The Content of Selected Bioactive Compounds in Wines Produced from Dehydrated Grapes of the Hybrid Variety ‘Hibernal’ as a Factor Determining the Method of Producing Straw Wines
Abstract
:1. Introduction
2. Materials and Methods
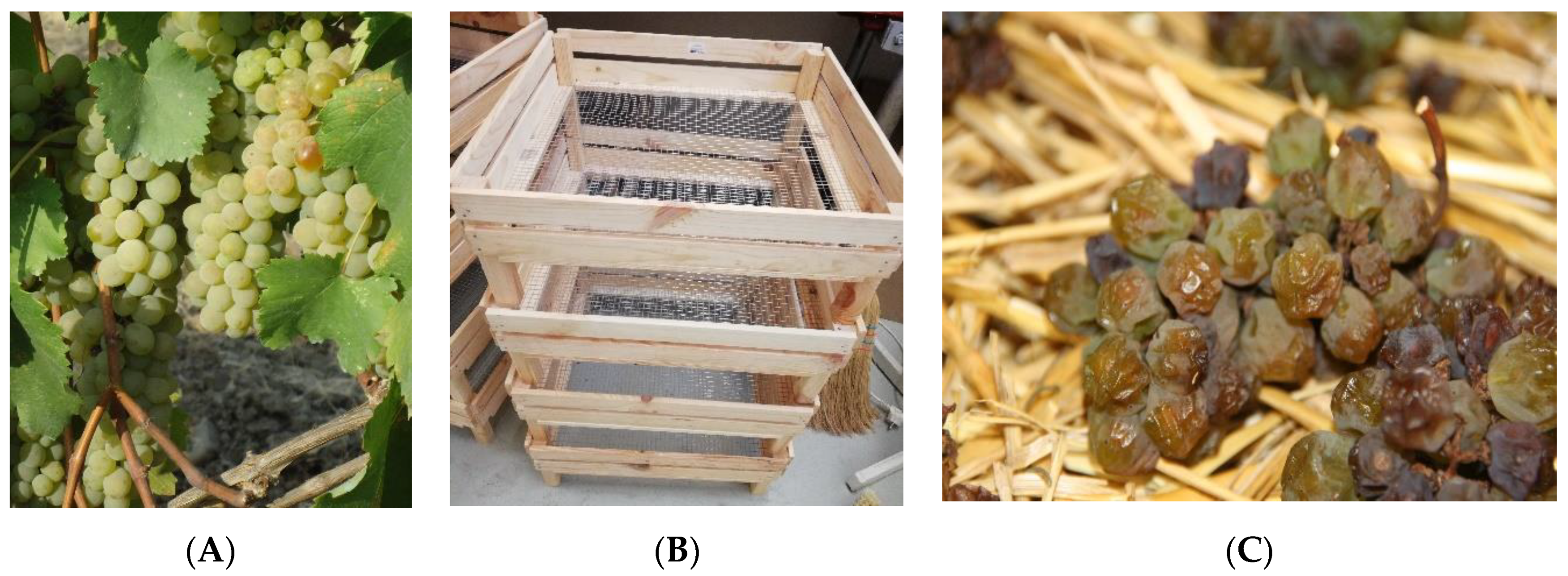
2.1. Biological Material
2.2. Physicochemical Analyzes
2.2.1. pH, the Total Acidity, the Extract Content
2.2.2. Ethanol Content
2.2.3. Acid Profile
2.2.4. Total Phenolic Content (TPC)
2.2.5. Antioxidant Capacity—A FRAP Assay
2.2.6. Antioxidant Capacity—A CUPRAC Assay
2.2.7. Radical Scavenging Capacity (RSC)—A DPPH Assay
2.2.8. Polyphenol Analysis
2.3. Organoleptic Analyses
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz, M.J.; Zea, L.; Moyano, L.; Medina, M. Aroma active compounds during the drying of grapes cv. Pedro Ximenez destined to the production of sweet Sherry wine. Eur. Food Res. Technol. 2010, 230, 429–435. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. Occurrence and infection of Cladosporium, Fusarium, Epicoccum and Aureobasidium in withered rotten grapes during post-harvest dehydration. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 108, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- de Lerma, N.L.; Martínez, T.G.; Moreno, J.; Mauricio, J.C.; Peinado, R.A. Sweet wines with great aromatic complexity obtained by partial fermentation of must from Tempranillo dried grapes. Eur. Food Res. Technol. 2012, 234, 695–701. [Google Scholar] [CrossRef]
- Pigeau, G.M.; Bozza, E.; Kaiser, K.; Inglis, D.L. Concentration effect of Riesling Icewine juice on yeast performance and wine acidity. J. Appl. Microbiol. 2007, 103, 1691–1698. [Google Scholar] [CrossRef] [Green Version]
- Cioch-Skoneczny, M.; Satora, P.; Skoneczny, S.; Skotniczny, M. Biodiversity of yeasts isolated during spontaneous fermentation of cool climate grape musts. Arch. Microbiol. 2021, 203, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Pazourek, J.; Gajdošová, D.; Spanilá, M.; Farková, M.; Novotná, K.; Havel, J. Analysis of polyphenols in wines: Correlation between total polyphenolic content and antioxidant potential from photometric measurements. Prediction of cultivars and vintage from capillary zone electrophoresis fingerprints using artificial neural network. J. Chromatogr. A 2005, 1081, 48–54. [Google Scholar] [CrossRef]
- Ristic, R.; Bindon, K.; Francis, L.I.; Herderich, M.J.; Iland, P.G. Flavonoids and C13-norisoprenoids in Vitis vinifera L. cv. Shiraz: Relationships between grape and wine composition, wine colour and wine sensory properties. Aust. J. Grape Wine Res. 2010, 16, 369–388. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. The effects of enological practices in anthocyanins, phenolic compounds and wine colour and their dependence on grape characteristics. J. Food Compos. Anal. 2007, 20, 546–552. [Google Scholar] [CrossRef]
- Raczkowska, J. Wpływ Polifenoli z Czerwonego Wina na Właściwości Antyoksydacyjne i Przeciwzakrzepowe u Pacjentów z Rakiem Płuc; Wielkopolska Biblioteka Cyfrowa: Poznań, Poland, 2010. [Google Scholar]
- Croce, R.; Malegori, C.; Oliveri, P.; Medici, I.; Cavaglioni, A.; Rossi, C. Prediction of quality parameters in straw wine by means of FT-IR spectroscopy combined with multivariate data processing. Food Chem. 2020, 305, 125512. [Google Scholar] [CrossRef] [PubMed]
