trogh, I.; Courtin, C.; Andersson, A.; Åman, P.; Sørensen, J.; Delcour, J. The combined use of hull-less barley flour and xylanase as a strategy for wheat/hull-less barley flour breads with increased arabinoxylan and (1→3,1→4)-β-D-glucan levels. J. Cereal Sci. 2004, 40, 257–267. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, X.; Yang, S.; Li, L.; Tan, S. Improvement of the breadmaking quality of wheat flour by the hyperthermophilic xylanase B from Thermotoga maritima. Food Res. Int. 2005, 38, 37–43. [Google Scholar] [CrossRef]
- Buksa, K.; Nowotna, A.; Ziobro, R. Application of cross-linked and hydrolyzed arabinoxylans in baking of model rye bread. Food Chem. 2016, 192, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Primo-Martín, C.; Wang, M.; Lichtendonk, W.J.; Plijter, J.J.; Hamer, R.J. An Explanation for the Combined Effect of Xy-lanase-Glucose Oxidase in Dough Systems. Proc. J. Sci. Food Agric. 2005, 85, 1186–1196. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, X.; Niu, M.; Xiang, X.; Chang, Y.; Zhao, Z.; Xiong, L.; Zhao, S.; Rong, J.; Tang, C.; et al. Gluten development and water distribution in bread dough influenced by bran components and glucose oxidase. LWT 2020, 137, 110427. [Google Scholar] [CrossRef]
- Yang, M.; Yue, Y.; Liu, L.; Tong, L.; Wang, L.; Ashraf, J.; Li, N.; Zhou, X.; Zhou, S. Investigation of combined effects of xylanase and glucose oxidase in whole wheat buns making based on reconstituted model dough system. LWT 2020, 135, 110261. [Google Scholar] [CrossRef]
- Flander, L.; Rouau, X.; Morel, M.-H.; Autio, K.; Seppänen-Laakso, T.; Kruus, K.; Buchert, J. Effects of Laccase and Xylanase on the Chemical and Rheological Properties of Oat and Wheat Doughs. J. Agric. Food Chem. 2008, 56, 5732–5742. [Google Scholar] [CrossRef]
- Laaksonen, T.J.; Labuza, T.P. Effects of moisture, sucrose, nacl, and arabinoxylan on relaxation in wheat dough as measured by dmta. Int. J. Food Prop. 2001, 4, 311–325. [Google Scholar] [CrossRef]
- Wang, P.; Hou, C.; Zhao, X.; Tian, M.; Gu, Z.; Yang, R. Molecular characterization of water-extractable arabinoxylan from wheat bran and its effect on the heat-induced polymerization of gluten and steamed bread quality. Food Hydrocoll. 2019, 87, 570–581. [Google Scholar] [CrossRef]
- Whitney, K.; Simsek, S. Reduced Gelatinization, Hydrolysis, and Digestibility in Whole Wheat Bread in Comparison to White Bread. Cereal Chem. 2017, 94, 991–1000. [Google Scholar] [CrossRef]
- Döring, C.; Number, C.; Stukenborg, F.; Jekle, M.; Becker, T. Impact of arabinoxylan addition on protein microstructure formation in wheat and rye dough. J. Food Eng. 2015, 154, 10–16. [Google Scholar] [CrossRef]
- Meeus, Y.; Janssen, F.; Wouters, A.G.; Delcour, J.A.; Moldenaers, P. The role of arabinoxylan in determining the non-linear and linear rheology of bread doughs made from blends of wheat (Triticum aestivum L.) and rye (Secale cereale L.) flour. Food Hydrocoll. 2021, 120, 106990. [Google Scholar] [CrossRef]
- Buksa, K.; Krystyjan, M. Arabinoxylan–starch–protein interactions in specially modified rye dough during a simulated baking process. Food Chem. 2019, 287, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, F.; Wang, Y.; Li, J.; Teng, C.; Wang, C.; Li, X. Effects of different molecular weight water-extractable arabinoxylans on the physicochemical properties and structure of wheat gluten. J. Food Sci. Technol. 2019, 56, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Domenek, S.; Morel, M.-H.; Bonicel, J.; Guilbert, S. Polymerization Kinetics of Wheat Gluten upon Thermosetting. A Mechanistic Model. J. Agric. Food Chem. 2002, 50, 5947–5954. [Google Scholar] [CrossRef]
- Santos, D.M.J.; Monteiro, S.R.; da Silva, J.A.L. Small strain viscoelastic behaviour of wheat gluten–pentosan mixtures. Eur. Food Res. Technol. 2005, 221, 398–405. [Google Scholar] [CrossRef]
