Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Method
2.2.1. Nonvolatile Compounds Extraction
2.2.2. Quality Control Samples Processing
2.2.3. LC-ESI-MS/MS Analysis of Nonvolatile Compounds
2.2.4. Quantitative Evaluation of Taste Attributes of Oolong Tea from Different Producing Areas
2.3. Statistical Analysis
3. Results and Discussion
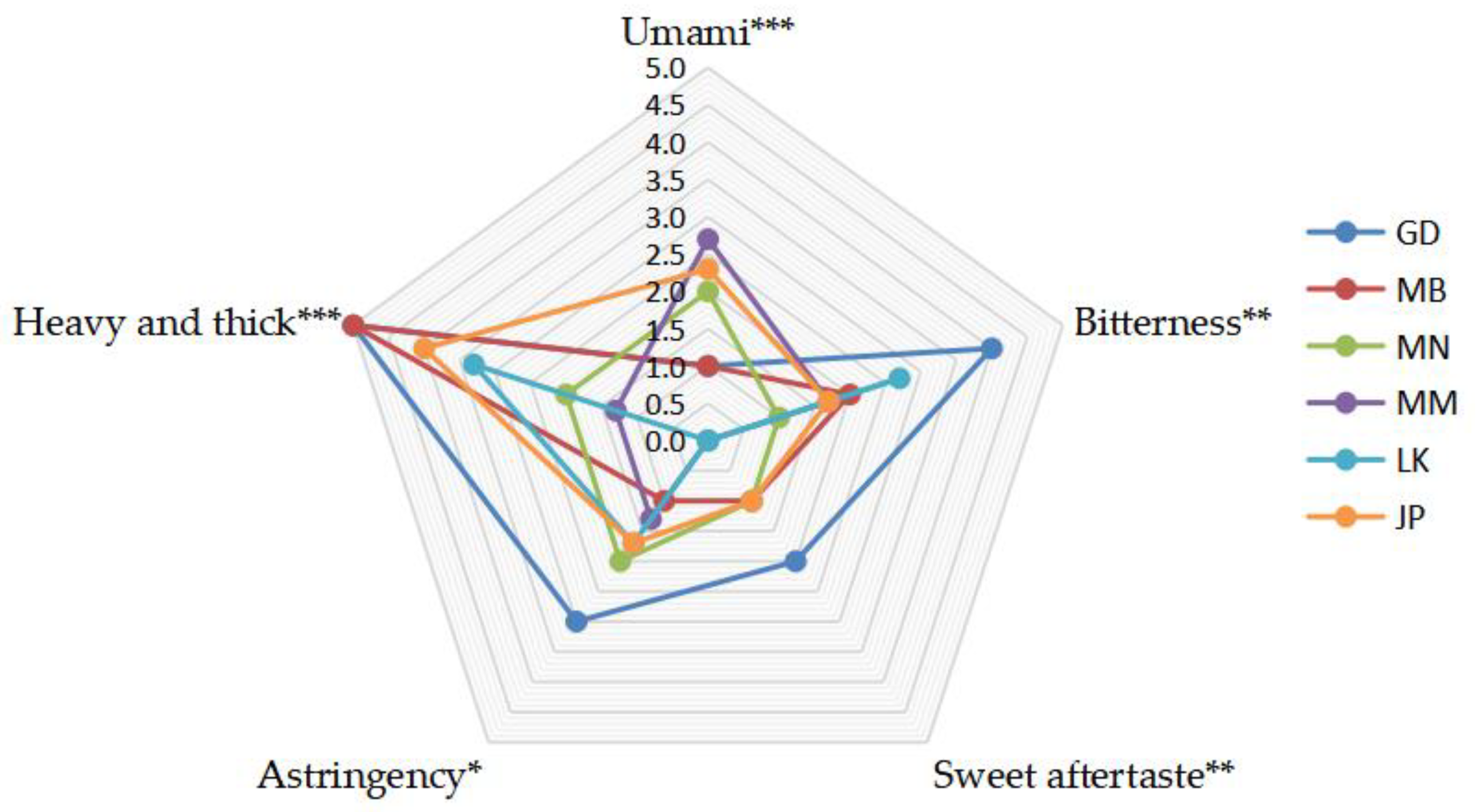
3.1. Profile Analysis of Taste Characteristics of Oolong Tea from Different Producing Areas
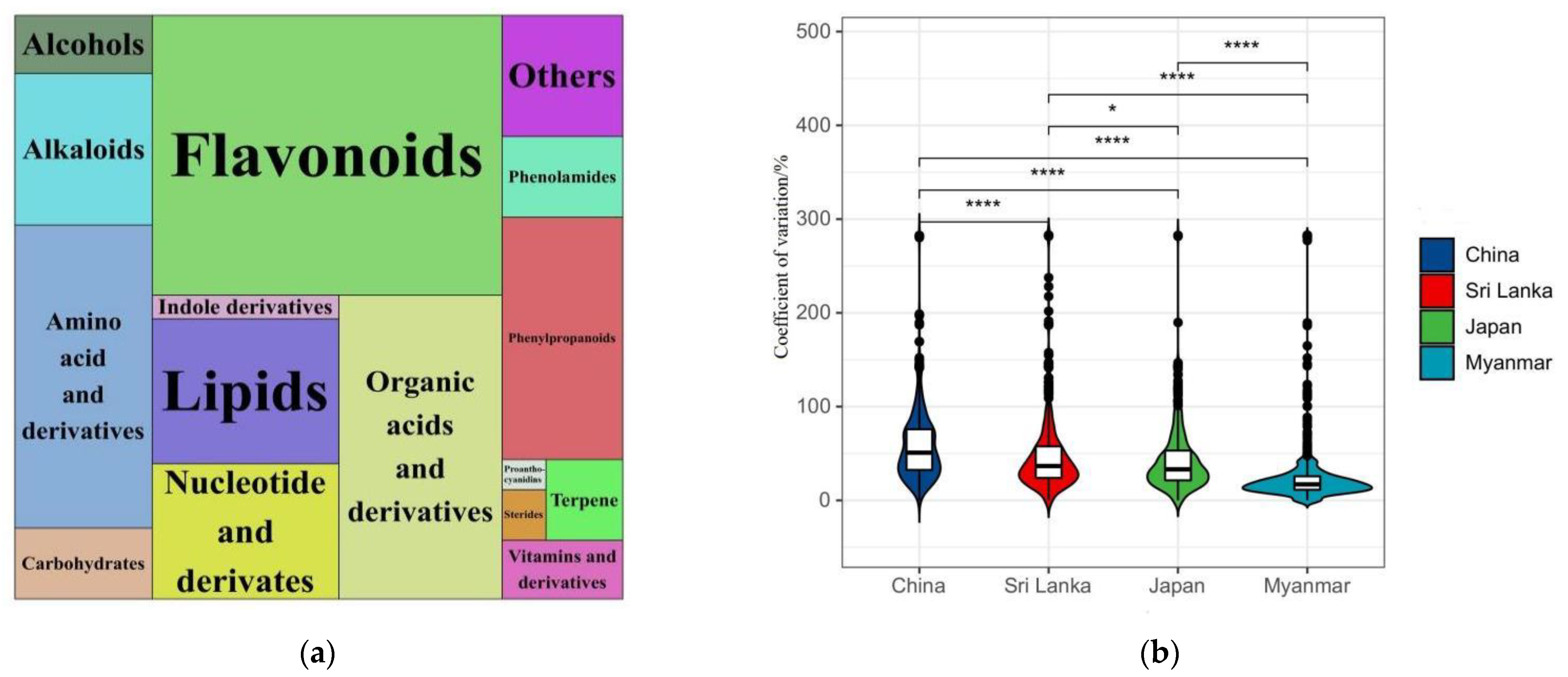
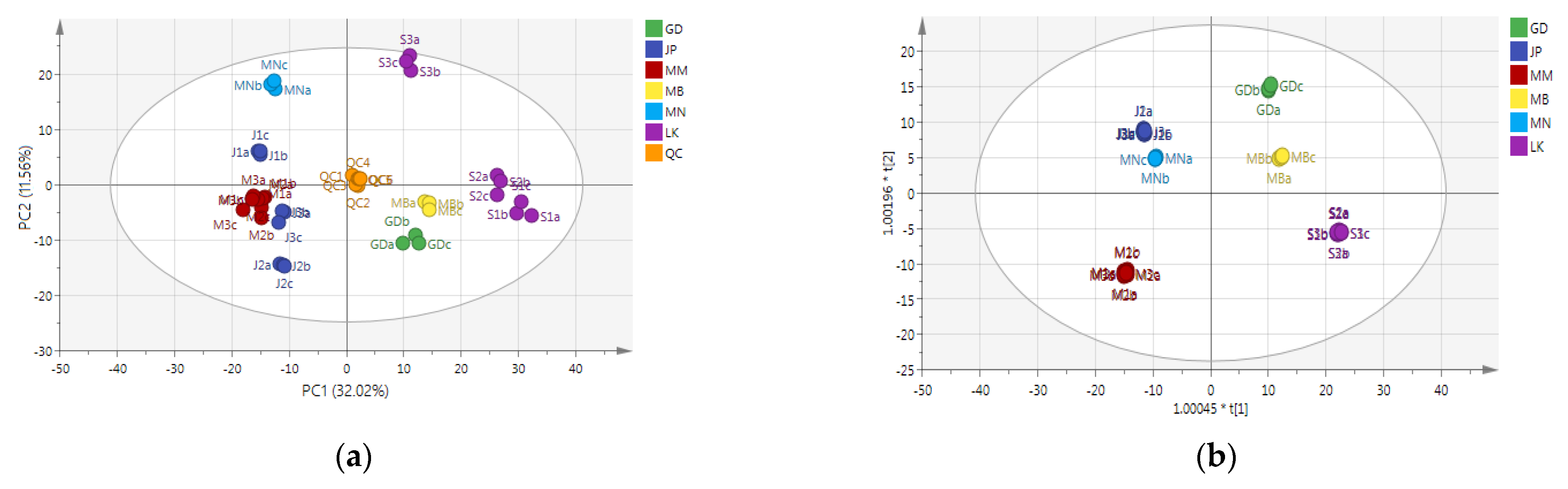
3.2. Identification of Nonvolatile Compounds of Oolong Tea from Different Producing Areas
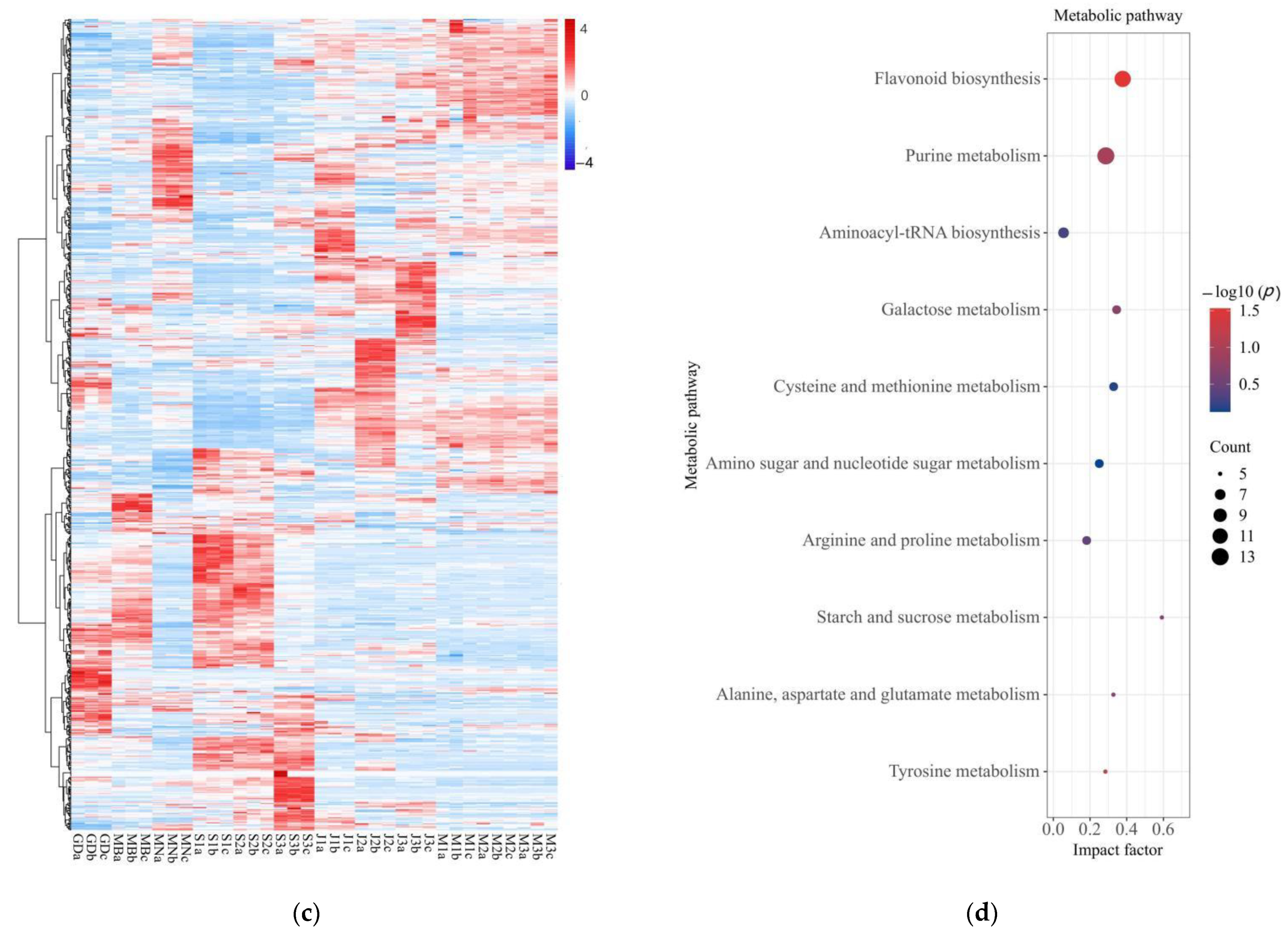
3.3. Analysis of Differential Nonvolatile Compounds and Metabolic Pathways in Oolong Tea from Different Producing Areas