- Bellincontro, A.; De Santis, D.; Botondi, R.; Villa, I.; Mencarelli, F. Different postharvest dehydration rates affect quality characteristics and volatile compounds of Malvasia, Trebbiano and Sangiovese grapes for wine production. J. Sci. Food Agric. 2004, 84, 1791–1800. [Google Scholar] [CrossRef]
- Costantini, V.; Bellincontro, A.; De Santis, D.; Botondi, R.; Mencarelli, F. Metabolic changes of Malvasia grapes for wine production during postharvest drying. J. Agric. Food Chem. 2006, 54, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- López de Lerma, N.; Peinado, R.A. Use of two osmoethanol tolerant yeast strain to ferment must from Tempranillo dried grapes. Effect on wine composition. Int. J. Food Microbiol. 2011, 145, 342–348. [Google Scholar] [CrossRef]
- Farkaš, J.; Koval, M. Use of isotachophoresis for identification and determination of acids in wine. Kvas. PRŮMYSL 1982, 28, 256–260. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoǧlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Mpelasoka, B.S.; Schachtman, D.P.; Treeby, M.T.; Thomas, M.R. A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Aust. J. Grape Wine Res. 2003, 9, 154–168. [Google Scholar] [CrossRef]
- Bondada, B.; Harbertson, E.; Shrestha, P.M.; Keller, M. Temporal extension of ripening beyond its physiological limits imposes physical and osmotic challenges perturbing metabolism in grape (Vitis vinifera L.) berries. Sci. Hortic. 2017, 219, 135–143. [Google Scholar] [CrossRef]
- Aponte, M.; Blaiotta, G. Selection of an autochthonous Saccharomyces cerevisiae strain for the vinification of “Moscato di Saracena”, a southern Italy (Calabria Region) passito wine. Food Microbiol. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Soyer, Y.; Koca, N.; Karadeniz, F. Organic acid profile of Turkish white grapes and grape juices. J. Food Compos. Anal. 2003, 16, 629–636. [Google Scholar] [CrossRef]
- Panceri, C.P.; Bordignon-Luiz, M.T. Off-vine grape dehydration process under controlled conditions: Effect on organic acid content of musts and wines. Acta Horticulturae. Int. Soc. Hortic. Sci. 2017, 1188, 391–398. [Google Scholar] [CrossRef]
- Sochorova, L.; Torokova, L.; Baron, M.; Sochor, J. Electrochemical and others techniques for the determination of malic acid and tartaric acid in must and wine. Int. J. Electrochem. Sci. 2018, 13, 9145–9165. [Google Scholar] [CrossRef]
- Domizio, P.; Lencioni, L. Vin Santo. Adv. Food Nutr. Res. 2011, 63, 41–100. [Google Scholar] [PubMed]
- Laureati, M.; Cattaneo, C.; Tateo, F.; Bononi, M. Identification of the Volatile Compounds and Sensory Attributes of Long-Term Aging Vin Santo Wine from Malvasia di Candia Aromatic Grapes. Foods 2020, 9, 1736. [Google Scholar] [CrossRef]
- Giordano, M.; Rolle, L.; Zeppa, G.; Gerbi, V. Chemical and volatile composition of three italian sweet white Passito wines. OENO ONE 2009, 43, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Naumov, G.I.; Naumova, E.S.; Martynenko, N.N.; Masneuf-Pomaréde, I. Taxonomy, ecology, and genetics of the yeast Saccharomyces bayanus: A new object for science and practice. Microbiology 2011, 80, 735–742. [Google Scholar] [CrossRef]