- Noort, M.W.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre–protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Li, J.; Li, F.; Teng, C.; Li, X. Effects of Water-Extractable Arabinoxylan on the Physicochemical Properties and Structure of Wheat Gluten by Thermal Treatment. J. Agric. Food Chem. 2017, 65, 4728–4735. [Google Scholar] [CrossRef]
- Li, J.; Kang, J.; Wang, L.; Li, Z.; Wang, R.; Chen, Z.X.; Hou, G.G. Effect of Water Migration between Arabinoxylans and Gluten on Baking Quality of Whole Wheat Bread Detected by Magnetic Resonance Imaging (MRI). J. Agric. Food Chem. 2012, 60, 6507–6514. [Google Scholar] [CrossRef]

| Source of Arabinoxylan | Tissue Type | Total AXs (%) | WEAXs (%) | References | Main AX Structure * | References |
|---|---|---|---|---|---|---|
| Wheat | Endosperm | 1.52–1.75 | 0.42–0.68 | [14] | Side chains linked by α-(1→2) and/or α-(1→3) bonds along the xylan backbone. Xyloses are most commonly mono-substituted. Side chains formed mainly by single arabinose units but can contain other short sugar sidechains. | [12,15,16,17] |
| Bran | 11.0–16.4 | 0.54–0.95 | [14] | |||
| Barley | Endosperm | 1.2–1.3 | 0.42–0.47 | [18] | Similar structure to wheat AXs. Side chains of xylose units in the 2 and/or 3 carbon of the xyloses, which form the backbones of these AXs. Consists of more arabinose side chains than wheat AXs. | [19,20,21,22] |
| Bran | 10.26 | - | [22] | |||
| Corn | Cob | 26.24 | - | [23] | Highly branched structures with a xylose backbone. Side chains of arabinose residues on primary and secondary hydroxyl groups. Glucuronic acid, galactose, and xylose residues can also be present. | [24,25,26] |
| Bran | 26.0 | 0.71 | [27] | |||
| Rice | Endosperm | 1.83 | 0.05 | [28] | Characteristic sugar linkages and non-reducing end xylose and galactose. (1→2)-, (1→3)- or (1→5)-linked arabinose residues also present. | [29,30] |
| Bran | 6.82 | 011 | [28] | |||
| Rye | Endosperm | 3.56–4.25 | [31] | Main chain of 4-linked β-D-xylopyranosyl residues. A terminal α-L-arabinofuranosyl residue substitutes (on average) every second unit at position 3 and a small portion of the xylose units at position 2 and 3. | [32,33,34] | |
| Bran | 12.6 | 2.1 | [31] | |||
| Oat | Endosperm | 1.2 | 0.2 | [35] | (1–4)-linked β-D-xylopyranosyl residues making up the main chain, with terminal L-arabinofuranosyl residues substituting at O-3, but also at both O-2 and O-3. | [35,36] |
| Bran | 5.2 | 0.7 | [35] |
| Source | Extraction | Solvent/Enzyme | AXs Yield * | A/X Ratio | Reference |
|---|---|---|---|---|---|
| De-starched wheat bran | Alkali | 0.44 M NaOH | 20.80 | 0.94 | [83] |
| Corn fibre | Alkali | 0.25–50 M NaOH | 26.80 ** | n.d. | [84] |
| De-starched plan materials | Alkali | NaOH (pH 11.5) | 14.30–59.9 *** | n.d. | [57] |
| Chinese, black-grained wheat bran residue (after removal of water-extractable polysaccharides) | Alkali | Saturated Ba(OH)2, 1% NaBH4 | ~5.8 | 0.6 | [85] |
| Wheat bran | Alkali | Saturated Ba(OH)2, 0.26 M NaBH4 | 24 | 0.7 | [86] |
| Corn husk | Alkali | 0.9% (w/v) Ca(OH)2 | n.d. | 0.75 | [87] |
| De-starched wheat | Alkali/Enzymatic + alkali | 0.16 mol/L NaOH, 0.5% H2O2//xylanase and cellulase (sodium acetate buffer) + 0.16 mol/L NaOH, 0.5% H2O2 | 19.83//5.27 and 14.95 | 1.14//0.25 and 1.52 | [13] |
| Rye bran | Alkali + enzymatic | First extraction: 0.17 M Na2CO3 or 0.17 M Ca (OH)2 or water Second extraction: xylanase | First extraction: 2.92–3.85 Second extraction: 7.5–9.85 | First extraction: 0.48–0.59 Second extraction: 0.23–0.28 | [88] |
| Wheat and barley straw | Alkali and steam pretreatment + enzymatic | 1–2 wt% NaOH (steam pretreatment) + β-glucosidase and xylanase | 18–35 (Wheat) 17–47 (Barley) | n.d. | [89] |
| Wheat bran | Ultrasound + Enzymatic | Xylanase (sodium acetate buffer) | 4.25–12.88 | n.d. | [66] |
| Wheat bran | Enzymatic | Xylanase | 23.1 | 0.44 | [90] |
| Corn fibre | Enzymatic | Xylanase and cellulase (sodium acetate buffer) | 30–45 | n.d. | [90] |