3.4. Differential Nonvolatile Compounds Analysis and Characteristic Nonvolatile Compounds Identification of Oolong Tea from Different Producing Areas
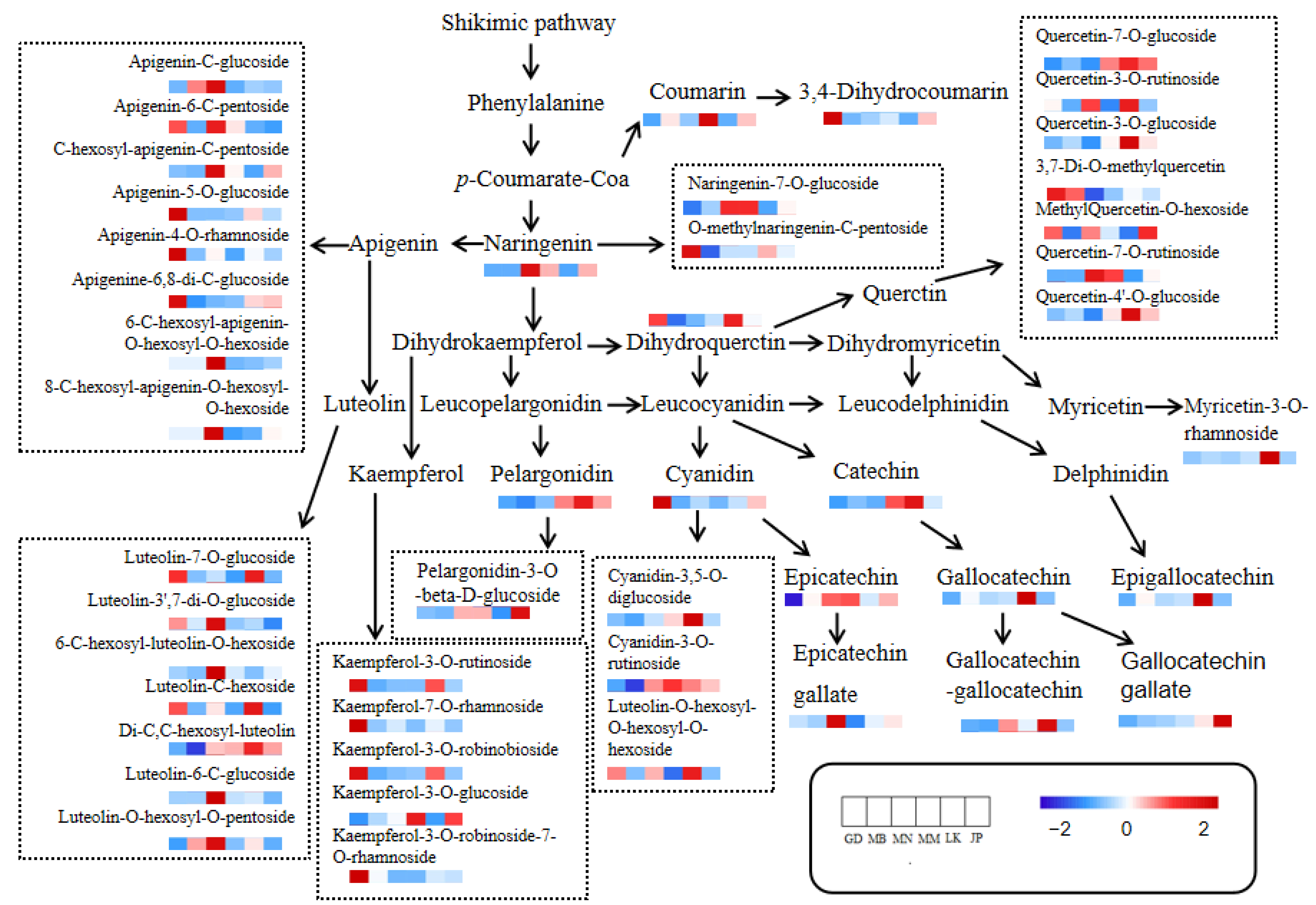
3.4.1. Flavonoids
3.4.2. Amino Acids and Their Derivatives
3.4.3. Saccharides
3.4.4. Alkaloids
3.4.5. Organic Acids and Their Derivatives
3.4.6. Phenylpropanoids, Nucleotides, Lipids and Others
3.5. Relationship between Differential Nonvolatile Compounds and Taste Characteristics of Oolong Tea from Different Producing Areas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, H.L.; Chen, S.S.; Zhou, Z.W.; Li, X.; Chen, S.; Hu, J.; Lai, Z.X.; Sun, Y. Transcriptome analysis reveals the effect of short-term sunlight on aroma metabolism in postharvest leaves of oolong tea (Camellia sinensis). Food Res. Int. 2020, 137, 109347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Kan, Z.P.; Thompson, H.J.; Ling, T.J.; Ho, C.T.; Li, D.X.; Wan, X.C. Impact