- Znamirowska, A.; Rożek, P.; Buniowska, M.; Kalicka, D. Dynamika fermentacji serwatki niskolaktozowej przez saccharomyces bayanus (bayanus G995) oraz jakość napojów serwatkowych. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2017, 24, 109–120. [Google Scholar] [CrossRef]
- Valášková, I.; Havránek, E. Isotachophoretic analysis of inorganic ions. J. Chromatogr. A 1999, 836, 201–208. [Google Scholar] [CrossRef]
- Nurgel, C.; Pickering, G.J.; Inglis, D.L. Sensory and chemical characteristics of Canadian ice wines. J. Sci. Food Agric. 2004, 84, 1675–1684. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bonesi, M.; Di Lecce, G.; Boselli, E.; Tundis, R.; Pugliese, A.; Menichini, F.; Frega, N.G. Phenolics, Aroma Profile, and In Vitro Antioxidant Activity of Italian Dessert Passito Wine from Saracena (Italy). J. Food Sci. 2013, 78, C703–C708. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A. Grape and wine phenolics: Observations and recent findings. Ciencia e Investigación Agraria 2008, 35, 77–90. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Wine-making of high coloured wines: Extended pomace contact and run-off of juice prior to fermentation. Food Sci. Technol. Int. 2004, 10, 287–295. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Application of pulsed electric fields for improving the maceration process during vinification of red wine: Influence of grape variety. Eur. Food Res. Technol. 2008, 227, 1099–1107. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Suraski, A.; Aviram, M. White Wine with Red Wine-like Properties: Increased Extraction of Grape Skin Polyphenols Improves the Antioxidant Capacity of the Derived White Wine. J. Agric. Food Chem. 2001, 49, 3164–3168. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef] [Green Version]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. Trends Anal. Chem. 2011, 4, 652–664. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and Health-Beneficial Nutrients in Fruits of Eighteen Cucurbita Cultivars: Analysis of Diversity and Dietary Implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Pachón, M.S.; Villaño, D.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Panceri, C.P.; De Gois, J.S.; Borges, D.L.G.; Bordignon-Luiz, M.T. Effect of grape dehydration under controlled conditions on chemical composition and sensory characteristics of Cabernet Sauvignon and Merlot wines. LWT Food Sci. Technol. 2015, 63, 228–235. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Merkyte, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef]
- Avizcuri-Inac, J.M.; González-Hernández, M.; Rosáenz-Oroz, D.; MartínezRuiz, R.; Vaquero-Fernández, L. Chemical and sensory characterisation of sweet wines obtained by different techniques. Ciência Técnica Vitivinícola 2018, 33, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Delgado, M.Á.; González-Hernández, G.; Conde-González, J.E.; Pérez-Trujillo, J.P. Principal component analysis of the polyphenol content in young red wines. Food Chem. 2002, 78, 523–532. [Google Scholar] [CrossRef]