| AXs Source | Type of Study | AXs Structure | Studied Parameters | Observed Effect | Reference |
|---|---|---|---|---|---|
| Triticale AXs extracted by different methods | In vitro | A/X ratio: 0.25–1.52 | Ferulic acid content Antioxidant activity Hypoglycaemic activity | Esterified and free ferulic acid (FE) content was influenced by AX structure. Enzymatically or water-extracted AXs had higher levels of esterified FE, whereas alkali-extracted AXs had higher free FE levels. AXs with a lower degree of substitution contributed to higher antioxidant capacity. Alkali-extracted AXs performed better than other AXs in the inhibition of α-amylase, something that the authors correlated with the higher levels of free FE. Glucose absorption capacity by AXs was higher for enzymatically and water-extracted AXs, in contrast with the results of α-amylase inhibition. | [101] |
| Hard and soft wheat (whole grains) | In vitro-Human faecal fermentation | Water extractable AXs A/X ratio: 0.5 and 0.47 (soft and hard wheat, respectively) Mw: 410–4 kDa. Hard wheat had a higher % of AXs in the higher range. | Stimulation of Bifidobacterium and Lactobacillus growth SCFAs production Gas production | Significant stimulation of Bifidobacterium with AXs from hard wheat. Improvement of SCFAs contents:
| [102] |
| Hard and soft wheat (whole grains) | In vitro and in vivo (mice) | Water-extractable AXs A/X ratio: 0.5 and 0.47 (soft and hard wheat, respectively) Mw: 410–4 kDa. Hard wheat had a higher % of AXs in the higher range. | Relative growth of Lactobacillus, Bifidobacterium, Bacteroides, Enterococcus, and Clostridium Prebiotic activity SCFAs production | Increased growth and prebiotic activity of Lactobacillus, Bifidobacterium, and Bacteroides (only in vitro), decreased growth of Clostridium, no effect for Enterococcus (no data for in vitro) and Bacteroides (in vivo) for both AXs. Effects were higher with AXs from hard wheat. In vitro prebiotic activity was enhanced by AXs. In vivo results showed an improvement of SCFAs content (increased acetic acid and butyric acid (only with AXs from soft wheat) concentration) | [5] |
| Commercial corn bran AXs | In vivo (class-I obesity humans) | Long-chain AXs alkali extracted. A/X ratio: 0.56 | Stool consistency and bowel movement frequency Faecal pH SCFA and moisture content Microbiota analysis | AXs altered global bacteria community and reduced bacterial diversity from week 1 of consumption, with no further changes with time. Bacterial shifts were highly individualised. AXs did not influence moisture content and faecal pH. AX consumption resulted in softer faecal consistencies and increased bowel movements. AXs did not modify total SCFAs concentration. AXs increased propionate relative abundance, and butyrate relative abundance decreased. Among the participants, two groups could be differentiated regarding propionate concentration along the intervention: group 1: concentration increased after one week but decreased after six weeks; group 2: concentration did not increase much after one week but sharply increased after six weeks. These two groups showed differences in microbiota between each other, although differences were not significant compared with the baseline. | [5] |
| Triticale bran | In vitro | Alkali-extractable AXs Hydrolysed alkali-extractable AXs Mw: AXs: 747 kDa AXs hydrolysed: 2.63–15.1 kDa. A/X ratio: 0.99 and 0.77–0.15 (hydrolysed AXs) Free and bound ferulic acid (FA and BA): FA > BA for all AXs. | Antioxidant activity Hypoglycaemic effect | Antioxidant capacity was increased when increasing AX concentration. For AXs with similar Mw, AX with a low degree of substitution (DS) had higher antioxidant activity. For AXs with similar DS, high Mw of AX was negatively correlated with its antioxidant activity at high DS. FA and BA in AXs were also important factors affecting its antioxidant activity. Hypoglycaemic effect: Positive, increased by AX concentration. Better for AXs with higher Mw (probably related to viscosity). | [107] |