of six typical processing methods on the chemical composition of tea leaves using a single camellia sinensis cultivar, Longjing 43. J. Agric. Food Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Duan, J.; Jiang, Y.M.; Shi, J.; Peng, L.T.; Xue, S.; Kakuda, Y. Production, quality, and biological effects of Oolong Tea (Camellia sinensis). Food Rev. Int. 2010, 27, 1–15. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Liang, J.; Luo, P.; Qing, L.S.; Ding, L.S. Determination of contents of catechins in Oolong teas by quantitative analysis of multi-components via a single marker (QAMS) method. Food Anal. Meth. 2017, 10, 363–368. [Google Scholar] [CrossRef]
- Guo, Y.L.; Lai, L.L.; Liao, Z.M.; Lin, Y.L. Advances in Oolong Tea Industry. Chin. J. Trop. Crops 2016, 37, 208–212. [Google Scholar]
- Wang, Z.H.; Gan, S.; Sun, W.J.; Chen, Z.D. Quality characteristics of Oolong tea products in different regions and the contribution of thirteen phytochemical components to its taste. Horticulturae 2022, 8, 278. [Google Scholar] [CrossRef]
- Zeng, L.T.; Zhou, X.C.; Su, X.G.; Yang, Z.Y. Chinese Oolong tea: An aromatic beverage produced under multiple stresses. Trends Food Sci. Technol. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Yue, C.N.; Yang, P.X.; Qin, D.D.; Cai, H.L.; Wang, Z.H.; Li, C.; Wu, H.L. Identification of volatile components and analysis of aroma characteristics of Jiangxi Congou black tea. Int. J. Food Prop. 2020, 23, 2160–2173. [Google Scholar] [CrossRef]
- Li, S.B.; Tian, Y.F.; Jiang, P.Y.Z.; Lin, Y.; Liu, X.L.; Yang, H.S. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.Z.; Liu, Z.H.; Liu, Z.X.; Lin, H.Y. Advances in application of metabolomics to Camellia sinensis research. Plant Physiol. Commun. 2017, 53, 1591–1597. [Google Scholar]