- Pavez, C.; González-Muñoz, B.; O’Brien, J.A.; Laurie, V.F.; Osorio, F.; Núñez, E.; Vega, R.E.; Bordeu, E.; Brossard, N. Red wine astringency: Correlations between chemical and sensory features. LWT 2022, 154, 112656. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Norton, E.L. Chemistry and Reactivity of Tannins in Vitis spp.: A Review. Molecules 2020, 25, 2110. [Google Scholar] [CrossRef] [PubMed]
- Budić-Leto, I.; Zdunić, G.; Gajdoš-Kljusurić, J.; Mucalo, A.; Vrhovšek, U. Differentiation between Croatian dessert wine Prošek and dry wines based on phenolic composition. J. Food Compos. Anal. 2017, 62, 211–216. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007, 101, 449–457. [Google Scholar] [CrossRef]
- Tadmor, R. Marangoni flow revisited. J. Colloid Interface Sci. 2009, 332, 451–454. [Google Scholar] [CrossRef] [PubMed]

| Fresh Grapes | Withered Grapes | |
|---|---|---|
| pH | 3.14 ± 0.01 | 3.31 ± 0.01 |
| Ta (TAE, g/L,) | 8.7 ± 0.15 | 17.9 ± 0.71 |
| TSS (°Brix) | 17.63 ± 0.01 | 40.10 ± 0.00 |
| APPEARANCE | |
| Clarity | clear-hazy (faulty?); intensity: pale, medium, deep |
| Colour | lemon, green, lemon, gold, amber, brown |
| Other observations | e.g., legs/tears, deposit, petillance, bubbles |
| NOSE | |
| Condition | clean, unclean (faulty?); intensity: light, medium (−), medium, medium (+), pronounced |
| Aroma characteristics | e.g., fruits, flowers, spices, vegetables, oak aromas, other |
| Development | youthful, developing, fully developed, tired/past its best |
| PALATE | |
| Sweetness | dry, off-dry, medium-dry, medium-sweet, sweet, luscious |
| Acidity | low, medium (−), medium, medium (+), high |
| Tannin | low, medium (−), medium, medium (+), high |
| Alcohol | low, medium (−), medium, medium (+), high |
| Body | light, medium (−), medium, medium (+), full |
| Flavour | intensity light, medium (−), medium, medium (+), pronounced |
| Flavour characteristics | e.g., fruits, flowers, spices, vegetables, oak flavours, other |
| Length | short, medium (−), medium, medium (+), long |
| CONCLUSIONS | |
| Quality level | faulty, poor, acceptable, good, very good, outstanding |
| Level of readiness for drinking/potential for an ageing | can drink now, drink now, not for drinking, too young but has potential, suitable for ageing, too old for ageing, for ageing or further ageing. |
| Wine A | Wine B | Wine C | |
|---|---|---|---|
| pH | 3.91 a ± 0.18 | 3.53 b ± 0.01 | 3.1 c ± 0.01 |
| TA (g/L) | 8.18 c ± 0.11 | 10.34 a ± 0.41 | 8.95 b ± 0.05 |
| TSS (°Brix) | 18.2 a ± 0.00 | 15.9 b ± 0.06 | 6.9 c ± 0.00 |
| ethanol (vol %) | 18.0 a ± 0.00 | 18.0 a ± 0.00 | 15.0 b ± 0.00 |
| tartaric acid (g/L) | 2.23 b ± 0.03 | 4.61 a ± 0.33 | 2.27 b ± 0.45 |
| citric acid (g/L) | 0.50 a ± 0.04 | 0.55 a ± 0.05 | 0.28 b ± 0.04 |
| malic acid (g/L) | 2.47 b ± 0.10 | 2.99 a ± 0.19 | 1.25 c ± 0.10 |
| succinic acid (g/L) | 1.60 b ± 0.03 | 2.40 a ± 0.07 | 0.57 c ± 0.20 |
| lactic acid (g/L) | 0.52 a ± 0.27 | 0.54 a ± 0.14 | 0.58 a ± 0.25 |
| acetic acid (g/L) | 1.50 a ± 0.01 | 0.95 b ± 0.01 | 0.19 c ± 0.01 |
| Wine A | Wine B | Wine C | |
|---|---|---|---|
| TPC | 1 082.2 b ± 90.76 | 3 748.5 a ± 354.74 | 907.5 b ± 33.23 |
| FRAP | 2.4 b ± 0.28 | 10.2 a ± 1.20 | 3.4 b ± 0.39 |
| CuPRAC | 4.9 b ± 0.22 | 24.6 a ± 1.66 | 5.6 b ± 0.42 |
| RSC | 1.8 c ± 0.05 | 5.0 a ± 0.74 | 1.9 b ± 0.04 |
| Phenolic Compound, mg/L | Wine A | Wine B | Wine C |
|---|---|---|---|
| FLAVONOLS | |||