| Corn bran (4 different genotypes) | In vitro-human faecal fermentation | Alkali extractable AXs A/X ratio: 0.46–0.54 | SCFAs production Relative growth of bacteria | All AXs improved SCFAs production. Differences in SCFAs production (rate, abundance, and distribution) were related to corn genotypes. Different distributions of SCFAs among genotypes were correlated with the abundance of certain bacteria. | [101] |
| Food Product | Main Observations | References or Patent Numbers |
|---|---|---|
| Pasta | Water-soluble AXs increase water absorption. AX structure is modified during elaboration process. AX loss drastically increases when cooking over the optimal cooking time. AX loss in the cooking water is higher when lower Mw of the AXs. The presence of added water-soluble AXs decreases pasta hardness. | [127,128,129,130] |
| Cookies | In general, AXs decrease spread ratio and increase hardness, although the effect is influenced by AX structure. AXs with very low Mw (oligosaccharides) increase the spread ratio. AXs of low Mw can be used to substitute sugar, increase the plasticity of the dough, and reduce the baking time. | [131,132,133,134,135,136,137] |
| Bread | AXs with high Mw have a detrimental effect on bread. AXs with ferulic acid increases dough extensibility. The combined use of AXs and enzymes can be an interesting strategy to increase specific volume and decrease staling. In addition, fermentation, extrusion, or sprouting of the flours/grains can also have a positive affect. AX modulates starch gelatinisation and retards the retrogradation of bread. AXs interfere with the gluten network. AXs with high Mw are more disruptive. Water-soluble AXs can increase bread volume, reduce rejuvenation, and extend bread’s shelf-life, inhibit the growth of ice crystal, and protect the dough network structure. Extending the shelf-life of bread also improves the flour performance. | [52,101,118,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163] |
| CN110938665A CN110938664A | ||
| Beer | AXs sourced from unmalted barley, rye, or oats improve the viscosity, fullness, and taste for low-alcohol beer. AXs promote the stability of wheat beer foam characteristics. | [124,164] |
| Cakes | β-glucan and arabinoxylans increase cake batter consistency and cell density and produce uniform crumbs while slowing down the movement of moisture from crumb to crust. | [165,166,167] |
| Infant formula milk powder | Promotes the growth and development of infants and toddlers. | CN108112702A |
| Infant and follow-on formulae | Controls the levels of glycemic index (Gl) and insulin index (II) in composite meal for infants and small children. | WO2015057151A1 |
| Non-alcoholic beverages | Improves the mouthfeel of sugar and qualities of low calories beverages. Enhances the biological activities of the wheat-based drink. Lowers the glycemic responses on instant tea. Improves smoothness of oat-base beverages. | CN109843086A CN104522811A CN110897023A WO2014177304A1 |
| Fish meal | Improves freezing resistance and nutritive value of fish ball. | CN112841568A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as Functional Food Ingredients: A Review. Foods 2022, 11, 1026. https://doi.org/10.3390/foods11071026
Zannini E, Bravo Núñez Á, Sahin AW, Arendt EK. Arabinoxylans as Functional Food Ingredients: A Review. Foods. 2022; 11(7):1026. https://doi.org/10.3390/foods11071026
Chicago/Turabian StyleZannini, Emanuele, Ángela Bravo Núñez, Aylin W. Sahin, and Elke K. Arendt. 2022. "Arabinoxylans as Functional Food Ingredients: A Review" Foods 11, no. 7: 1026. https://doi.org/10.3390/foods11071026
APA StyleZannini, E., Bravo Núñez, Á., Sahin, A. W., & Arendt, E. K. (2022). Arabinoxylans as Functional Food Ingredients: A Review. Foods, 11(7), 1026. https://doi.org/10.3390/foods11071026