- Wang, Z.H.; Ma, B.S.; Ma, C.Q.; Zheng, C.Q.; Zhou, B.X.; Guo, G.Y.; Xia, T. Region identification of Xinyang Maojian tea using UHPLC-Q-TOF/MS-based metabolomics coupled with multivariate statistical analyses. J. Food Sci. 2021, 86, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.J.; Sun, W.J.; Rao, R.; Shyama, P.; Ye, N.X.; Yang, Z.B.; Chen, M.J. Non-targeted metabolomics reveals distinct chemical compositions among different grades of Bai Mudan white tea. Food Chem. 2019, 277, 289–297. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.H.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liang, X.J.; Li, Y.K.; Fan, Y.F.; Li, Y.L.; Cao, Y.L.; An, W.; Shi, Z.G.; Zhao, J.H.; Guo, S.J. Changes in metabolome and nutritional quality of Lycium barbarum fruits from three typical growing areas of China as revealed by widely targeted metabolomics. Metabolites 2020, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.C.; Wu, J.C.; Shahid, M.Q.; He, Y.H.; Lin, S.Q.; Liu, Z.H.; Yang, X.H. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Wang, H.J.; Hua, J.J.; Yu, Q.Y.; Li, J.; Wang, J.J.; Deng, Y.L.; Yuan, H.B.; Jiang, Y.W. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Huang, X.J.; Liu, S.R.; Liu, J.H.; Guo, Y.Q.; Sun, Y.; Lin, J.K.; Guo, Y.L.; Wei, S. Understanding the formation mechanism of oolong tea characteristic non-volatile chemical constitutes during manufacturing processes by using integrated widely-targeted metabolome and DIA proteome analysis. Food Chem. 2020, 310, 125941. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organizationof the United Nations. Available online: http://www.fao.org/faostat/en (accessed on 5 January 2019).