| (+)-catechin | 56.91 a ± 10.12 | 64.29 a ± 3.65 | 37.82 b ± 0.30 |
| quercetin | 0.22 b ± 0.06 | 0.32 a ± 0.01 | 0.32 a ± 0.01 |
| HYDROXYBENZOIC ACIDS | |||
| vanilin acid | 1.97 b ± 0.30 | 2.83 a ± 0.32 | 1.38 b ± 0.32 |
| syringic acid | 1.55 c ± 0.20 | 2.25 a ±0.19 | 1.27 b ± 0.20 |
| HYDROXYCINNAMIC ACIDS | |||
| chlorogenic acid | 0.2 b ± 0.02 | 0.57 a ± 0.04 | 0.51 a ± 0.02 |
| caffeic acid | 0.04 c ± 0.01 | 7.10 a ± 0.71 | 3.43 b ± 0.03 |
| p-coumaric acid | 1.28 b ± 0.04 | 3.00 a ± 0.25 | 1.58 b ± 0.03 |
| trans-cinnamic acid | 0.28 b ± 0.06 | 0.43 a ± 0.01 | 0.26 b ± 0.01 |
| ferulic acid | 0.19 c ± 0.03 | 2.68 a ± 0.13 | 0.64 b ± 0.04 |
| STILBENES | |||
| trans-resveratrol | 0.03 b ± 0.003 | nd. | 0.18 a ± 0.05 |
| APPERANCE | Wine A | Wine B | Wine C |
|---|---|---|---|
| Clarity | Clear | Clear | Clear |
| Intensity | Medium to deep | Deep | Medium |
| Colour | Amber | Intese amber | Amber |
| Other observations | Tears | Tears | Tears |
| NOSE | |||
| Condition | Clean | Clean | Clean |
| Intensity | Pronounced | Pronounced | Medium |
| Aroma characteristics | Almonds, walnuts, golden apples, honey, wood, dried plum | Almonds, walnuts, golden apples, honey, wood, dried plum | Green apples, pear, hay, vanilla, herbs |
| Development | Developed | Fully developed | Fully developed |
| PALATE | |||
| Sweetness | Medium sweet to sweet | Medium sweet to sweet | Dry to medium dry |
| Acidity | Medium | Medium (−) | Medium |
| Alkohol | High | High | Medium |
| Body | Full | Full | Medium |
| Flovour intensity | Pronounced | Pronounced | Medium |
| Flavour characteristics | Pear, jam, caramel, dried fruit | Apple, jam, caramel, dried fruit | Apple, lemon, grape, pear |
| Lenght | Long | Long | Medium (+) |
| CONCLUSIONS | |||
| Quality level | Good | Very good | Very good |
| Level of readiness for drinking/potential for an ageing | Can drink now, but has potential for ageing | Can drink now, but has potential for ageing | Can drink now, not suitable for ageing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, B.; Bieniasz, M.; Kostecka-Gugała, A. The Content of Selected Bioactive Compounds in Wines Produced from Dehydrated Grapes of the Hybrid Variety ‘Hibernal’ as a Factor Determining the Method of Producing Straw Wines. Foods 2022, 11, 1027. https://doi.org/10.3390/foods11071027
Kowalczyk B, Bieniasz M, Kostecka-Gugała A. The Content of Selected Bioactive Compounds in Wines Produced from Dehydrated Grapes of the Hybrid Variety ‘Hibernal’ as a Factor Determining the Method of Producing Straw Wines. Foods. 2022; 11(7):1027. https://doi.org/10.3390/foods11071027
Chicago/Turabian StyleKowalczyk, Barbara, Monika Bieniasz, and Anna Kostecka-Gugała. 2022. "The Content of Selected Bioactive Compounds in Wines Produced from Dehydrated Grapes of the Hybrid Variety ‘Hibernal’ as a Factor Determining the Method of Producing Straw Wines" Foods 11, no. 7: 1027. https://doi.org/10.3390/foods11071027
APA StyleKowalczyk, B., Bieniasz, M., & Kostecka-Gugała, A. (2022). The Content of Selected Bioactive Compounds in Wines Produced from Dehydrated Grapes of the Hybrid Variety ‘Hibernal’ as a Factor Determining the Method of Producing Straw Wines. Foods, 11(7), 1027. https://doi.org/10.3390/foods11071027