- GB/T 23776-2018; Methodology of Sensory Evaluation of Tea. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 16291.1-2012; Sensory Analysis-General Guidance for the Selection, Training and Monitoring of Assessors—Part 1: Selected Assessors. Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiong, L.Z.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Hiplot. Available online: https://hiplot.com.cn (accessed on 8 October 2021).
- Cai, L.W.; Xu, Y.Q.; Zhou, Y.H.; Wu, S.B. Analysis of sensory quality and chemical composition of Oolong tea in different producing areas. Tea Fujian 2016, 38, 17–19. [Google Scholar]
- Wang, Z.H.; Yang, P.X.; Peng, H.; Li, C.; Yue, C.N.; Li, W.J.; Jiang, X.F. Comprehensive evaluation of 47 tea [Camellia sinensis (L.) O. Kuntze] germplasm based on entropy weight method and grey relational degree. Genet. Resour. Crop Evol. 2021, 68, 3257–3270. [Google Scholar]
- Li, T.H.; Xu, S.S.; Wang, Y.J.; Wei, Y.M.; Shi, L.Y.; Xiao, Z.P.; Liu, Z.Q.; Deng, W.W.; Ning, J.M. Quality chemical analysis of crush–tear–curl (CTC) black tea from different geographical regions based on UHPLC-Orbitrap-MS. J. Food Sci. 2021, 86, 3909–3925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, X.F.; Qiu, J.Q.; Zhang, L.; Zhang, Y.T.; Qiu, X.H.; Huang, Z.H.; Xu, W. Comprehensive comparison on the chemical profile of Guang Chen Pi at different ripeness stages using untargeted and pseudotargeted metabolomics. J. Agric. Food Chem. 2020, 68, 8483–8495. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Shen, Y.; Ling, T.J.; Ho, C.T.; Li, D.X.; Guo, H.M.; Xie, Z.W. Analysis of Differentiated chemical components between Zijuan purple tea and Yunkang green tea by UHPLC-Orbitrap-MS/MS combined with chemometrics. Foods 2021, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, P.H.; Xia, T.; Wan, X.C. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef]
- Yu, Z.M.; Yang, Z.Y. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef]
- Wen, M.C.; Cui, Y.Q.; Dong, C.X.; Zhang, L. Quantitative changes in monosaccharides of Keemun black tea and qualitative analysis of theaflavins-glucose adducts during processing. Food Res. Int. 2021, 148, 110588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Zhao, W.J.; Xu, H.C. Analysis of the fingerprints of different orange varieties and their differential metabolites based on Ultra-Performance Liquid Chromatography coupled with Quadrupole Time-of-Flight Mass Spectrometry and Progenesis QI. Sci. Agric. Sin. 2018, 51, 2551–2560. [Google Scholar]
- Zheng, X.Q.; Nie, Y.; Gao, Y.; Huang, B.; Ye, J.H.; Lu, J.L.; Liang, Y.R. Screening the cultivar and processing factors based on the flavonoid profiles of dry teas using principal component analysis. J. Food Compos. Anal. 2018, 67, 29–37. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yue, C.N.; Tong, H.R. Analysis of taste characteristics and identification of key chemical components of fifteen Chinese yellow tea samples. J. Food Sci. Technol. 2021, 58, 1378–1388. [Google Scholar] [CrossRef]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef]
- Chen, G.H.; Yang, C.Y.; Lee, S.J.; Wu, C.C.; Tzen, J.T.C. Catechin content and the degree of its galloylation in oolong tea are inversely correlated with cultivation altitude. J. Food Drug Anal. 2014, 22, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.N.; Wang, Z.H.; Mao, S.H.; Li, X.Y.; Tong, H.R. The main taste substances in tea research progress. Food Res. Dev. 2017, 38, 219–224. [Google Scholar]
- Guo, X.Y. Function and Mechanism of Theanine in the Formation of Tea Baking Aroma; Anhui Agricultural University: Hefei, China, 2019. [Google Scholar]
- Wan, X.C. Tea Biochemistry, 3rd ed.; China Agricultural Press: Beijing, China, 2007; p. 52. [Google Scholar]
- Liu, Y.X.; Tian, X.; Yang, X.; Zhang, X.J.; Duan, G.F.; Liu, Q.L. Analysis of the differences in bitter and astringent substances in the pelli-cle of different walnut varieties. J. Fruit Sci. 2021, 38, 222–230. [Google Scholar]
- Xie, G.; He, R.R.; Li, Y.B. Research progress on biosynthesis and catabolism of tea alkaloids. Chin. J. Nat. Med. 2010, 8, 153–160. [Google Scholar] [CrossRef]
- Yu, F.; Chen, C.; Chen, S.N.; Wang, K.X.; Huang, H.T.; Wu, Y.Y.; He, P.M.; Tu, Y.Y.; Li, B. Dynamic changes and mechanisms of organic acids during black tea manufacturing process. Food Control 2022, 132, 108535. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Fu, Y.Q.; Wang, J.Q.; Zhang, L.; Wang, F.; Yin, J.F.; Xu, Y.Q. Sensory and chemical characteristics of Tieguanyin oolong tea after roasting. Food Chem. X 2021, 12, 100178. [Google Scholar] [CrossRef]
- Chen, X.; Tian, Y.Y.; Zhang, L.X. The changes of metabolites during the withering process of white tea based on HILIC LC-QqQ MS method. J. Tea Sci. 2020, 40, 238–249. [Google Scholar]
- Zeng, L.T.; Yang, Z.Y. Biosyntheses of volatile phenylpropanoids/benzenoids in tea (Camellia sinensis) and their formations in response to stresses. J. Trop. Subtrop. Bot. 2019, 27, 591–600. [Google Scholar]
- Wang, H.B. Research overview of lipids in tea. Tea Commun. 1988, 4, 26–30. [Google Scholar]
- Cheng, L.Z.; Wang, Y.F.; Zhang, J.R.; Xu, L.R.; Zhou, H.; Wei, K.; Peng, L.L.; Zhang, J.; Liu, Z.H.; Wei, X.L. Integration of non-targeted metabolomics and E-tongue evaluation reveals the chemical variation and taste characteristics of five typical dark teas. LWT-Food Sci. Technol. 2021, 150, 111875. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.L.; Jiang, R.G.; Xu, R.Q.; Zhang, Y.Y.; Luo, Y.; Huang, J.L.; Wang, K.B.; Liu, Z.H. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2020, 344, 128576. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, M.H.; Zheng, G.Y.; Wang, T.T.; Lin, J.; Wang, S.S.; Wang, X.X.; Chao, Q.L.; Cao, S.X.; Yang, Z.B.; et al. Metabolite profiling of 14 Wuyi Rock tea cultivars using UPLC-QTOF MS and UPLC-QqQ MS combined with chemometrics. Molecules 2018, 81, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gan, S.; Sun, W.; Chen, Z. Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas. Foods 2022, 11, 1057. https://doi.org/10.3390/foods11071057
Wang Z, Gan S, Sun W, Chen Z. Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas. Foods. 2022; 11(7):1057. https://doi.org/10.3390/foods11071057
Chicago/Turabian StyleWang, Zhihui, Shuang Gan, Weijiang Sun, and Zhidan Chen. 2022. "Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas" Foods 11, no. 7: 1057. https://doi.org/10.3390/foods11071057
APA StyleWang, Z., Gan, S., Sun, W., & Chen, Z. (2022). Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas. Foods, 11(7), 1057. https://doi.org/10.3390/foods11071057






